Implantable Immunosuppressant Delivery to Prevent Rejection in Transplantation
Abstract
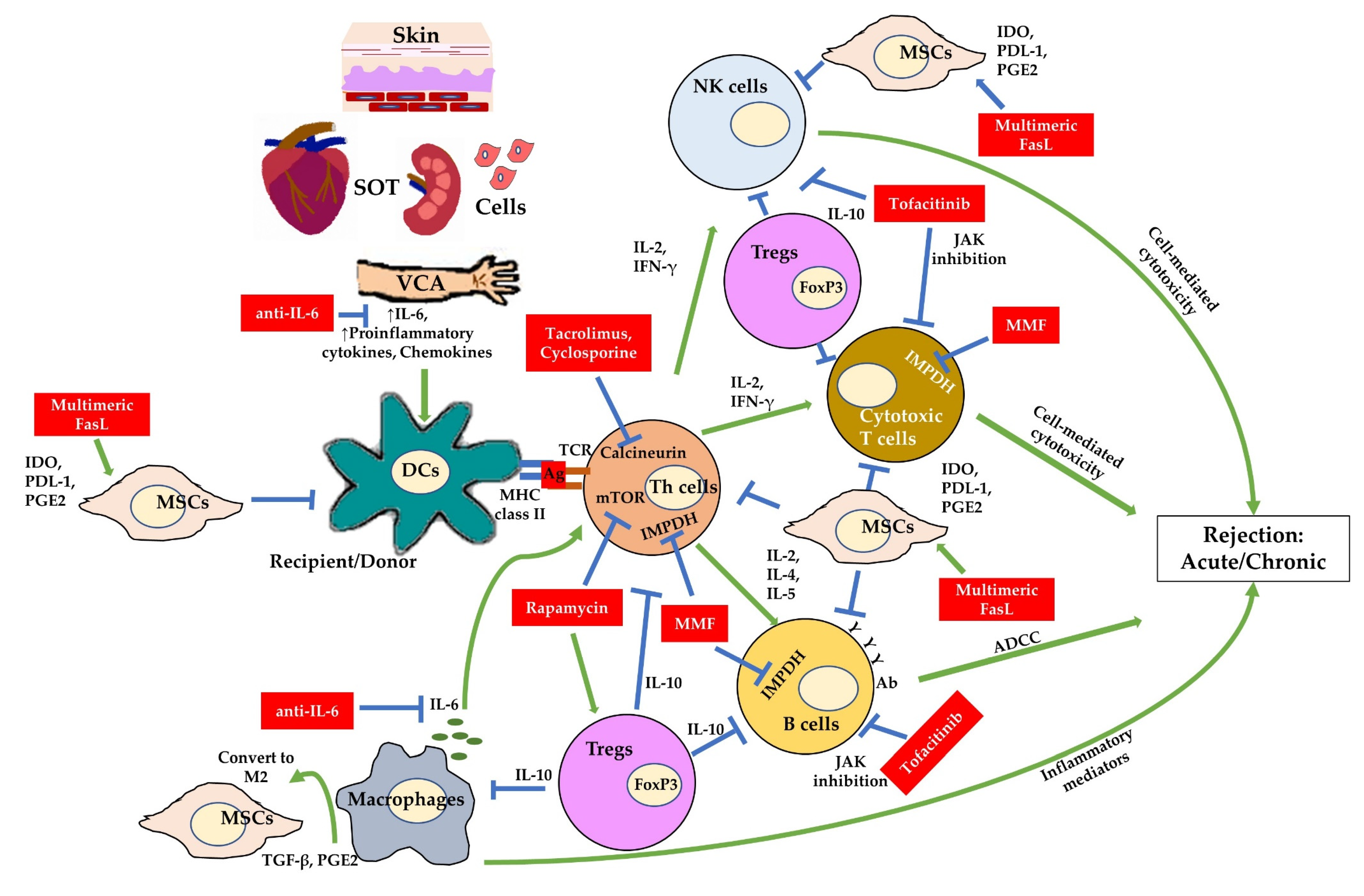
:1. Introduction
2. Immunosuppressants to Prevent Allograft Rejection in Transplantation
3. Types of Implantable Immunosuppressant Delivery System in Transplantation
3.1. Hydrogel
3.2. Microsphere
3.3. Nanoparticles
3.4. Micelles and Liposomes
3.5. Scaffold
4. Applications and Mechanisms of Implantable Immunosuppressant Delivery to Prevent Allograft Rejection
4.1. Immunosuppressant Drugs
4.2. Biological Immunosuppressant
5. Efficacy and Efficiency Compared to Conventional Administration
6. Biocompatibility and Safety
7. Conclusions
8. Challenge and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Flaherty, D.K. Transplantation; Elsevier: St. Louis, MO, USA, 2012. [Google Scholar]
- Rouchi, A.H.; Mahdavi-Mazdeh, M. Regenerative Medicine in Organ and Tissue Transplantation: Shortly and Practically Achievable? Int. J. Organ Transplant. Med. 2015, 6, 93–98. [Google Scholar]
- Kaufman, C.L.; Bhutiani, N.; Ramirez, A.; Tien, H.Y.; Palazzo, M.D.; Galvis, E.; Farner, S.; Ozyurekoglu, T.; Jones, C.M. Current Status of Vascularized Composite Allotransplantation. Am. Surg. 2019, 85, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Gill, R.G. Direct and indirect allograft recognition: Pathways dictating graft rejection mechanisms. Curr. Opin. Organ Transplant. 2016, 21, 40–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.D.; Way, S.S.; Abbas, A.K. Regulatory T cell memory. Nat. Rev. Immunol. 2016, 16, 90–101. [Google Scholar] [CrossRef]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Jiang, S.; Herrera, O.; Lechler, R.I. New spectrum of allorecognition pathways: Implications for graft rejection and transplantation tolerance. Curr. Opin. Immunol. 2004, 16, 550–557. [Google Scholar] [CrossRef]
- Allison, T.L. Immunosuppressive Therapy in Transplantation. Nurs. Clin. N. Am. 2016, 51, 107–120. [Google Scholar] [CrossRef]
- Yeung, M.Y.; Grimmig, T.; Sayegh, M.H. Costimulation Blockade in Transplantation. Adv. Exp. Med. Biol. 2019, 1189, 267–312. [Google Scholar] [CrossRef]
- Citro, A.; Cantarelli, E.; Piemonti, L. Anti-Inflammatory Strategies to Enhance Islet Engraftment and Survival. Curr. Diabetes Rep. 2013, 13, 733–744. [Google Scholar] [CrossRef]
- Jordan, S.C.; Choi, J.; Kim, I.; Wu, G.; Toyoda, M.; Shin, B.; Vo, A. Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade. Transplantation 2017, 101, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Jasiak, N.M.; Park, J.M. Immunosuppression in Solid-Organ Transplantation: Essentials and Practical Tips. Crit. Care Nurs. Q. 2016, 39, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Bunnell, B.A. Adipose Tissue-Derived Stem Cells: Immunomodulatory Effects and Therapeutic Potential. Physiology 2020, 35, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Pallet, N.; Fernández-Ramos, A.A.; Loriot, M.-A. Impact of Immunosuppressive Drugs on the Metabolism of T Cells. Int. Rev. Cell Mol. Biol. 2018, 341, 169–200. [Google Scholar] [CrossRef]
- Naik, R.H.; Shawar, S.H. Renal Transplantation Rejection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Zhuang, Q.; Lakkis, F.G. Dendritic cells and innate immunity in kidney transplantation. Kidney Int. 2015, 87, 712–718. [Google Scholar] [CrossRef] [Green Version]
- Ingulli, E. Mechanism of cellular rejection in transplantation. Pediatr. Nephrol. 2010, 25, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Bevington, S.L.; Cauchy, P.; Withers, D.R.; Lane, P.J.L.; Cockerill, P.N. T Cell Receptor and Cytokine Signaling Can Function at Different Stages to Establish and Maintain Transcriptional Memory and Enable T Helper Cell Differentiation. Front. Immunol. 2017, 8, 204. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, R.; Fitch, Z.W.; Schroder, P.M.; Choi, A.Y.; Jackson, A.M.; Knechtle, S.J.; Kwun, J. B cells in transplant tolerance and rejection: Friends or foes? Transpl. Int. 2019, 33, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Von Borstel, A.; Abdulahad, W.H.; Dekkema, G.; Rutgers, A.; Stegeman, C.A.; Veldman, J.; Heeringa, P.; Sanders, J.S. Mycophenolic acid and 6-mercaptopurine both inhibit B-cell proliferation in granulomatosis with polyangiitis patients, whereas only mycophenolic acid inhibits B-cell IL-6 production. PLoS ONE 2020, 15, e0235743. [Google Scholar] [CrossRef]
- Zand, M.S. Tofacitinab in Renal Transplantation. Transplant. Rev. 2013, 27, 85–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Aubert, O.; Vo, A.; Loupy, A.; Haas, M.; Puliyanda, D.; Kim, I.; Louie, S.; Kang, A.; Peng, A.; et al. Assessment of Tocilizumab (Anti-Interleukin-6 Receptor Monoclonal) as a Potential Treatment for Chronic Antibody-Mediated Rejection and Transplant Glomerulopathy in HLA-Sensitized Renal Allograft Recipients. Am. J. Transplant. 2017, 17, 2381–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.L.; Madsen, J.C. IL-6 Directed Therapy in Transplantation. Curr. Transplant. Rep. 2021, 8, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Velez, M.; Enam, S.F.; Mehta, N.; Lyon, J.G.; LaPlaca, M.C.; Bellamkonda, R.V. Immuno-suppressive hydrogels enhance allogeneic MSC survival after transplantation in the injured brain. Biomaterials 2021, 266, 120419. [Google Scholar] [CrossRef]
- Akiyama, K.; Chen, C.; Wang, D.; Xu, X.; Qu, C.; Yamaza, T.; Cai, T.; Chen, W.; Sun, L.; Shi, S. Mesenchymal-Stem-Cell-Induced Immunoregulation Involves FAS-Ligand-/FAS-Mediated T Cell Apoptosis. Cell Stem Cell 2012, 10, 544–555. [Google Scholar] [CrossRef] [Green Version]
- Balani, S.S.; Jensen, C.J.; Kouri, A.M.; Kizilbash, S.J. Induction and maintenance immunosuppression in pediatric kidney transplantation—Advances and controversies. Pediatr. Transplant. 2021, 25, e14077. [Google Scholar] [CrossRef]
- Enderby, C.; Keller, C.A. An overview of immunosuppression in solid organ transplantation. Am. J. Manag. care 2015, 21, S12–S23. [Google Scholar]
- Dhanasekaran, R. Management of Immunosuppression in Liver Transplantation. Clin. Liver Dis. 2017, 21, 337–353. [Google Scholar] [CrossRef]
- Casati, C.; Menegotto, A.; Querques, M.L.; Ravera, F.; Colussi, G. Immunosuppression in kidney transplantation: A way between efficacy and toxicity. G. Ital. Nefrol. 2017, 34, 29–39. [Google Scholar]
- Fuehner, T.; Benden, C.; Gottlieb, J. Initial immunosuppression and managing rejection. Intensiv. Care Med. 2019, 45, 388–390. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.-B.; Yuan, Y.-B.; Jiang, W.; Liu, J.; Tian, E.-J.; Shun, H.-M.; Huang, D.-H.; Yuan, X.-Y.; Li, H.; Sheng, J. Preparation of rapamycin-loaded chitosan/PLA nanoparticles for immunosuppression in corneal transplantation. Int. J. Pharm. 2008, 349, 241–248. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Fu, S.; Liu, Q.; Dou, D.; Lv, H.; Fan, M.; Guo, G.; Luo, F.; Qian, Z. Preparation of Tacrolimus loaded micelles based on poly(ε-caprolactone)–poly(ethylene glycol)–poly(ε-caprolactone). Int. J. Pharm. 2011, 407, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, A.Y.; Sadi, D.; McAlister, V.C.; Mendez, I. Liposomal Formulations of Tacrolimus and Rapamycin Increase Graft Survival and Fiber Outgrowth of Dopaminergic Grafts. Cell Transplant. 2004, 13, 263–271. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzhonova, D.V.; Olariu, R.; Leckenby, J.; Banz, Y.; Prost, J.-C.; Dhayani, A.; Vemula, P.K.; Voegelin, E.; Taddeo, A.; Rieben, R. Local Injections of Tacrolimus-loaded Hydrogel Reduce Systemic Immunosuppression-related Toxicity in Vascularized Composite Allotransplantation. Transplantation 2018, 102, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Gajanayake, T.; Olariu, R.; Leclère, F.M.; Dhayani, A.; Yang, Z.; Bongoni, A.K.; Banz, Y.; Constantinescu, M.A.; Karp, J.M.; Vemula, P.K.; et al. A single localized dose of enzyme-responsive hydrogel improves long-term survival of a vascularized composite allograft. Sci. Transl. Med. 2014, 6, 249ra110. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-C.; Anggelia, M.R.; Cheng, C.-C.; Ku, K.-L.; Cheng, H.-Y.; Wen, C.-J.; Wang, A.Y.L.; Lin, C.-H.; Chu, I.-M. A Mixed Thermosensitive Hydrogel System for Sustained Delivery of Tacrolimus for Immunosuppressive Therapy. Pharmaceutics 2019, 11, 413. [Google Scholar] [CrossRef] [Green Version]
- Majumder, P.; Zhang, Y.; Iglesias, M.; Fan, L.; Kelley, J.A.; Andrews, C.; Patel, N.; Stagno, J.R.; Oh, B.C.; Furtmüller, G.J.; et al. Multiphase Assembly of Small Molecule Microcrystalline Peptide Hydrogel Allows Immunomodulatory Combination Therapy for Long-Term Heart Transplant Survival. Small 2020, 16, 2002791. [Google Scholar] [CrossRef]
- Li, R.; Liang, J.; He, Y.; Qin, J.; He, H.; Lee, S.; Pang, Z.; Wang, J. Sustained Release of Immunosuppressant by Nanoparticle-anchoring Hydrogel Scaffold Improved the Survival of Transplanted Stem Cells and Tissue Regeneration. Theranostics 2018, 8, 878–893. [Google Scholar] [CrossRef]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef]
- Mohamed, F.; van der Walle, C.F. Engineering Biodegradable Polyester Particles with Specific Drug Targeting and Drug Release Properties. J. Pharm. Sci. 2008, 97, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiong, Y.; Wang, Y.; Chen, J.; Yang, J.; Sun, B. Evaluation of PLGA microspheres with triple regimen on long-term survival of vascularized composite allograft—An experimental study. Transpl. Int. 2020, 33, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fang, K.; Wang, X.; Li, M.; Wu, Y.; Chen, F.; Shahzad, K.A.; Gu, N.; Shen, C. Antigen-Specific Killer Polylactic-Co-Glycolic Acid (PLGA) Microspheres Can Prolong Alloskin Graft Survival in a Murine Model. Immunol. Investig. 2015, 44, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Unadkat, J.V.; Schnider, J.T.; Feturi, F.G.; Tsuji, W.; Bliley, J.M.; Venkataramanan, R.; Solari, M.G.; Marra, K.G.; Gorantla, V.S.; Spiess, A.M. Single Implantable FK506 Disk Prevents Rejection in Vascularized Composite Allotransplantation. Plast. Reconstr. Surg. 2017, 139, 403e–414e. [Google Scholar] [CrossRef] [PubMed]
- Eshita, Y.; Uemoto, S.; Tabata, Y.; Sakamoto, S.; Egawa, H.; Hashida, T.; Inui, K.; Tanaka, K. Drug delivery system using microspheres that contain tacrolimus in porcine small bowel transplantation. Transpl. Int. 2005, 17, 841–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, R.; Yoshida, T.; Tasaki, H.; Umejima, H.; Maeda, M.; Higashi, Y.; Watanabe, S.; Oku, N. Release mechanisms of tacrolimus-loaded PLGA and PLA microspheres and immunosuppressive effects of the microspheres in a rat heart transplantation model. Int. J. Pharm. 2015, 492, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Boverhof, D.R.; Bramante, C.M.; Butala, J.H.; Clancy, S.F.; Lafranconi, M.; West, J.; Gordon, S.C. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 2015, 73, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Shahbaz, S.K.; Foroughi, F.; Soltaninezhad, E.; Jamialahmadi, T.; Penson, P.E.; Sahebkar, A. Application of PLGA nano/microparticle delivery systems for immunomodulation and prevention of allotransplant rejection. Expert Opin. Drug Deliv. 2020, 17, 767–780. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Shirali, A.C.; Look, M.; Du, W.; Kassis, E.; Stout-Delgado, H.W.; Fahmy, T.M.; Goldstein, D.R. Nanoparticle Delivery of Mycophenolic Acid Upregulates PD-L1 on Dendritic Cells to Prolong Murine Allograft Survival. Am. J. Transplant. 2011, 11, 2582–2592. [Google Scholar] [CrossRef]
- Deng, C.; Chen, Y.; Zhang, L.; Wu, Y.; Li, H.; Wu, Y.; Wang, B.; Sun, Z.; Li, Y.; Lv, Q.; et al. Delivery of FK506-loaded PLGA nanoparticles prolongs cardiac allograft survival. Int. J. Pharm. 2020, 575, 118951. [Google Scholar] [CrossRef] [PubMed]
- Giles, A.J.; Hutchinson, M.-K.N.D.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-induced immunosuppression: Mechanisms and implications for immunotherapy. J. Immunother. Cancer 2018, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Zhang, L. Cyclosporine Nanomicelle Eye Drop: A Novel Medication for Corneal Graft Transplantation Treatment. Biol. Pharm. Bull. 2015, 38, 893–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Wang, Y.; Ma, L.; Wang, X.; Chi, H.; Zhang, S.; Liu, T.; Li, Z.; Xiang, D.; Dong, Y.; et al. Rapamycin Nano-Micelle Ophthalmic Solution Reduces Corneal Allograft Rejection by Potentiating Myeloid-Derived Suppressor Cells’ Function. Front. Immunol. 2018, 9, 2283. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, Q.; Chen, W.; Lin, H.; Zhu, Y.; Liu, Y.; Liang, H.; Zhu, F. A novel FK506 loaded nanomicelles consisting of amino-terminated poly(ethylene glycol)-block-poly(D,L)-lactic acid and hydroxypropyl methylcellulose for ocular drug delivery. Int. J. Pharm. 2019, 562, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Luo, Y.; Li, B.; Xia, Y.; Wang, H.; Fu, C. Implantable and Injectable Biomaterial Scaffolds for Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2020, 8, 612950. [Google Scholar] [CrossRef] [PubMed]
- Wahl, E.A.; Fierro, F.A.; Peavy, T.R.; Hopfner, U.; Dye, J.F.; Machens, H.-G.; Egaña, J.T.; Schenck, T.L. In Vitro Evaluation of Scaffolds for the Delivery of Mesenchymal Stem Cells to Wounds. BioMed Res. Int. 2015, 2015, 108571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paez-Mayorga, J.; Capuani, S.; Hernandez, N.; Farina, M.; Chua, C.Y.X.; Blanchard, R.; Sizovs, A.; Liu, H.-C.; Fraga, D.W.; Niles, J.A.; et al. Neovascularized implantable cell homing encapsulation platform with tunable local immunosuppressant delivery for allogeneic cell transplantation. Biomaterials 2020, 257, 120232. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef]
- Ayenehdeh, J.M.; Niknam, B.; Rasouli, S.; Hashemi, S.M.; Rahavi, H.; Rezaei, N.; Soleimani, M.; Liaeiha, A.; Niknam, M.H.; Tajik, N. Immunomodulatory and protective effects of adipose tissue-derived mesenchymal stem cells in an allograft islet composite transplantation for experimental autoimmune type 1 diabetes. Immunol. Lett. 2017, 188, 21–31. [Google Scholar] [CrossRef]
- Fries, C.A.; Lawson, S.D.; Wang, L.C.; Slaughter, K.V.; Vemula, P.K.; Dhayani, A.; Joshi, N.; Karp, J.M.; Rickard, R.F.; Gorantla, V.S.; et al. Graft-implanted, enzyme responsive, tacrolimus-eluting hydrogel enables long-term survival of orthotopic porcine limb vascularized composite allografts: A proof of concept study. PLoS ONE 2019, 14, e0210914. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.; Li, X.; Sheikhi, A.; Zandi, N.; Walker, B.; Saleh, B.; Banouni, N.; Jiang, L.; Ordikhani, F.; Dai, L.; et al. Anti-IL-6 eluting immunomodulatory biomaterials prolong skin allograft survival. Sci. Rep. 2019, 9, 6535. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.-E.; Anggelia, M.R.; Lin, S.-Y.; Chen, C.-Y.; Chu, I.-M.; Lin, C.-H. Thermosensitive Polyester Hydrogel for Application of Immunosuppressive Drug Delivery System in Skin Allograft. Gels 2021, 7, 229. [Google Scholar] [CrossRef]
- Kuppan, P.; Kelly, S.; Polishevska, K.; Hojanepesov, O.; Seeberger, K.; Korbutt, G.S.; Pepper, A.R. Co-localized immune protection using dexamethasone-eluting micelles in a murine islet allograft model. Am. J. Transplant. 2019, 20, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Daneshmandi, S.; Hughes, K.R.; Yu, S.; Bedoya, A.M.; Shea, L.D.; Luo, X. Optimizing PLG nanoparticle-peptide delivery platforms for transplantation tolerance using an allogeneic skin transplant model. Biomaterials 2019, 210, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Jin, Q.; Wu, Y.; Li, H.; Yi, L.; Chen, Y.; Gao, T.; Wang, W.; Wang, J.; Lv, Q.; et al. Immunosuppressive effect of PLGA-FK506-NPs in treatment of acute cardiac rejection via topical subcutaneous injection. Drug Deliv. 2021, 28, 1759–1768. [Google Scholar] [CrossRef]
- Taube, D.; Jones, G.; O’Beirne, J.; Wennberg, L.; Connor, A.; Rasmussen, A.; Bäckman, L. Generic tacrolimus in solid organ transplantation. Clin. Transplant. 2014, 28, 623–632. [Google Scholar] [CrossRef]
- Saunders, R.N.; Metcalfe, M.S.; Nicholson, M.L. Rapamycin in transplantation: A review of the evidence. Kidney Int. 2001, 59, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Woodhead, J.L.; Hall, C.K. Encapsulation Efficiency and Micellar Structure of Solute-Carrying Block Copolymer Nanoparticles. Macromolecules 2011, 44, 5443–5451. [Google Scholar] [CrossRef] [Green Version]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Lai, P.-L.; Lin, Y.-K.; Peng, S.; Lee, L.-Y.; Chen, C.-N.; Chu, I.-M. A poloxamer-polypeptide thermosensitive hydrogel as a cell scaffold and sustained release depot. Polym. Chem. 2016, 7, 2976–2985. [Google Scholar] [CrossRef]
- Gerber, D.A.; Oettinger, C.W.; D’Souza, M.; Milton, G.V.; Larsen, C.P.; Pearson, T.C. Prolongation of Murine Cardiac Allograft Survival by Microspheres Containing TNFα and IL1-β Neutralizing Antibodies. J. Drug Target. 1995, 3, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Wattendorf, U.; Koch, M.C.; Walter, E.; Vörös, J.; Textor, M.; Merkle, H.P. Phagocytosis of poly(L-lysine)-graft-poly(ethylene glycol) coated microspheres by antigen presenting cells: Impact of grafting ratio and poly(ethylene glycol) chain length on cellular recognition. Biointerphases 2006, 1, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tietjen, G.T.; Bracaglia, L.G.; Saltzman, W.M.; Pober, J.S. Focus on Fundamentals: Achieving Effective Nanoparticle Targeting. Trends Mol. Med. 2018, 24, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Greineder, C.F.; Villa, C.H.; Walsh, L.R.; Kiseleva, R.Y.; Hood, E.D.; Khoshnejad, M.; Warden-Rothman, R.; Tsourkas, A.; Muzykantov, V.R. Site-Specific Modification of Single-Chain Antibody Fragments for Bioconjugation and Vascular Immunotargeting. Bioconjugate Chem. 2018, 29, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Shuvaev, V.V.; Pardi, N.; Khoshnejad, M.; Kiseleva, R.Y.; Brenner, J.S.; Uhler, T.; Tuyishime, S.; Mui, B.L.; Tam, Y.K.; et al. PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake. J. Control. Release 2018, 291, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shahzad, K.A.; Li, M.; Zhang, A.; Zhang, L.; Xu, T.; Wan, X.; Shen, C. An Antigen-Presenting and Apoptosis-Inducing Polymer Microparticle Prolongs Alloskin Graft Survival by Selectively and Markedly Depleting Alloreactive CD8+ T Cells. Front. Immunol. 2017, 8, 657. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, K.A.; Wan, X.; Zhang, L.; Pei, W.; Zhang, A.; Younis, M.; Wang, W.; Shen, C. On-target and direct modulation of alloreactive T cells by a nanoparticle carrying MHC alloantigen, regulatory molecules and CD47 in a murine model of alloskin transplantation. Drug Deliv. 2018, 25, 703–715. [Google Scholar] [CrossRef] [Green Version]
- Diehl, R.; Ferrara, F.; Müller, C.; Dreyer, A.Y.; McLeod, D.D.; Fricke, S.; Boltze, J. Immunosuppression for in vivo research: State-of-the-art protocols and experimental approaches. Cell. Mol. Immunol. 2017, 14, 146–179. [Google Scholar] [CrossRef] [Green Version]
- Brandacher, G.; Lee, W.P.A.; Schneeberger, S. Minimizing immunosuppression in hand transplantation. Expert Rev. Clin. Immunol. 2012, 8, 673–684. [Google Scholar] [CrossRef]
- Kumar, R.; Ison, M.G. Opportunistic Infections in Transplant Patients. Infect. Dis. Clin. N. Am. 2019, 33, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Dalmau, A.; Campistol, J.M. Immunosuppressive Therapy and Malignancy in Organ Transplant Recipients: A systematic review. Drugs 2007, 67, 1167–1198. [Google Scholar] [CrossRef] [PubMed]
- Busca, A.; Locatelli, F.; Moscato, D.; Falda, M. Sirolimus-Related Toxicity in Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2005, 11, 647–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hucker, A.; Bunn, F.; Carpenter, L.; Lawrence, C.; Farrington, K.; Sharma, S. Non-adherence to immunosuppressants following renal transplantation: A protocol for a systematic review. BMJ Open 2017, 7, e015411. [Google Scholar] [CrossRef]
- Patel, K.; Atkinson, C.; Tran, D.; Nadig, S.N. Nanotechnological Approaches to Immunosuppression and Tolerance Induction. Curr. Transplant. Rep. 2017, 4, 159–168. [Google Scholar] [CrossRef]
- Sikma, M.A.; van Maarseveen, E.M.; van de Graaf, E.A.; Kirkels, J.H.; Verhaar, M.C.; Donker, D.W.; Kesecioglu, J.; Meulenbelt, J. Pharmacokinetics and Toxicity of Tacrolimus Early After Heart and Lung Transplantation. Am. J. Transplant. 2015, 15, 2301–2313. [Google Scholar] [CrossRef]
- Sutter, D.; Dzhonova, D.V.; Prost, J.-C.; Bovet, C.; Banz, Y.; Rahnfeld, L.; Leroux, J.-C.; Rieben, R.; Vögelin, E.; Plock, J.A.; et al. Delivery of Rapamycin Using In Situ Forming Implants Promotes Immunoregulation and Vascularized Composite Allograft Survival. Sci. Rep. 2019, 9, 9269. [Google Scholar] [CrossRef] [Green Version]
- Taddeo, A.; Tsai, C.; Vögelin, E.; Rieben, R. Novel targeted drug delivery systems to minimize systemic immunosuppression in vascularized composite allotransplantation. Curr. Opin. Organ Transplant. 2018, 23, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, L.B.; Zdravković, A.S.; Nikolić, V.D.; Ilić-Stojanović, S.S. Synthetic Hydrogels and Their Impact on Health and Environment. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1363–1391. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of polymer-based biomaterials and medical devices–regulations, in vitro screening and risk-management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef]
- Liu, C.; Shi, Y.; Hou, L.; Sun, X. Current Progress on Biological Evaluation for Medical Devices. Zhongguo Yi Liao Qi Xie Za Zhi 2021, 45, 72–75. [Google Scholar]
- Hsu, S.-H.; Tang, C.-M.; Lin, C.-C. Biocompatibility of poly(ε-caprolactone)/poly(ethylene glycol) diblock copolymers with nanophase separation. Biomaterials 2004, 25, 5593–5601. [Google Scholar] [CrossRef] [PubMed]
- De Mel, A.; Cousins, B.G.; Seifalian, A.M. Surface Modification of Biomaterials: A Quest for Blood Compatibility. Int. J. Biomater. 2012, 2012, 707863. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Liu, Y.; Liu, C.; Li, X.; Lin, J.; Lanzon, A.L.; Zhang, H.; Chen, M. Biological compatibility, thermal and in vitro simulated degradation for poly(p-dioxanone)/poly(lactide-co-glycolide)/poly(ethylene succinate-co-glycolide). J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1817–1835. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, Y.T.; Zu, Z.H.; Ju, R.K.; Guo, M.Y.; Wang, X.M.; Xu, Q.Y.; Cui, F.Z. Combination of Hyaluronic Acid Hydrogel Scaffold and PLGA Microspheres for Supporting Survival of Neural Stem Cells. Pharm. Res. 2011, 28, 1406–1414. [Google Scholar] [CrossRef]
- Li, D.; Sun, H.; Jiang, L.; Zhang, K.; Liu, W.; Zhu, Y.; Fangteng, J.; Shi, C.; Zhao, L.; Sun, H.; et al. Enhanced Biocompatibility of PLGA Nanofibers with Gelatin/Nano-Hydroxyapatite Bone Biomimetics Incorporation. ACS Appl. Mater. Interfaces 2014, 6, 9402–9410. [Google Scholar] [CrossRef]
- Trcin, M.T.; Zdraveva, E.; Dolenec, T.; Zimić, I.V.; Mihica, M.B.; Batarilo, I.; Dekaris, I.; Blažević, V.; Slivac, I.; Grgurić, T.H.; et al. Poly(ε-caprolactone) Titanium Dioxide and Cefuroxime Antimicrobial Scaffolds for Cultivation of Human Limbal Stem Cells. Polymers 2020, 12, 1758. [Google Scholar] [CrossRef]
- Landgraf, L.; Müller, I.; Ernst, P.; Schäfer, M.; Rosman, C.; Schick, I.; Köhler, O.; Oehring, H.; Breus, V.V.; Basché, T.; et al. Comparative evaluation of the impact on endothelial cells induced by different nanoparticle structures and functionalization. Beilstein J. Nanotechnol. 2015, 6, 300–312. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers–A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef]
- Heirani-Tabasi, A.; Hosseinzadeh, S.; Rabbani, S.; Tafti, S.H.A.; Jamshidi, K.; Soufi-Zomorrod, M.; Soleimani, M. Cartilage tissue engineering by co-transplantation of chondrocyte extracellular vesicles and mesenchymal stem cells, entrapped in chitosan-hyaluronic acid hydrogel. Biomed. Mater. 2021, 16, 055003. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.; Xu, J.; Liu, M.; Li, P.; Li, X.; Fan, Y. A biomimetic hierarchical small intestinal submucosa–chitosan sponge/chitosan hydrogel scaffold with a micro/nano structure for dural repair. J. Mater. Chem. B 2021, 9, 7821–7834. [Google Scholar] [CrossRef]
- Oliveira, J.P.R.; Melendez-Ortiz, H.I.; Bucio, E.; Alves, P.T.; Lima, M.I.S.; Goulart, L.R.; Mathor, M.B.; Varca, G.H.C.; Lugao, A.B. Current Methods Applied to Biomaterials–Characterization Approaches, Safety Assessment and Biological International Standards. Curr. Top. Med. Chem. 2018, 18, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Huhtala, A.; Pohjonen, T.; Salminen, L.; Salminen, A.; Kaarniranta, K.; Uusitalo, H. In vitro biocompatibility of degradable biopolymers in cell line cultures from various ocular tissues: Extraction studies. J. Mater. Sci. Mater. Electron. 2008, 19, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Olariu, R.; Denoyelle, J.; Leclère, F.M.; Dzhonova, D.V.; Gajanayake, T.; Banz, Y.; Hayoz, M.; Constantinescu, M.; Rieben, R.; Vögelin, E.; et al. Intra-graft injection of tacrolimus promotes survival of vascularized composite allotransplantation. J. Surg. Res. 2017, 218, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Italia, J.L.; Bhatt, D.K.; Bhardwaj, V.; Tikoo, K.; Kumar, M.N.V.R. PLGA nanoparticles for oral delivery of cyclosporine: Nephrotoxicity and pharmacokinetic studies in comparison to Sandimmune Neoral®. J. Control. Release 2007, 119, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-H.; Zhang, L.; Zhao, Z.-L.; Liu, J.-H. Preparation and in vitro and in vivo characterization of cyclosporin A-loaded, PEGylated chitosan-modified, lipid-based nanoparticles. Int. J. Nanomed. 2013, 8, 601–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdy, S.; Haddadi, A.; Shayeganpour, A.; Alshamsan, A.; Aliabadi, H.M.; Lavasanifar, A. The Immunosuppressive Activity of Polymeric Micellar Formulation of Cyclosporine A: In Vitro and In Vivo Studies. AAPS J. 2011, 13, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Pan, J.; Li, H.; Yu, D.; Wu, T.; Wang, L.; Wang, Y.; Zhou, L.; Zheng, S. Albumin Based Nanomedicine for Enhancing Tacrolimus Safety and Lymphatic Targeting Efficiency. J. Biomed. Nanotechnol. 2019, 15, 1313–1324. [Google Scholar] [CrossRef]

| Literature, Year | Delivery Systems | Polymer | Transplantation | Animal Model | Immunosuppressant, Dose | Outcome (Survival Rate) |
|---|---|---|---|---|---|---|
| Gajanayake et al., 2014 [38] | Enzyme responsive hydrogel | TGMS | VCA (Orthotopic hindlimb) | Rat | TAC, 7 mg | 6/6, >100 days |
| Dzhonova et al., 2018 [37] | Enzyme responsive hydrogel | TGMS | VCA (Orthotopic hindlimb) | Rat | TAC, 7 mg per 70 days | 5/6, >280 days |
| Li et al., 2018 [41] | Nanoparticle-anchoring hydrogel scaffold | PLGA-PEG-maleimide | Stem cells (EPCs) | Mouse | TAC, 1 mg | 12/12, >14 days |
| Fries et al., 2019 [63] | Enzyme responsive hydrogel | TGMS | VCA (Orthotopic forelimb) | Porcine | TAC, 49 mg | 4/4, 56–93 days |
| Uehara et al., 2019 [64] | Photocrosslinkable hydrogel | Gelatin- methacryloyl | Skin | Porcine | Anti-IL-6, (0.4% w/v) | 5/5, MST: 23 days |
| Majumder et al., 2020 [40] | Microcrystalline hydrogel 1 | TFA/ thioanisole/ ethanedithiol | Heart | Mouse | Tofacitinib, 750 μg | 5/5, up to 160 days |
| Wu et al., 2021 [65] | Thermosensitive hydrogel | mPEG-PLCL/PVP | Skin | Rat | TAC, 10 mg | 2/6, >30 days |
| Alvarado-Velez et al., 2021 [26] | Lipid microtubes agarose hydrogel | Agarose | MSC | Rat | Multimeric Fas ligand 12 μg | 8/8, >6 days |
| Literature, Year | Delivery Systems | Polymer | Transplantation | Animal Model | Immunosuppressant, Dose | Outcome (Survival Rate) |
|---|---|---|---|---|---|---|
| Wang et al., 2015 [45] | Microsphere | PLGA | Skin | Mouse | H-2Kb/OVA257–264 monomers, 100 μg | 16/16, 14–19 days |
| Unadkat et al., 2017 [46] | Disk microsphere | PLGA | VCA (Orthotopic hindlimb) | Rat | TAC, 40 mg | 6/6, >180 days |
| Wei et al., 2018 [56] | Nanomicelles | PVCL-PVA-PEG | Cornea | Mouse | RAPA, 3 × 0.05 mg/day | 18/20, >60 days |
| Kuppan et al., 2019 [66] | Micelles 1 | PLGA | Islet | Mouse | Dexametasone, 2 mg | 8/10, >60 days |
| Liu et al., 2019 [57] | Nanomicelles | NH2-PEG-b-PLA and mPEG-b-PLA | Cornea | Rat | TAC, 87.5 μg/day | 10/10, MST: 27.5 days |
| Shah et al., 2019 [67] | Nanoparticles | PLG | Skin | Mouse | ECDI peptide, 3 mg | 6/6, >90 days |
| Wang et al., 2020 [44] | Microsphere | PLGA | VCA (Orthotopic hind-limb) | Rat | TAC, MMF, PDNN, 6 mg, 300 mg, 60 mg | 6/6, >150 days |
| Deng et al., 2021 [68] | Nanoparticles | PLGA | Heterotopic abdominal heart | Rat | TAC, 3 mg/kg | 3/6, >28 days |
| Systemic Administration | Local Delivery | Ref | |
|---|---|---|---|
| Concentration in the blood | Fluctuate | Initial burst then stable | [38,63,82,87] |
| Side effects | More | Less | [37,82,84,87,88,89] |
| Patient compliance issue | More | Less | [86,89,90] |
| Dosing frequency | High | Low | [37,38,64,81,89] |
| Drug efficacy | Low | High | [38,63,81] |
| Drug dosage | More | Less | [38,63,81] |
| Additional cost | No | Yes (with biomaterial production) | [91,92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anggelia, M.R.; Huang, R.-W.; Cheng, H.-Y.; Lin, C.-H.; Lin, C.-H. Implantable Immunosuppressant Delivery to Prevent Rejection in Transplantation. Int. J. Mol. Sci. 2022, 23, 1592. https://doi.org/10.3390/ijms23031592
Anggelia MR, Huang R-W, Cheng H-Y, Lin C-H, Lin C-H. Implantable Immunosuppressant Delivery to Prevent Rejection in Transplantation. International Journal of Molecular Sciences. 2022; 23(3):1592. https://doi.org/10.3390/ijms23031592
Chicago/Turabian StyleAnggelia, Madonna Rica, Ren-Wen Huang, Hui-Yun Cheng, Chih-Hung Lin, and Cheng-Hung Lin. 2022. "Implantable Immunosuppressant Delivery to Prevent Rejection in Transplantation" International Journal of Molecular Sciences 23, no. 3: 1592. https://doi.org/10.3390/ijms23031592






