Functional Role of Mitochondrial DNA in Cancer Progression
Abstract
:1. Introduction
2. The Genetic Information of mtDNA and Nuclear DNA on Cancer Progression
2.1. The Role of mtDNA (SNP/Mutation) in Cancer
2.2. The Association between Mitochondrial-Related Noncoding RNA and Cancer Progression
2.3. Dysregulation of Mitochondrial-Encoded Protein-Coding Genes in Cancer Progression
3. Nuclear DNA-Encoded Genes Function as Modulators to Coordinate Mitochondrial Gene Expression and Mitochondrial Functions
3.1. Action of Peroxisome Proliferator-Activated Receptor-γ Coactivatior-1α (PGC-1α) in Mitochondria
3.2. Action of NF-E2-Related Factor 2 (NRF2) in Mitochondria
3.3. Action of lncRNAs in Mitochondria
4. Strategy for Investigating Mitochondrial Function in Cell Lines
5. Mitochondria Target Therapies and Their Application
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; De Bruijn, M.H.L.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shan, G. Mitochondria Encoded Non-coding RNAs in Cell Physiology. Front. Cell Dev. Biol. 2021, 9, 713729. [Google Scholar] [CrossRef] [PubMed]
- Gusic, M.; Prokisch, H. ncRNAs: New players in mitochondrial health and disease? Front. Genet. 2020, 11, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Jin, Y.; Fan, Z. The mechanism of Warburg effect-induced chemoresistance in cancer. Front. Oncol. 2021, 11, 698023. [Google Scholar] [CrossRef]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-L.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Indo, H.P.; Davidson, M.; Yen, H.-C.; Suenaga, S.; Tomita, K.; Nishii, T.; Higuchi, M.; Koga, Y.; Ozawa, T.; Majima, H.J. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion 2007, 7, 106–118. [Google Scholar] [CrossRef]
- Indo, H.P.; Yen, H.-C.; Nakanishi, I.; Matsumoto, K.-I.; Tamura, M.; Nagano, Y.; Matsui, H.; Gusev, O.; Cornette, R.; Okuda, T.; et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J. Clin. Biochem. Nutr. 2015, 56, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef]
- Huang, B.-F.; Tzeng, H.-E.; Chen, P.-C.; Wang, C.-Q.; Su, C.-M.; Wang, Y.; Hu, G.-N.; Zhao, Y.-M.; Wang, Q.; Tang, C.-H. HMGB1 genetic polymorphisms are biomarkers for the development and progression of breast cancer. Int. J. Med. Sci. 2018, 15, 580–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Guo, J.; Cheng, G.; Wei, Y.; Liu, S.; Qi, Y.; Wang, G.; Xiao, R.; Qi, W.; Qiu, W. Identification and validation of SNP-containing genes with prognostic value in gastric cancer via integrated bioinformatics analysis. Front. Oncol. 2021, 11, 564296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, W.; Gu, D.; Du, M.; Gong, W.; Tan, Y.; Wang, M.; Wen, J.; Zhai, Y.; Xu, Z. Association of antioxidative enzymes polymorphisms with efficacy of platin and fluorouracil-based adjuvant therapy in gastric cancer. Cell. Physiol. Biochem. 2018, 48, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtney, K.D.; Bezwada, D.; Mashimo, T.; Pichumani, K.; Vemireddy, V.; Funk, A.; Wimberly, J.; McNeil, S.S.; Kapur, P.; Lotan, Y.; et al. Isotope tracing of human clear cell renal cell carcinomas demonstrates suppressed glucose oxidation in vivo. Cell Metab. 2018, 28, 793–800.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, E.-H.; Sung, J.; Lee, S.-I.; Hong, J.-H. Mitochondrial NADH Dehydrogenase Subunit 3 (MTND3) polymorphisms are associated with gastric cancer susceptibility. Int. J. Med. Sci. 2018, 15, 1329–1333. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.-H.; Wu, C.-C.; Lin, J.-C.; Chi, C.-W.; Wei, Y.-H.; Lee, H.-C. Somatic mutations of mitochondrial genome in hepatocellular carcinoma. Mitochondrion 2010, 10, 174–182. [Google Scholar] [CrossRef]
- Akouchekian, M.; Houshmand, M.; Akbari, M.H.H.; Kamalidehghan, B.; Dehghan, M. Analysis of mitochondrial ND1 gene in human colorectal cancer. J. Res. Med. Sci. 2011, 16, 50–55. [Google Scholar]
- Sun, W.; Zhou, S.; Chang, S.S.; McFate, T.; Verma, A.; Califano, J.A. Mitochondrial mutations contribute to HIF1 accumulation via increased reactive oxygen species and up-regulated pyruvate dehydrogenease kinase 2 in head and neck squamous cell carcinoma. Clin. Cancer Res. 2009, 15, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Horibe, S.; Ishikawa, K.; Nakada, K.; Wake, M.; Takeda, N.; Tanaka, T.; Kawauchi, S.; Sasaki, N.; Rikitake, Y. Mitochondrial DNA mutations are involved in the acquisition of cisplatin resistance in human lung cancer A549 cells. Oncol. Rep. 2021, 47, 1–12. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J.-I. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K.; Hayashi, J.-I. A novel function of mtDNA: Its involvement in metastasis. Ann. N. Y. Acad. Sci. 2010, 1201, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, H.; Hattori, K.; Wada, R.; Ishikawa, K.; Fukuda, S.; Takenaga, K.; Nakada, K.; Hayashi, J.-I. Mitochondrial DNA mutations regulate metastasis of human breast cancer cells. PLoS ONE 2011, 6, e23401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beadnell, T.C.; Scheid, A.D.; Vivian, C.J.; Welch, D.R. Roles of the mitochondrial genetics in cancer metastasis: Not to be ignored any longer. Cancer Metastasis Rev. 2018, 37, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Myers, L.; Ravussin, E.; Cherry, K.E.; Jazwinski, S.M. Single nucleotide polymorphisms linked to mitochondrial uncoupling protein genes UCP2 and UCP3 affect mitochondrial metabolism and healthy aging in female nonagenarians. Biogerontology 2016, 17, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Shidara, Y.; Yamagata, K.; Kanamori, T.; Nakano, K.; Kwong, J.Q.; Manfredi, G.; Oda, H.; Ohta, S. Positive contribution of pathogenic mutations in the mitochondrial genome to the promotion of cancer by prevention from apoptosis. Cancer Res. 2005, 65, 1655–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Zheng, L.; Xie, D.; Yu, S.; Zhao, J. Mutational analysis of mitochondrial tRNA genes in patients with lung cancer. Balk. J. Med. Genet. 2016, 19, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.-L.; Meng, H.; Zhang, W.; Qin, Y.-H.; Zhao, N.-M. The role of mitochondrial tRNA variants in female breast cancer. Mitochondrial DNA Part A 2015, 27, 3199–3201. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chu, Y.-D.; Lim, S.-N.; Chen, C.-W.; Yeh, C.-T.; Lin, W.-R. Impact of an MT-RNR1 gene polymorphism on hepatocellular carcinoma progression and clinical characteristics. Int. J. Mol. Sci. 2021, 22, 1119. [Google Scholar] [CrossRef]
- Han, C.-B.; Ma, J.-M.; Xin, Y.; Mao, X.-Y.; Zhao, Y.-J.; Wu, D.-Y.; Zhang, S.-M.; Zhang, Y.-K. Mutations of mitochondrial 12S rRNA in gastric carcinoma and their significance. World J. Gastroenterol. 2005, 11, 31–35. [Google Scholar] [CrossRef]
- Watson, C.N.; Belli, A.; Di Pietro, V. Small Non-coding RNAs: New class of biomarkers and potential therapeutic targets in neurodegenerative disease. Front. Genet. 2019, 10, 364. [Google Scholar] [CrossRef]
- Fan, S.; Tian, T.; Chen, W.; Lv, X.; Lei, X.; Zhang, H.; Sun, S.; Cai, L.; Pan, G.; He, L.; et al. Mitochondrial miRNA determines chemoresistance by reprogramming metabolism and regulating mitochondrial transcription. Cancer Res. 2019, 79, 1069–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Wang, P.; Lu, Y.; Jin, T.; Lei, X.; Liu, M.; Zhuang, P.; Liao, J.; Lin, Z.; Li, B.; et al. Decreased expression of mitochondrial miR-5787 contributes to chemoresistance by reprogramming glucose metabolism and inhibiting MT-CO3 translation. Theranostics 2019, 9, 5739–5754. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Cirilli, I.; Prattichizzo, F.; Mensà, E.; Fulgenzi, G.; Sabbatinelli, J.; Graciotti, L.; Olivieri, F.; Procopio, A.D.; Tiano, L.; et al. The mitomiR/Bcl-2 axis affects mitochondrial function and autophagic vacuole formation in senescent endothelial cells. Aging 2018, 10, 2855–2873. [Google Scholar] [CrossRef]
- Pang, Y.; Mao, C.; Liu, S. Encoding activities of non-coding RNAs. Theranostics 2018, 8, 2496–2507. [Google Scholar] [CrossRef]
- Mercer, T.R.; Neph, S.; Dinger, M.E.; Crawford, J.; Smith, M.A.; Shearwood, A.-M.J.; Haugen, E.; Bracken, C.P.; Rackham, O.; Stamatoyannopoulos, J.A.; et al. The human mitochondrial transcriptome. Cell 2011, 146, 645–658. [Google Scholar] [CrossRef] [Green Version]
- Rackham, O.; Shearwood, A.-M.J.; Mercer, T.R.; Davies, S.M.; Mattick, J.S.; Filipovska, A. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA 2011, 17, 2085–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M.A. PPR (pentatricopeptide repeat) proteins in mammals: Important aids to mitochondrial gene expression. Biochem. J. 2008, 416, e5–e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jusic, A.; Devaux, Y. Mitochondrial noncoding RNA-regulatory network in cardiovascular disease. Basic Res. Cardiol. 2020, 115, 23. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Bauters, C.; Volkmann, I.; Maury, F.; Fetisch, J.; Holzmann, A.; Lemesle, G.; de Groote, P.; Pinet, F.; Thum, T. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ. Res. 2014, 114, 1569–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Wen, S.; Zhou, H.; Feng, S. De-methylation of displacement loop of mitochondrial DNA is associated with increased mitochondrial copy number and nicotinamide adenine dinucleotide subunit 2 expression in colorectal cancer. Mol. Med. Rep. 2015, 12, 7033–7038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Tian, J.; Peng, F.; Wang, J. Hypermethylation of mitochondrial DNA facilitates bone metastasis of renal cell carcinoma. J. Cancer 2022, 13, 304–312. [Google Scholar] [CrossRef]
- Li, N.; Zhao, J.; Ma, Y.; Roy, B.; Liu, R.; Kristiansen, K.; Gao, Q. Dissecting the expression landscape of mitochondrial genes in lung squamous cell carcinoma and lung adenocarcinoma. Oncol. Lett. 2018, 16, 3992–4000. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, S.; Hoque, M.O.; Upadhyay, S.; Sidransky, D. Mitochondrial Cytochrome B gene mutation promotes tumor growth in bladder cancer. Cancer Res. 2008, 68, 700–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, P.D. The ATP synthase—A splendid molecular machine. Annu. Rev. Biochem. 1997, 66, 717–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, L.B.; Gui, D.Y.; Hosios, A.M.; Bush, L.N.; Freinkman, E.; Vander Heiden, M.G. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 2015, 162, 552–563. [Google Scholar] [CrossRef] [Green Version]
- Wallace, L.; Mehrabi, S.; Bacanamwo, M.; Yao, X.; Aikhionbare, F.O. Expression of mitochondrial genes MT-ND1, MT-ND6, MT-CYB, MT-COI, MT-ATP6, and 12S/MT-RNR1 in colorectal adenopolyps. Tumor Biol. 2016, 37, 12465–12475. [Google Scholar] [CrossRef] [Green Version]
- Boland, M.L.; Chourasia, A.H.; MacLeod, K.F. Mitochondrial dysfunction in cancer. Front. Oncol. 2013, 3, 292. [Google Scholar] [CrossRef] [Green Version]
- Austin, S.; St-Pierre, J. PGC1α and mitochondrial metabolism–emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef] [Green Version]
- Uguccioni, G.; Hood, D.A. The importance of PGC-1alpha in contractile activity-induced mitochondrial adaptations. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E361–E371. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.N.; Clark, J.P.; Anderson, R.M. Mitochondrial regulator PGC-1a—Modulating the modulator. Curr. Opin. Endocr. Metab. Res. 2019, 5, 37–44. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; O’Connell, J.T.; Gonzalez Herrera, K.N.; Wikman, H.; Pantel, K.; Haigis, M.C.; de Carvalho, F.M.; Damascena, A.; Chinen, L.T.D.; Rocha, R.M.; et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014, 16, 992–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feichtinger, R.G.; Schäfer, G.; Seifarth, C.; Mayr, J.A.; Kofler, B.; Klocker, H. Reduced levels of ATP synthase subunit ATP5F1A correlate with earlier-onset prostate cancer. Oxidative Med. Cell. Longev. 2018, 2018, 1347174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuezva, J.M.; Krajewska, M.; De Heredia, M.L.; Krajewski, S.; Santamaría, G.; Kim, H.; Zapata, J.M.; Marusawa, H.; Chamorro, M.; Reed, J.C. The bioenergetic signature of cancer: A marker of tumor progression. Cancer Res. 2002, 62, 6674–6681. [Google Scholar]
- Cuezva, J.M.; Chen, G.; Alonso, A.M.; Isidoro, A.; Misek, D.E.; Hanash, S.M.; Beer, D.G. The bioenergetic signature of lung adenocarcinomas is a molecular marker of cancer diagnosis and prognosis. Carcinogenesis 2004, 25, 1157–1163. [Google Scholar] [CrossRef] [Green Version]
- Li, R.J.; Zhang, G.S.; Chen, Y.H.; Zhu, J.F.; Lu, Q.J.; Gong, F.J.; Kuang, W.Y. Down-regulation of mitochondrial ATPase by hypermethylation mechanism in chronic myeloid leukemia is associated with multidrug resistance. Ann. Oncol. 2009, 21, 1506–1514. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, J.H.; Lee, S.H. Hypoxia-induced PGC-1α regulates mitochondrial function and tumorigenesis of colorectal cancer cells. Anticancer Res. 2019, 39, 4865–4876. [Google Scholar] [CrossRef]
- Evans, M.J.; Scarpulla, R.C. NRF-1: A trans-activator of nuclear-encoded respiratory genes in animal cells. Genes Dev. 1990, 4, 1023–1034. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Antonucci, L.; Karin, M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis 2020, 41, 405–416. [Google Scholar] [CrossRef]
- Zimta, A.-A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The role of Nrf2 activity in cancer development and progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Mimura, J.; Yamamoto, M. Discovery of the negative regulator of Nrf2, Keap1: A historical overview. Antioxidants Redox Signal. 2010, 13, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Happel, C.; Manna, S.K.; Acquaah-Mensah, G.; Carrerero, J.; Kumar, S.; Nasipuri, P.; Krausz, K.W.; Wakabayashi, N.; Dewi, R.; et al. Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. J. Clin. Investig. 2013, 123, 2921–2934. [Google Scholar] [CrossRef] [Green Version]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmstrom, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Piantadosi, C.A.; Carraway, M.S.; Babiker, A.; Suliman, H.B. Heme Oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory Factor-1. Circ. Res. 2008, 103, 1232–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blesa, J.R.; Prieto-Ruiz, J.A.; Hernandez, J.M.; Hernandez-Yago, J. NRF-2 transcription factor is required for human TOMM20 gene expression. Gene 2007, 391, 198–208. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1 alpha. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Lin, N.; Fu, J.; Xu, L.; Luo, H.; Jin, Y.; Liu, Y.; Sun, L.; Su, J. The Nrf2/PGC1alpha pathway regulates antioxidant and proteasomal activity to alter cisplatin sensitivity in ovarian cancer. Oxid. Med. Cell. Longev. 2020, 2020, 4830418. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of mitochondrial biogenesis as a way for active longevity: Interaction between the Nrf2 and PGC-1α signaling pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Kadomatsu, T.; Miyata, K.; Warren, J.S.; Tian, Z.; Zhu, S.; Horiguchi, H.; Makaju, A.; Bakhtina, A.; Morinaga, J.; et al. The lncRNA Caren antagonizes heart failure by inactivating DNA damage response and activating mitochondrial biogenesis. Nat. Commun. 2021, 12, 2529. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Long, B.; Zhou, L.-Y.; Liu, F.; Zhou, Q.-Y.; Liu, C.-Y.; Fan, Y.-Y.; Li, P.-F. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat. Commun. 2014, 5, 3596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Xiong, R.; Li, C.; Xu, M.; Guo, M. LncRNA TUG1 promotes cisplatin resistance in esophageal squamous cell carcinoma cells by regulating Nrf2. Acta Biochim. Biophys. Sin. 2019, 51, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Fogal, V.; Richardson, A.; Karmali, P.P.; Scheffler, I.E.; Smith, J.W.; Ruoslahti, E. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol. Cell. Biol. 2010, 30, 1303–1318. [Google Scholar] [CrossRef] [Green Version]
- Leucci, E.; Vendramin, R.; Spinazzi, M.; Laurette, P.; Fiers, M.; Wouters, J.; Radaelli, E.; Eyckerman, S.; Leonelli, C.; Vanderheyden, K.; et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature 2016, 531, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Sirey, T.M.; Roberts, K.; Haerty, W.; Bedoya-Reina, O.; Rogatti-Granados, S.; Tan, J.Y.; Li, N.; Heather, L.C.; Carter, R.N.; Cooper, S.; et al. The long non-coding RNA Cerox1 is a post transcriptional regulator of mitochondrial complex I catalytic activity. eLife 2019, 8, e45051. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, L.; Sun, L.-H.; Li, Y.; Gao, Y.; Xu, C.; Shao, Y.; Li, M.; Li, C.; Lu, Y.; et al. LncRNA ZFAS1 as a SERCA2a Inhibitor to cause intracellular Ca2+ overload and contractile dysfunction in a mouse model of myocardial infarction. Circ. Res. 2018, 122, 1354–1368. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, L.; Li, H.; Sun, T.; Wen, X.; Li, X.; Meng, Y.; Li, Y.; Liu, M.; Liu, S.; et al. Nuclear-encoded lncRNA MALAT1 epigenetically controls metabolic reprogramming in HCC cells through the mitophagy pathway. Mol. Ther.-Nucleic Acids 2020, 23, 264–276. [Google Scholar] [CrossRef]
- Sang, L.; Ju, H.-Q.; Yang, Z.; Ge, Q.; Zhang, Z.; Liu, F.; Yang, L.; Gong, H.; Shi, C.; Qu, L.; et al. Mitochondrial long non-coding RNA GAS5 tunes TCA metabolism in response to nutrient stress. Nat. Metab. 2021, 3, 90–106. [Google Scholar] [CrossRef]
- Correia-Melo, C.; Ichim, G.; Tait, S.W.G.; Passos, J. Depletion of mitochondria in mammalian cells through enforced mitophagy. Nat. Protoc. 2016, 12, 183–194. [Google Scholar] [CrossRef]
- Hussain, S.-R.A.; Yalvac, M.E.; Khoo, B.; Eckardt, S.; McLaughlin, K.J. Adapting CRISPR/Cas9 System for Targeting Mitochondrial Genome. Front. Genet. 2021, 12, 627050. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.J.; Porteous, C.M.; Coulter, C.V.; Murphy, M.P. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 1999, 263, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.J.; Porteous, C.M.; Gane, A.M.; Murphy, M.P. Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 5407–5412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, M.; Vakili, M.R.; Soleymani Abyaneh, H.; Molavi, O.; Lai, R.; Lavasanifar, A. Mitochondrial delivery of doxorubicin via triphenylphosphine modification for overcoming drug resistance in MDA-MB-435/DOX cells. Mol. Pharm. 2014, 11, 2640–2649. [Google Scholar] [CrossRef] [PubMed]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in cardiovascular and metabolic diseases: Current state of the problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef]
- Gutierrez-Mariscal, F.M.; Larriva, A.P.A.-D.; Limia-Perez, L.; Romero-Cabrera, J.L.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q10 Supplementation for the reduction of oxidative stress: Clinical implications in the treatment of chronic diseases. Int. J. Mol. Sci. 2020, 21, 7870. [Google Scholar] [CrossRef]
- Sato, Y.; Nakamura, T.; Yamada, Y.; Harashima, H. Development of a multifunctional envelope-type nano device and its application to nanomedicine. J. Control. Release 2016, 244 Pt B, 194–204. [Google Scholar] [CrossRef]
- Abe, J.; Yamada, Y.; Harashima, H. Validation of a strategy for cancer therapy: Delivering aminoglycoside drugs to mitochondria in HeLa cells. J. Pharm. Sci. 2016, 105, 734–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreux, P.A.; Houtkooper, R.H.; Auwerx, J. Pharmacological approaches to restore mitochondrial function. Nat. Rev. Drug Discov. 2013, 12, 465–483. [Google Scholar] [CrossRef] [Green Version]
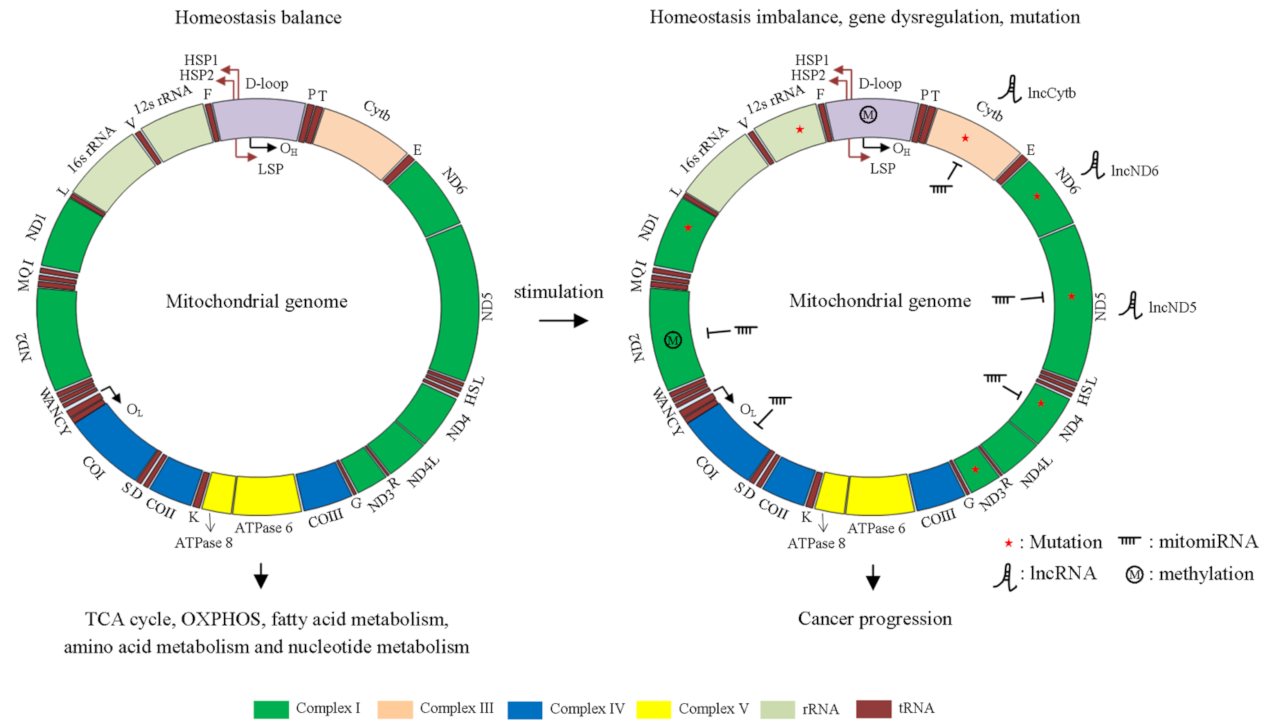
| Gene Name | SNP/Mutation | Mediated-Cellular Functions | Cancer | Reference |
|---|---|---|---|---|
| ND1 | G3842A | - | HCC | [17] |
| ND1 | T4216C | - | CRC | [18] |
| ND1 | T3394C | Metastasis | - | [24] |
| ND1 | C3497T | Metastasis | - | [24] |
| ND2 | G4776A | Cell growth and ROS production | Head and neck cancer | [19] |
| ND2 | T4587C | Drug resistance and Mitochondrial complex I activity | RCC | [20] |
| ND3 | rs28358278, rs2853826, and rs41467651 | - | Gastric Cancer | [16] |
| ND4 | A11708G | - | HCC | [17] |
| ND5 | 12418insA | - | HCC | [17] |
| ND6 | 13885insC | Complex I activity and ROS production | Lung and Breast Cancer | [21,22] |
| ND6 | C12084T | Metastasis | Breast Cancer | [23] |
| UCP2 | rs591758 and rs675547 | - | - | [25] |
| UCP3 | rs1626521 | - | - | [25] |
| MT-ATP6 | T8993G and T9176C | Cell growth and Apoptosis | Head and neck squamous cell carcinoma | [26] |
| MT-RNR1 | G709A | Metastasis | HCC | [29] |
| MT-RNR1 | 652G insertion and 716G | - | Gastric cancer | [30] |
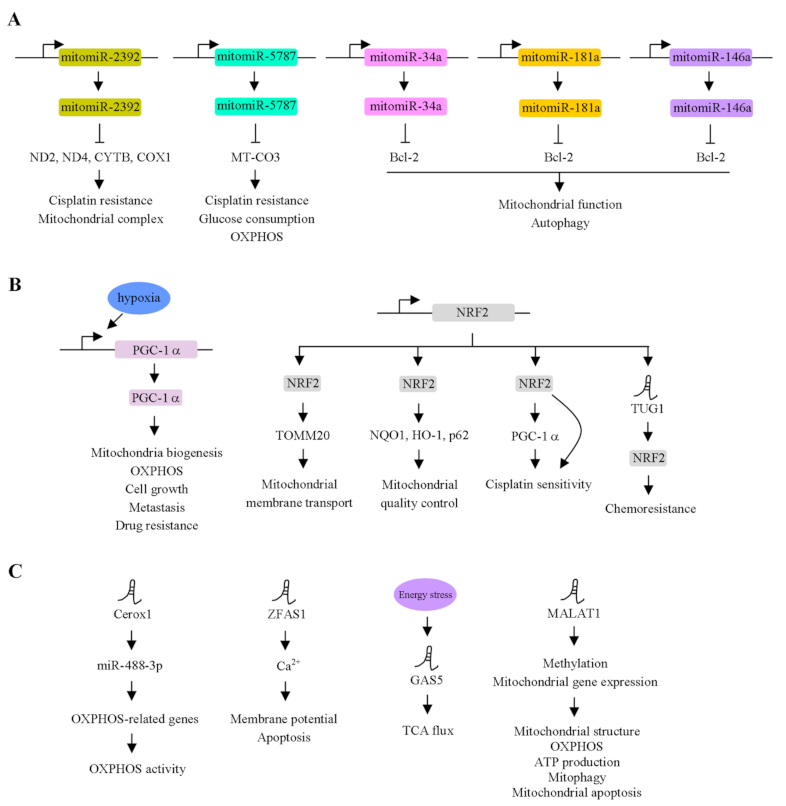
| Gene Name | Principal Functions | Molecules and Signaling Pathways Involved | Study Model | Prognostic Markers in Cancer | Cancer Development | Reference |
|---|---|---|---|---|---|---|
| mitomiR-2392 | Cisplatin resistance, mitochondrial complex activity | ND2, ND4, ND5, CYTB, COX1 | TSCC | ✓ | Progression | [32] |
| mitomiR-5787 | Glucose metabolism, chemoresistance | MT-CO3 | TSCC | ✓ | Repression | [33] |
| mitomiR-34a | Autophagy | Bcl-2 | HUVEC | - | - | [34] |
| mitomiR-181a | Autophagy | Bcl-2 | HUVEC | - | - | [34] |
| mitomiR-146a | Autophagy | Bcl-2 | HUVEC | - | - | [34] |
| lncND5 | Mitochondrial gene expression | PTCD1, mitochondria RNase P protein 3 | - | - | - | [36,37] |
| lncND6 | Mitochondrial gene expression | PTCD1, PTCD2, mitochondria RNase P protein 3 | - | - | - | [36,37] |
| lncCytb | Mitochondrial gene expression | Mitochondria RNase P protein 3 | - | - | - | [36,37] |
| LIPCAR | - | - | Heart failure | ✓ | [39] | |
| ND2 | Epigenetic regulation, mtDNA copy number | Methylation in D-loop | Colorectal cancer | - | Progression | [41] |
| ND5 | - | - | LUSC, LUAD | ✓ | Repression | [43] |
| ND6 | - | - | LUSC, LUAD | ✓ | Repression | [43] |
| CYTB | ROS production, oxygen utilization, lactate production, metastasis, angiogenesis | NFκB2 signaling pathway | Bladder cancer | - | Repression | [44] |
| PGC-1α | Mitochondria biogenesis, OXPHOS, metastasis | ATP synthase | Breast cancerColorectal cancer | ✓ | Progression | [52] |
| ATP5F1A | OXPHOS | - | Prostate cancer | ✓ | Progression | [53] |
| ATPase | HSP60 | - | LUAD | ✓ | Repression | [56] |
| ATP5B | Hypermethylation, drug resistance | - | Chronic myeloid leukemia | - | - | [56] |
| NRF1 | Metabolic homeostasis | TFAM, TFB1M, TFB2M | - | ✓ | - | [66] |
| NRF2 | Oxygen consumption, ATP level, mitochondrial membrane potential, mitochondria membrane transport | TOMM20 | Glial cells, mouse embryonic fibroblast | ✓ | Progression/Repression | [69] |
| lncRNA Caren | Mitochondrial biogenesis and fission | ATM/DDR pathway, Hint1 expression | Cardiomyocyte | - | - | [71] |
| lncRNA CARL | Mitochondrial fission and apoptosis | miR-539, PHB2 | Cardiomyocyte | - | - | [72] |
| TUG1 | Chemoresistance | NRF2 interaction | Esophageal carcinoma | - | - | [73] |
| SAMMSON | OXPHOS, glycolysis, survival | P32 | Melanoma | ✓ | - | [75] |
| Cerox1 | OXPHOS, enzymatic activity | OXPHOS-related genes expressions, miR-488-3p | Mouse Neuro-2a neuroblastoma cells, HEK293 cells | - | - | [76] |
| ZFAS1 | Mitochondria membrane potential, mitochondria apoptosis | Ca2+ homeostasis | cardiomyocyte | - | - | [77] |
| GAS5 | Energy stress, TCA flux | Citrate synthase, fumarate hydratase and malate dehydrogenase | breast cancer | ✓ | Repression | [79] |
| MALAT1 | Metabolic reprogramming | D-loop, ND3, COX2, CYTB | HCC | ✓ | Progression | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Lim, S.-N.; Chen, C.-Y.; Chi, H.-C.; Yeh, C.-T.; Lin, W.-R. Functional Role of Mitochondrial DNA in Cancer Progression. Int. J. Mol. Sci. 2022, 23, 1659. https://doi.org/10.3390/ijms23031659
Lin Y-H, Lim S-N, Chen C-Y, Chi H-C, Yeh C-T, Lin W-R. Functional Role of Mitochondrial DNA in Cancer Progression. International Journal of Molecular Sciences. 2022; 23(3):1659. https://doi.org/10.3390/ijms23031659
Chicago/Turabian StyleLin, Yang-Hsiang, Siew-Na Lim, Cheng-Yi Chen, Hsiang-Cheng Chi, Chau-Ting Yeh, and Wey-Ran Lin. 2022. "Functional Role of Mitochondrial DNA in Cancer Progression" International Journal of Molecular Sciences 23, no. 3: 1659. https://doi.org/10.3390/ijms23031659
APA StyleLin, Y.-H., Lim, S.-N., Chen, C.-Y., Chi, H.-C., Yeh, C.-T., & Lin, W.-R. (2022). Functional Role of Mitochondrial DNA in Cancer Progression. International Journal of Molecular Sciences, 23(3), 1659. https://doi.org/10.3390/ijms23031659






