Modernistic and Emerging Developments of Nanotechnology in Glioblastoma-Targeted Theranostic Applications
Abstract
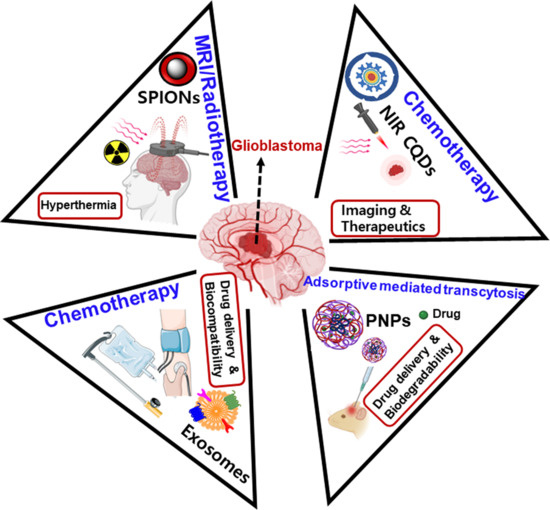
:1. Introduction
2. Challenges and Strategies of the Nanotechnology against Glioblastoma
3. Recent Developments in the Nanotechnology for Investigating Glioblastoma
3.1. Exosomes
3.2. Carbon-Based Nanomaterials
3.3. Liposomes
3.4. Metal-Based Nanoparticles
3.5. Magnetic Nanoparticles
3.6. Polymer-Based Nanoparticles
3.7. Clinical Trials-Related Studies of Nanomaterials for the Glioblastoma Treatment
4. Future Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BBB | blood–brain barrier |
| NIR | near-infrared |
| PA | photoacoustic |
| EPR | enhanced permeability and retention |
| PDT | photodynamic therapy |
| GBM | glioblastoma multiforme |
| AuNPs | gold nanoparticles |
| AgNPs | silver nanoparticles |
| PEG | polyethylene glycol |
| ROS | reactive oxygen species |
| PTT | photothermal therapy |
| AMF | alternating magnetic field |
| DR5 | death receptor 5 |
| SERS | surface-enhanced Raman scattering |
| PLGA | poly (lactic-co-glycolic acid) |
| CMT | carrier-mediated transport |
| RMT | receptor-mediated transcytosis |
| AMT | adsorption-mediated transcytosis |
| BTICs | brain tumor-initiating cells |
| PBAEs | poly (β-amino esters) |
| BSA | bovine serum albumin |
References
- Ganipineni, L.P.; Danhier, F.; Préat, V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J. Control. Release 2018, 281, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiniger, S.C.J.; Kreuter, J.; Khalansky, A.S.; Skidan, I.N.; Bobruskin, A.I.; Smirnova, Z.S.; Severin, S.E.; UHL, R.; Kock, M.; Geiger, K.D.; et al. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int. J. Cancer 2004, 109, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Kim, P. Cancer cell-sticky hydrogels to target the cell membrane of invading glioblastomas. ACS Appl. Mater. Interfaces 2021, 13, 31371–31378. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.B.; Qin, L.; Xiao, W.; Hu, C.; Zhou, Y.; Wang, R.R.; Sun, X.; Yu, W.Q.; He, Q.; Gao, H.L. Acid-responsive transferrin dissociation and glut mediated exocytosis for increased blood-brain barrier transcytosis and programmed glioma targeting delivery. Adv. Funct. Mater. 2018, 28, 1802227. [Google Scholar] [CrossRef]
- Abdo, J.; Cornell, D.L.; Mittal, S.K.; Agrawal, D.K. Immunotherapy plus cryotherapy: Potential augmented abscopal effect for advanced cancers. Front. Oncol. 2018, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Zhong, J.; Dou, N.N.; Visocchi, M.; Gao, G. One-Pot Aqueous Synthesization of near-infrared quantum dots for bioimaging and photodynamic therapy of gliomas. Acta Neurochir. Suppl. 2017, 124, 303–308. [Google Scholar]
- Mangraviti, A.; Tzeng, S.Y.; Kozielski, K.L.; Wang, Y.; Jin, Y.; Gullotti, D.; Pedone, M.; Buaron, N.; Liu, A.; Wilson, D.R.; et al. Polymeric nanoparticles for nonviral gene therapy extend brain tumor survival in vivo. ACS Nano 2015, 9, 1236–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Játiva, P.; Ceña, V. Use of nanoparticles for glioblastoma treatment: A new approach. Nanomedicine 2017, 12, 2533–2554. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.; Ward, S.M.; Nilsson, C.L.; Emrick, T. Augmenting glioblastoma chemotherapy with polymers. ACS Chem. Neurosci. 2018, 9, 8–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhunia, S.; Vangala, V.; Bhattacharya, D.; Ravuri, H.G.; Kuncha, M.; Chakravarty, S.; Sistla, R.; Chaudhuri, A. Large amino acid transporter 1 selective liposomes of L-dopa functionalized amphiphile for combating glioblastoma. Mol. Pharm. 2017, 14, 3834–3847. [Google Scholar] [CrossRef] [PubMed]
- Richiardi, J.; Eryilmaz, H.; Schwartz, S.; Vuilleumier, P.; Van De Ville, D. Decoding brain states from fMRI connectivity graphs. NeuroImage 2011, 56, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.R.; Muzi, M.; Peck, M.; Krohn, K.A. Multimodality brain tumor imaging: MR imaging, PET, and PET/MR imaging. J. Nucl. Med. 2015, 56, 1554–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbault, M.; Chauvet, D.; Gennisson, J.L.; Capelle, L.; Tanter, M. Intraoperative functional ultrasound imaging of human brain activity. Sci. Rep. 2017, 7, 7304. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Johnson, J.; Peck, A.; Xie, Q. Near infrared fluorescent imaging of brain tumor with IR780 dye incorporated phospholipid nanoparticles. J. Transl. Med. 2017, 15, 18. [Google Scholar] [CrossRef] [Green Version]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yong, W.H.; Sun, Y.; Vernier, P.T.; Koeffler, H.P.; Gundersen, M.A.; Marcu, L. Receptor-targeted quantum dots: Fluorescent probes for brain tumor diagnosis. J. Biomed. Opt. 2007, 12, 044021. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhou, W.; Li, X.; Ren, J.; Ji, G.; Du, J.; Tian, W.; Liu, Q.; Hao, A. Graphene oxide suppresses the growth and malignancy of glioblastoma stem cell-like spheroids via epigenetic mechanisms. J. Transl. Med. 2020, 18, 200. [Google Scholar] [CrossRef]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Penga, Z.; Leblanc, R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef]
- Rabha, B.; Bharadwaj, K.K.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Kari, Z.A.; Edinur, H.A.; Baishya, D.; Atanase, L.I. Development of polymer-based nanoformulations for glioblastoma brain cancer therapy and diagnosis: An update. Polymers 2021, 13, 4114. [Google Scholar] [CrossRef]
- Belhadj, Z.; Ying, M.; Cao, X.; Hu, X.; Zhan, C.; Wei, X.; Gao, J.; Wang, X.; Yan, Z.; Lu, W. Design of Y-shaped targeting material for liposome-based multifunctional glioblastoma-targeted drug delivery. J. Control. Release 2017, 255, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, G.; Cho, S.B.; Im, H.J. Radiosensitizing high-Z metal nanoparticles for enhanced radiotherapy of glioblastoma multiforme. J. Nanobiotechnol. 2020, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Marekova, D.; Turnovcova, K.; Sursal, T.H.; Gandhi, C.D.; Jendelova, P.; Uniyal, M.J. Potential for treatment of glioblastoma: New aspects of superparamagnetic iron oxide nanoparticles. Anticancer Res. 2020, 40, 5989–5994. [Google Scholar] [CrossRef] [PubMed]
- Orza, A.; Soriţău, O.; Tomuleasa, C.; Olenic, L.; Florea, A.; Pana, O.; Bratu, L.; Pall, E.; Florian, S.; Casciano, D.; et al. Reversing chemoresistance of malignant glioma stem cells using gold nanoparticles. Int. J. Nanomed. 2013, 8, 689–702. [Google Scholar] [CrossRef] [Green Version]
- Ruan, S.; Xie, R.; Qin, L.; Yu, M.; Xiao, W.; Hu, C.; Yu, W.; Qian, Z.; Ouyang, L.; He, Q.; et al. Aggregable nanoparticles-enabled chemotherapy and autophagy inhibition combined with anti-PD-L1 antibody for improved glioma treatment. Nano Lett. 2019, 19, 8318–8332. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Kim, J.; Mondal, S.K.; Tzeng, S.Y.; Rui, Y.; Al-kharboosh, R.; Kozielski, K.K.; Bhargav, A.G.; Garcia, C.A.; Hinojosa, A.Q.; Green, J.J. Poly(ethylene glycol)-poly(beta-amino ester)-based nanoparticles for suicide gene therapy enhance brain penetration and extend survival in a preclinical human glioblastoma orthotopic xenograft model. ACS Biomater. Sci. Eng. 2020, 6, 2943–2955. [Google Scholar] [CrossRef]
- Garcia, H.R.; Loera, C.R.; Malouff, T.D.; Seneviratne, D.S.; Palmer, J.D.; Trifiletti, D.M. Novel strategies for nanoparticle-based radiosensitization in glioblastoma. Int. J. Mol. Sci. 2021, 22, 9673. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Nikolaev, B.P.; Ryzhov, V.A.; Yakovleva, L.Y.; Marchenko, Y.Y.; Parr, M.A.; Rolich, V.I.; Mikhrina, A.L.; Dobrodumov, A.V.; Pitkin, E.; et al. Ionizing radiation improves glioma-specific targeting of superparamagnetic iron oxide nanoparticles conjugated with cmHsp70.1 monoclonal antibodies (SPION–cmHsp70.1). Nanoscale 2015, 7, 20652–20664. [Google Scholar] [CrossRef]
- Liu, P.; Jin, H.; Guo, Z.; Ma, J.; Zhao, J.; Li, D.; Wu, H.; Gu, N. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int. J. Nanomed. 2016, 11, 5003–5014. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Huang, Z.; Chen, Z.; Xu, R.; Wu, H.; Zang, F.; Wang, C.; Gu, N. Silver nanoparticles: A novel radiation sensitizer for glioma? Nanoscale 2013, 5, 11829–11836. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, M.; Locatelli, E.; Rasile, M.; Monaco, I.; Rodighiero, S.; Corradini, I.; Franchini, M.C.; Passoni, L.; Matteoli, M. A Combined approach employing chlorotoxin-nanovectors and low dose radiation to reach infiltrating tumor niches in glioblastoma. ACS Nano 2016, 10, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Cai, G.; Li, Q. Exosomes as actively targeted nanocarriers for cancer therapy. Int. J. Nanomed. 2020, 15, 4257–4273. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J.; et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chung, J.; Balaj, L.; Charest, A.; Bigner, D.D.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Weissleder, R.; Lee, H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat. Med. 2012, 18, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Wurdinger, T.; Rijn, S.V.; Meijer, D.; Gainche, L.; Esteves, M.S.; Curry Jr, W.T.; Carter, R.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Li, J.; Kong, J.; Ma, S.; Li, J.; Mao, M.; Chen, K.; Chen, Z.; Zhang, J.; Chang, Y.; Yuan, H.; et al. Exosome-coated 10B carbon dots for precise boron neutron capture therapy in a mouse model of glioma in situ. Adv. Funct. Mater. 2021, 31, 2100969. [Google Scholar] [CrossRef]
- Hendrix, M.J.C.; Seftor, E.A.; Seftor, R.E.B.; Kulesa, J.K.; Kulesa, P.M.; Postovit, L.M. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat. Rev. Cancer 2007, 7, 246–255. [Google Scholar] [CrossRef]
- Zhou, S.; Abdouh, M.; Arena, V.; Arena, M.; Arena, G.O. Reprogramming malignant cancer cells toward a benign phenotype following exposure to human embryonic stem cell microenvironment. PLoS ONE 2017, 12, e0169899. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.P.; Mardini, O.; Ericsson, M.; Prabhakar, S.; Maguire, C.A.; Chen, J.W.; Tannous, B.A.; Breakefield, X.O. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014, 8, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Ling, X.; Yang, Y.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Chen, B.; Li, H.; Wang, Y.; et al. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Adv. Sci. 2019, 6, 1801899. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Xu, H.; Ye, B.C. Membrane-decorated exosomes for combination drug delivery and improved glioma therapy. Langmuir 2022, 38, 299–308. [Google Scholar] [CrossRef]
- Hsieh, F.Y.; Zhilenkov, A.V.; Voronov, I.I.; Khakina, E.A.; Mischenko, D.V.; Troshin, P.A.; Hsu, S.H. Water-soluble fullerene derivatives as brain medicine: Surface chemistry determines if they are neuroprotective and antitumor. ACS Appl. Mater. Interfaces 2017, 9, 11482–11492. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, D.; Lu, W.; Hu, X.; Hong, H.; Cai, T. Positron emission tomography (PET) guided glioblastoma targeting by a fullerene-based nanoplatform with fast renal clearance. Acta Biomater. 2017, 61, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Usenko, C.Y.; Harper, S.L.; Tanguay, R.L. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon 2007, 45, 1891–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Zhang, X.; Zhang, X.; Zhang, G.; Zheng, J.; Guan, M.; Fang, X.; Wang, C.; Shu, C. C70-carboxyfullerenes as efficient antioxidants to protect cells against oxidative-induced stress. ACS Appl. Mater. Interfaces 2013, 5, 11101–11107. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, H.L.; Shultz, M.D.; Henderson, S.C.; Cooper, P.; Broaddus, W.C.; Chen, Z.J.; Shu, C.-Y.; Zhang, J.; Ge, J.; Dorn, H.C.; et al. Conjugation of functionalized gadolinium metallofullerenes with IL-13 peptides for targeting and imaging glial tumors. Nanomedicine 2011, 6, 449–458. [Google Scholar] [CrossRef]
- Shultz, M.D.; Duchamp, J.C.; Wilson, J.D.; Shu, C.-Y.; Ge, J.; Zhang, J.; Gibson, H.W.; Fillmore, H.L.; Hirsch, J.I.; Dorn, H.C.; et al. Encapsulation of a radiolabeled cluster inside a fullerene cage, 177LuxLu(3−x)N@C80: An interleukin-13-conjugated radiolabeled metallofullerene platform. J. Am. Chem. Soc. 2010, 132, 4980–4981. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Huang, R.; Liang, G.; Zhang, Z.; Zhang, P.; Yu, S.; Kong, J. MRI-visualized, dual-targeting, combined tumor therapy using magnetic graphene-based mesoporous silica. Small 2014, 10, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kargar, S.; Khoei, S.; Khoee, S.; Shirvalilou, S.; Mahdavi, S.R. Evaluation of the combined effect of NIR laser and ionizing radiation on cellular damages induced by IUdR-loaded PLGA-coated nano-graphene oxide. Photodiagn. Photodyn Ther. 2018, 21, 91–97. [Google Scholar] [CrossRef]
- Shirvalilou, S.; Khoei, S.; Khoee, S.; Mahdavi, S.R.; Raoufi, N.J.; Motevalian, M.; Karimi, M.Y. Enhancement radiation-induced apoptosis in C6 glioma tumor-bearing rats via pH responsive magnetic graphene oxide nanocarrier. J. Photochem. Photobiol. B Biol. 2020, 205, 111827. [Google Scholar] [CrossRef] [PubMed]
- Jovito, B.L.; Paterno, L.G.; Sales, M.J.A.; Gross, M.A.; Silva, L.P.; de Souza, P.; Bao, S.N. Graphene oxide/zinc oxide nanocomposite displaying selective toxicity to glioblastoma cell lines. ACS Appl. Bio Mater. 2021, 4, 829–843. [Google Scholar] [CrossRef]
- Perini, G.; Palmieri, V.; Ciasca, G.; D’Ascenzo, M.; Gervasoni, J.; Primiano, A.; Rinaldi, M.; Fioretti, D.; Prampolini, C.; Tiberio, F.; et al. Graphene quantum dots’ surface chemistry modulates the sensitivity of glioblastoma cells to chemotherapeutics. Int. J. Mol. Sci. 2020, 21, 6301. [Google Scholar] [CrossRef]
- Li, S.; Su, W.; Wu, H.; Yuan, T.; Yuan, C.; Liu, J.; Deng, G.; Gao, X.; Chen, Z.; Bao, Y.; et al. Targeted tumour theranostics in mice via carbon quantum dots structurally mimicking large amino acids. Nat. Biomed. Eng. 2020, 4, 704–716. [Google Scholar] [CrossRef]
- d’Angelo, M.; Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Benot, R.D.; Pitari, G.; Ippoliti, R.; Cimini, A. Theranostic nanocarriers for malignant gliomas. Front. Bioeng. Biotechnol. 2019, 7, 325. [Google Scholar] [CrossRef] [PubMed]
- Golubewa, L.; Timoshchenko, I.; Romanov, O.; Karpicz, R.; Kulahava, T.; Rutkauskas, D.; Shuba, M.; Dementjev, A.; Svirko, Y.; Kuzhir, P. Single walled carbon nanotubes as a photo thermo acoustic cancer theranostic agent: Theory and proof of the concept experiment. Sci. Rep. 2020, 10, 22174. [Google Scholar] [CrossRef]
- Golubewa, L.; Kulahava, T.; Timoshchenko, I.; Shuba, M.; Svirko, Y.; Kuzhir, P. Rapid and delayed effects of single-walled carbon nanotubes in glioma cells. Nanotechnology 2021, 32, 505103. [Google Scholar] [CrossRef]
- Wang, C.H.; Chiou, S.H.; Chou, C.P.; Chen, Y.C.; Huang, Y.J.; Peng, C.A. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 69–79. [Google Scholar] [CrossRef]
- Golubewa, L.; Kulahava, T.; Kunitskaya, Y.; Bulai, P.; Shuba, M.; Karpicz, R. Enhancement of single-walled carbon nanotube accumulation in glioma cells exposed to low-strength electric field: Promising approach in cancer nanotherapy. Biochem. Biophys. Res. Commun. 2020, 529, 647–651. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Shao, S.; Geng, J.; Yi, H.A.; Gogia, S.; Neelamegham, S.; Jacobs, A.; Lovell, J.F. Functionalization of cobalt porphyrin–phospholipid bilayers with his-tagged ligands and antigens. Nat. Chem. 2015, 7, 438–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Chen, Y.; Zhao, H.; Qiao, G.; Liu, M.; Zhang, C.; Cui, D.; Ma, L. Dual-modified cationic liposomes loaded with paclitaxel and survivin siRNA for targeted imaging and therapy of cancer stem cells in brain glioma. Drug Deliv. 2018, 25, 1718–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.; Wang, C.Z.; Fan, H.J.; Zhang, C.J.; Zhang, H.W.; LV, M.H.; Cu, S.D. A dual-targeting liposome conjugated with transferrin and arginine-glycine-aspartic acid peptide for glioma-targeting therapy. Oncol. Lett. 2014, 8, 2000–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Gao, J.; Zhan, C.; Xie, C.; Chai, Z.; Ran, D.; Ying, M.; Zheng, P.; Lu, W. Liposome-based glioma targeted drug delivery enabled by stable peptide ligands. J. Control. Release 2015, 218, 13–21. [Google Scholar] [CrossRef]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Han, T.L.V.; Hwang, M.K.; Maffa, A.; Sinclair, E.B.; Yaffe, M.B.; Floyd, S.R.; Hammond, P.T. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef]
- Hayward, S.L.; Francis, D.M.; Sis, M.J.; Kidambi, S. Ionic driven embedment of hyaluronic acid coated liposomes in polyelectrolyte multilayer films for local therapeutic delivery. Sci. Rep. 2015, 5, 14683. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Mi, G.; Shen, Y.; Webster, T.J. Glioma-targeted dual functionalized thermosensitive Ferri-liposomes for drug delivery through an in vitro blood–brain barrier. Nanoscale 2019, 11, 15057–15071. [Google Scholar] [CrossRef]
- Mende, C.H.; Mueller, M.M.; Bonsanto, M.M.; Schmitt, H.P.; Kunze, S.; Steiner, H.H. Clinical impact and functional aspects of tenascin-C expression during glioma progression. Int. J. Cancer 2002, 98, 362–369. [Google Scholar] [CrossRef]
- Weber, G.F. Why does cancer therapy lack effective anti-metastasis drugs? Cancer Lett. 2013, 328, 207–211. [Google Scholar] [CrossRef]
- Munson, J.M.; Fried, L.; Rowson, S.A.; Bonner, M.Y.; Karumbaiah, L.; Diaz, B.; Courtneidge, S.A.; Knaus, U.G.; Brat, D.J.; Arbiser, J.L.; et al. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci. Transl. Med. 2012, 4, 127ra36. [Google Scholar] [CrossRef]
- Hayashi, K.; Michiue, H.; Yamada, H.; Takata, K.; Nakayama, H.; Wei, F.Y.; Fujimura, A.; Tazawa, H.; Asai, A.; Ogo, N.; et al. Fluvoxamine, an anti-depressant, inhibits human glioblastoma invasion by disrupting actin polymerization. Sci. Rep. 2015, 6, 23372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Mason, R.P.; Sarkaria, J.N.; Zhao, D. Convertible MRI contrast: Sensing the delivery and release of antiglioma nano-drugs. Sci. Rep. 2015, 5, 9874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huwyler, J.; Wu, D.; Pardridge, W.M. Brain drug delivery of small molecules using immunoliposomes. Proc. Natl. Acad. Sci. USA 1996, 93, 14164–14169. [Google Scholar] [CrossRef] [Green Version]
- Ashrafzadeh, M.S.; Akbarzadeh, A.; Heydarinasab, A.; Ardjmand, M. In vivo glioblastoma therapy using targeted liposomal cisplatin. Int. J. Nanomed. 2020, 15, 7035–7049. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef] [PubMed]
- Eavarone, D.A.; Yu, X.; Bellamkonda, R.V. Targeted drug delivery to C6 glioma by transferrin-coupled liposomes. J. Biomed. Mater. Res. 2000, 51, 10–14. [Google Scholar] [CrossRef]
- Mu, L.M.; Bu, Y.Z.; Liu, L.; Xie, H.J.; Ju, R.J.; Wu, J.S.; Zeng, F.; Zhao, Y.; Zhang, J.Y.; Lu, W.L. Lipid vesicles containing transferrin receptor binding peptide TfR-T12 and octa-arginine conjugate stearyl-R8 efficiently treat brain glioma along with glioma stem cells. Sci. Rep. 2017, 7, 3487. [Google Scholar] [CrossRef] [Green Version]
- Koneru, T.; McCord, E.; Pawar, S.; Tatiparti, K.; Sau, S.; Iyer, A.K. Transferrin: Biology and use in receptor-targeted nanotherapy of gliomas. ACS Omega 2021, 6, 8727–8733. [Google Scholar] [CrossRef]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef]
- Hayward, S.L.; Wilson, C.L.; Kidambi, S. Hyaluronic acid-conjugated liposome nanoparticles for targeted delivery to CD44 overexpressing glioblastoma cells. Oncotarget 2016, 7, 34158–34171. [Google Scholar] [CrossRef]
- Jabali, S.M.; Farshbaf, M.; Walker, P.R.; Hemmati, S.; Fatahi, Y.; Milani, P.Z.; Sarfraz, M.; Valizadeh, H. An update on actively targeted liposomes in advanced drug delivery to glioma. Int. J. Pharm. 2021, 602, 120645. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Fan, L.; Pang, Z.; Ren, J.; Ren, Y.; Li, J.; Chen, J.; Wen, Z.; Jiang, X. TRAIL and doxorubicin combination enhances anti-glioblastoma effect based on passive tumor targeting of liposomes. J. Control. Release 2011, 154, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Leuthardt, E.C.; Duan, C.; Kim, M.J.; Campian, J.L.; Kim, A.H.; Miller-Thomas, M.M.; Shimony, J.S.; Tran, D.D. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS ONE 2016, 11, eo148613. [Google Scholar]
- Choi, J.; Yang, J.; Park, J.; Kim, E.; Suh, J.S.; Huh, Y.M.; Haam, S. Specific Near-IR absorption imaging of glioblastomas using integrin-targeting gold nanorods. Adv. Funct. Mater. 2011, 21, 1082–1088. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, C.; Chen, S.; Liu, J.; Yu, J.; Chilek, J.; Zhao, L.; Yu, M.; Vinluan, R.; Huang, B.; et al. Surface-chemistry effect on cellular response of luminescent plasmonic silver nanoparticles. Bioconjugate Chem. 2014, 25, 453–459. [Google Scholar] [CrossRef]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Nami, S.H.; Mehrzadi, S.; Zadeh, A.S.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef]
- Fauzia, R.P.; Denkova, A.G.; Djanashvili, K. Potential of MRI in radiotherapy mediated by small conjugates and nanosystems. Inorganics 2019, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Winsett, J.; Moilanen, A.; Paudel, K.; Kamali, S.; Ding, K.; Cribb, W.; Seifu, D.; Neupane, S. Quantitative determination of magnetite and maghemite in iron oxide nanoparticles using Mössbauer spectroscopy. SN Appl. Sci. 2019, 1, 1636. [Google Scholar] [CrossRef] [Green Version]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; An, X.; Cui, J.; Li, J.; Wen, S.; Li, K.; Shen, M.; Zheng, L.; Zhang, G.; Shi, X. Facile hydrothermal synthesis and surface functionalization of polyethyleneimine-coated iron oxide nanoparticles for biomedical applications. ACS Appl. Mater. Interfaces 2013, 5, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Gil, P.R.; Hühn, D.; del Mercato, L.L.; Sasse, D.; Parak, W.J. Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds. Pharmacol. Res. 2010, 62, 115–125. [Google Scholar]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar] [CrossRef]
- Du, C.; Liu, X.; Hu, H.; Li, H.; Yu, L.; Geng, D.; Chen, Y.; Zhang, J. Dual-targeting and excretable ultrasmall SPIONs for T1-weighted positive MR imaging of intracranial glioblastoma cells by targeting the lipoprotein receptor-related protein. J. Mater. Chem. B 2020, 8, 2296–2306. [Google Scholar] [CrossRef]
- Rachakatla, R.S.; Balivada, S.A.; Seo, G.; Myers, C.B.; Wang, K.H.; Samarakoon, T.N.; Dani, R.; Pyle, M.; Kroh, F.O.; Walker, B.; et al. Attenuation of mouse melanoma by a/c magnetic field after delivery of bi-magnetic nanoparticles by neural progenitor cells. ACS Nano 2010, 4, 7093–7104. [Google Scholar] [CrossRef]
- Habra, K.; McArdle, S.E.B.; Morris, R.H.; Cave, G.W.V. Synthesis and functionalisation of superparamagnetic nano-rods towards the treatment of glioblastoma brain tumours. Nanomaterials 2021, 11, 2157. [Google Scholar] [CrossRef]
- Wang, Y.X.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef] [Green Version]
- Marino, A.; Camponovo, A.; Degl’Innocenti, A.; Bartolucci, M.; Tapeinos, C.; Martinelli, C.; De Pasquale, D.; Santoro, F.; Mollo, V.; Arai, S.; et al. Multifunctional temozolomide-loaded lipid superparamagnetic nanovectors: Dual targeting and disintegration of glioblastoma spheroids by synergic chemotherapy and hyperthermia treatment. Nanoscale 2019, 11, 21227–21248. [Google Scholar] [CrossRef]
- Veiseh, O.; Sun, C.; Gunn, J.; Kohler, N.; Gabikian, P.; Lee, D.; Bhattarai, N.; Ellenbogen, R.; Sze, R.; Hallahan, A.; et al. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005, 5, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Kievit, F.M.; Sun, C.; Fang, C.; Lee, J.S.; Zhang, M. Inhibition of tumor-cell invasion with chlorotoxin-bound superparamagnetic nanoparticles. Small 2009, 5, 256–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshane, J.; Garner, C.C.; Sontheimer, H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J. Biol. Chem. 2003, 278, 4135–4144. [Google Scholar] [CrossRef] [Green Version]
- Christian, S.; Pilch, J.; Akerman, M.E.; Porkka, K.; Laakkonen, P.; Ruoslahti, E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J. Cell Biol. 2003, 163, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, K.; Hadjipanayis, C.G. The application of magnetic nanoparticles for the treatment of brain tumors. Front. Chem. 2014, 2, 109. [Google Scholar] [CrossRef] [Green Version]
- Senturk, F.; Cakmak, S.; Kocum, I.C.; Gumusderelioglu, M.; Ozturk, G.G. GRGDS-conjugated and curcumin-loaded magnetic polymeric nanoparticles for the hyperthermia treatment of glioblastoma cells. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126648. [Google Scholar] [CrossRef]
- Xu, H.L.; Mao, K.L.; Huang, Y.P.; Yang, J.-J.; Xu, J.; Chen, P.-P.; Fan, Z.-L.; Zou, S.; Gao, Z.-Z.; Yin, J.-Y.; et al. Glioma-targeted superparamagnetic iron oxide nanoparticles as drug-carrying vehicles for theranostic effects. Nanoscale 2016, 8, 14222–14236. [Google Scholar] [CrossRef]
- Jiang, W.; Xie, H.; Ghoorah, D.; Shang, Y.; Shi, H.; Liu, F.; Yang, X.; Xu, H. Conjugation of functionalized SPIONs with transferrin for targeting and imaging brain glial tumors in rat model. PLoS ONE 2012, 7, e37376. [Google Scholar] [CrossRef]
- Zhi, K.; Raji, B.; Nookala, A.R.; Khan, M.M.; Nguyen, X.H.; Sakshi, S.; Pourmotabbed, T.; Yallapu, M.M.; Kochat, H.; Tadrous, E.; et al. PLGA nanoparticle-based formulations to cross the blood-brain barrier for drug delivery: From R&D to cGMP. Pharmaceutics 2021, 13, 500. [Google Scholar]
- Cazares, H.G.; Tzeng, S.Y.; Young, N.P.; Abutaleb, A.O.; Hinojosa, A.Q.; Green, J.J. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano 2014, 8, 5141–5153. [Google Scholar] [CrossRef]
- Kou, L.; Hou, Y.; Yao, Q.; Guo, W.; Wang, G.; Wang, M.; Fu, Q.; He, Z.; Ganapathy, V.; Sun, J. L-Carnitine-conjugated nanoparticles to promote permeation across blood-brain barrier and to target glioma cells for drug delivery via the novel organic cation/carnitine transporter OCTN2. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, Q.; Chow, P.K.H.; Wang, D.; Wang, C.H. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials 2013, 34, 8511–8520. [Google Scholar] [CrossRef] [PubMed]
- Hartl, N.; Adams, F.; Merkel, O.M. From adsorption to covalent bonding: Apolipoprotein E functionalization of polymeric nanoparticles for drug delivery across the blood–brain barrier. Adv. Ther. 2021, 4, 2000092. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, Y.; Tan, Y.Z.; Hu, K.L.; Jiang, X.G.; Fu, S.K. Cationic albumin-conjugated pegylated nanoparticles as novel drug carrier for brain delivery. J. Control. Release 2005, 107, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Beier, C.P.; Schmid, C.; Gorlia, T.; Kleinletzenberger, C.; Beier, D.; Grauer, O.; Steinbrecher, A.; Hirschmann, B.; Brawanski, A.; Dietmaier, C.; et al. RNOP-09: Pegylated liposomal doxorubicine and prolonged temozolomide in addition to radiotherapy in newly diagnosed glioblastoma—A phase II study. BMC Cancer 2009, 9, 308. [Google Scholar] [CrossRef] [Green Version]
- Ananda, S. Phase 2 trial of temozolomide and pegylated liposomal doxorubicin in the treatment of patients with glioblastoma multiforme following concurrent radiotherapy and chemotherapy. J. Clin. Neurosci. 2011, 18, 1444–1448. [Google Scholar] [CrossRef]
- Ren, H.; Boulikas, T.; Soling, A.; Warnke, P.C.; Rainov, N.G. Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki forest virus vector carrying the human interleukin-12 gene—A phase l/lI clinical protocol. J. Neuro-Oncol. 2003, 64, 147–154. [Google Scholar] [CrossRef]
- Hauff, K.M.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [Green Version]




| Nanomaterials | Ligands or Precursors | Targeted Drugs or Radiation Source | Expressed Receptors or Markers | Approaches | Ref. |
| ESC-derived exosomes | c(RGDyK) | PTX | αvβ3integrins | Chemotherapy | [41] |
| Carboxyfullerenes (C70) | Malonic acid | - | - | ROS prevention | [46] |
| Radio-fullerenes (C80) | IL-13 peptide | β-emitters | Radio-immunotherapy (RIT) | [48] | |
| Magnetic nano GO SPIONs | Poly lactic-co-glycolic acid (PLGA) | 5-Iodo-2-deoxyuridine (IUdR) | Bax/Bcl-2 | MRI/radiotherapy | [51] |
| CQDs | TAAQ and CA | DOX, hydroxycamptothecin, topotecan hydrochloride (TPTC) | - | Chemotherapy and NIR/PAimaging | [54] |
| SWCNTs | CD133 MoAb | TMZ | CD133 | Photothermolysis by NIR laser irradiation | [58] |
| LNPs | Hyaluronic acid | DOX | CD44 | Chemotherapy | [80] |
| Liposomes | TNF | DOX/ TRAIL | DR5 | Chemotherapy | [82] |
| Gold nanorods | PEG and RGD peptide | NIR | αvβ3integrins | NIR imaging | [84] |
| Ag NPs | PEG and c-RGD peptide | Fluorescence and SERS | αvβ3integrins | Fluorescence and SERS | [85] |
| SPIONs | PEG/PEI/polysorbate 80 | DOX | Caspase-3 | Chemotherapy | [107] |
| PNPs | BSA/PEG/PLA | 6-coumarin | BCECs | AMT | [114] |
| Nanomaterials | Disease | Loaded Drugs/ Therapy | Phase | Results | Ref |
| PEGylated liposomes | Glioblastoma | DOX and TMZ | Phase II | Median overall survival (17.6 months) | [115] |
| PEGylated liposomes | Glioblastoma | DOX and TMZ | Phase II | Median overall survival (13.4 months) | [116] |
| Cationic liposomes | Recurrent glioblastoma | Interleukin-12 | Phase I, II | Convection-enhanced delivery through virus vector | [117] |
| Magnetic iron-oxide nanoparticles | Recurrent glioblastoma | Thermotherapy and lower radiotherapy dose (30 Gy) | Phase II | Effective and prolonged overall survival | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakshmi, B.A.; Kim, Y.-J. Modernistic and Emerging Developments of Nanotechnology in Glioblastoma-Targeted Theranostic Applications. Int. J. Mol. Sci. 2022, 23, 1641. https://doi.org/10.3390/ijms23031641
Lakshmi BA, Kim Y-J. Modernistic and Emerging Developments of Nanotechnology in Glioblastoma-Targeted Theranostic Applications. International Journal of Molecular Sciences. 2022; 23(3):1641. https://doi.org/10.3390/ijms23031641
Chicago/Turabian StyleLakshmi, Buddolla Anantha, and Young-Joon Kim. 2022. "Modernistic and Emerging Developments of Nanotechnology in Glioblastoma-Targeted Theranostic Applications" International Journal of Molecular Sciences 23, no. 3: 1641. https://doi.org/10.3390/ijms23031641
APA StyleLakshmi, B. A., & Kim, Y.-J. (2022). Modernistic and Emerging Developments of Nanotechnology in Glioblastoma-Targeted Theranostic Applications. International Journal of Molecular Sciences, 23(3), 1641. https://doi.org/10.3390/ijms23031641