Nanoparticles in Clinical Translation for Cancer Therapy
Abstract
1. Introduction
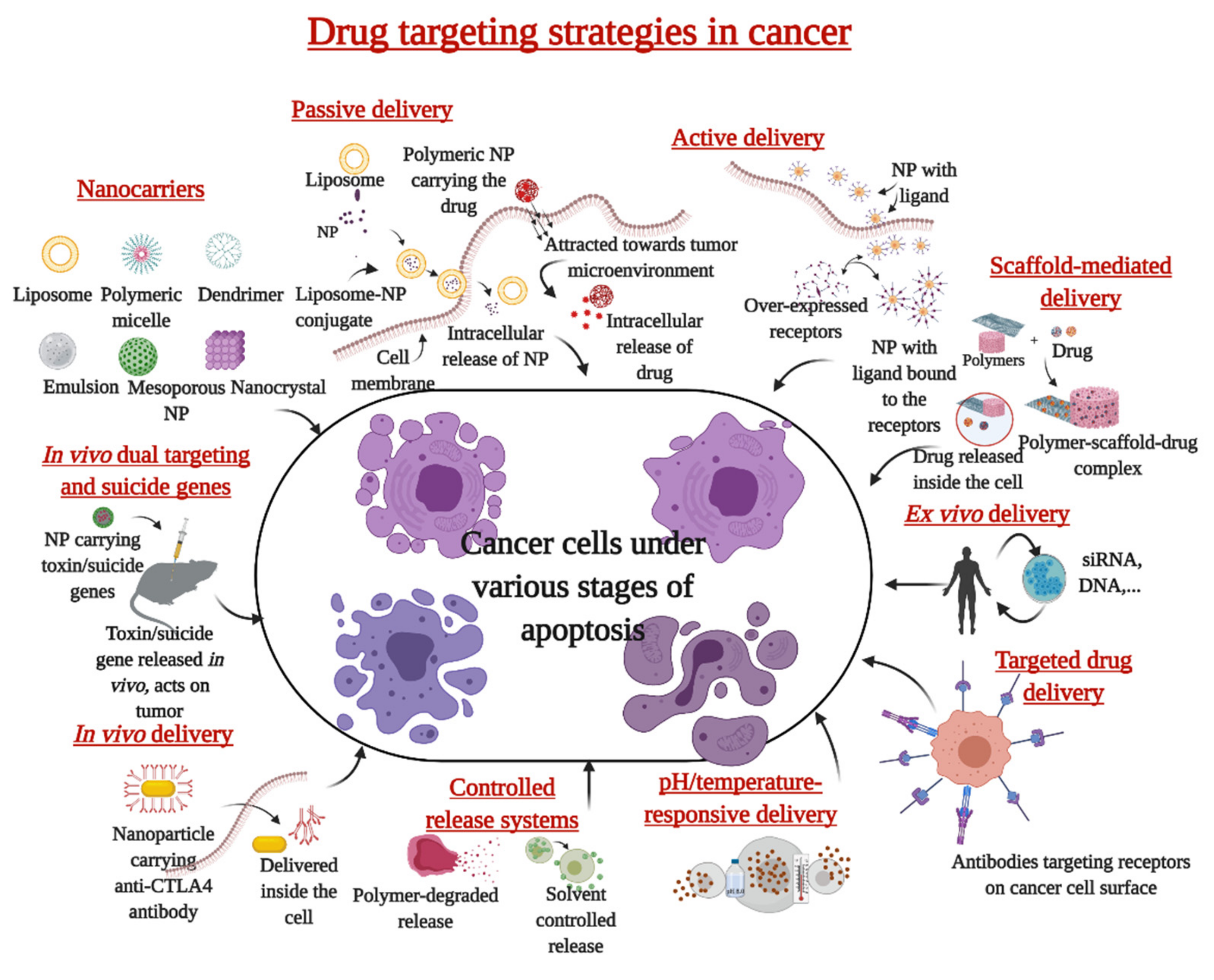
2. Nanoparticles in the Treatment of Cancer
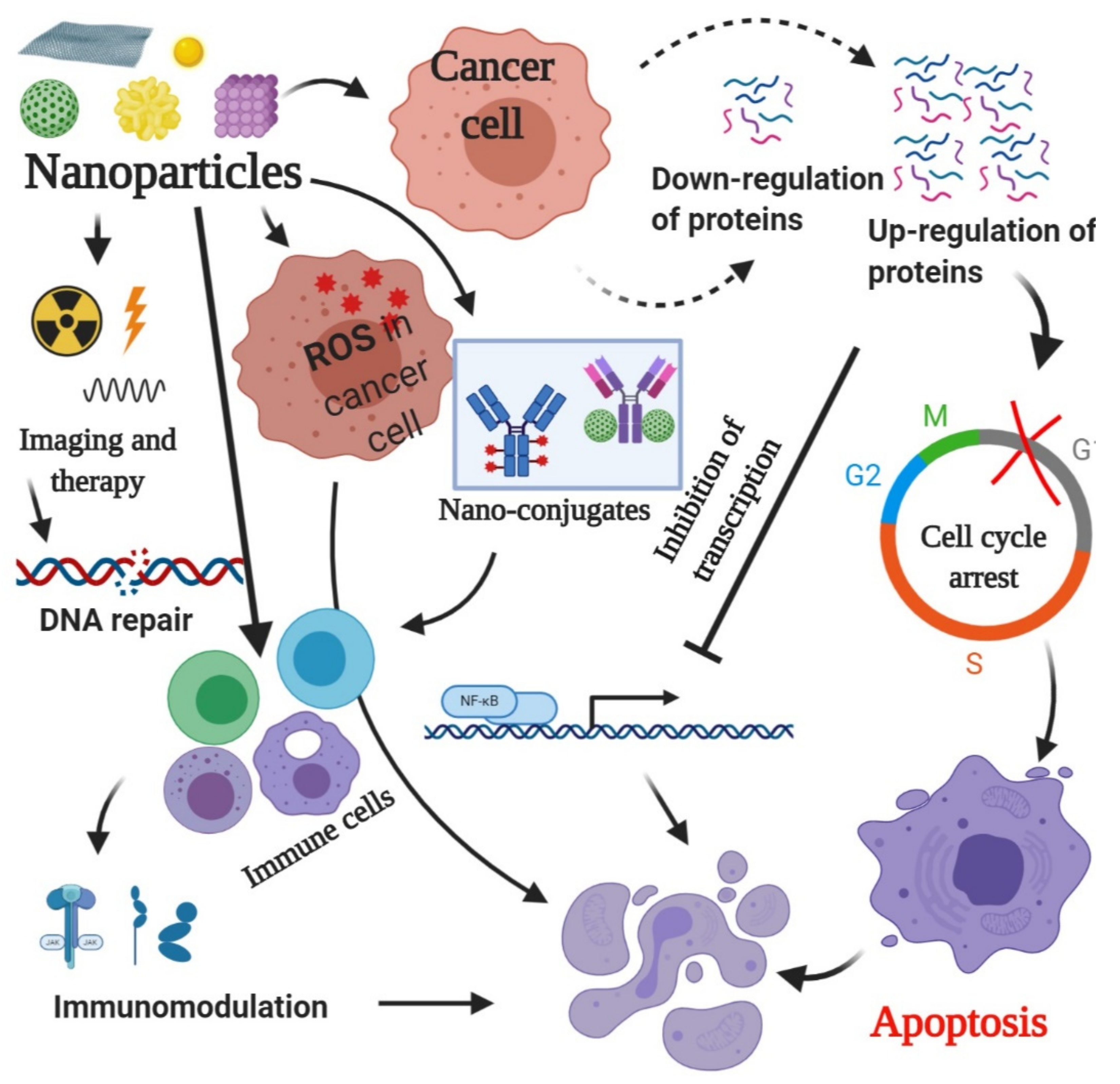
3. Mechanism of Action of Nanoparticles
3.1. Generation of ROS
3.2. Regulation of Proteins
3.3. Radiation Therapy
3.4. Phototherapy
3.5. Triggering Immunological Reactions
3.6. Site-Specific Cytotoxicity
3.7. Gene Therapy for Cancer Cell Growth Inhibition
4. Nanoparticles in Clinical Translation
4.1. Liposomal Nanoparticles
4.2. Metal and Metal Oxide Nanoparticles, Polymeric Micelles, Polymer/Lipids, and Other Conjugates
5. Obstacles in the Clinical Translation of Nanoparticles
5.1. The Difficulty in Predicting the Predisposal of the Patient to Allergic Reactions
5.2. Endotoxin Quantification
5.3. The Cellular Internalization of the Drug
5.4. Sustained Release
5.5. Overcoming Biological Barriers along with Increased Bioavailability
5.6. Increasing the Functional Capability to Target Only Tumor Cells
5.7. Controlling Immune System Response to the New Drug
6. Cutting-Edge Developments in Nanochemotherapy
6.1. CRISPR—The Gene-Editing Tool
6.2. ThermoResponsive-NanoVelcro Purification System
6.3. PROTAC—A Novel Proteolysis Targeting Entity
6.4. Proton Therapy—An Alternate Approach to Conventional Radiation Therapy
6.5. Functionalized DNA—A Programmable Way to Deliver Cancer Therapeutics
6.6. Avatar—A Real-Time Data Based Translational Therapeutic Approach
6.7. Protein Catenation—A Novel Approach to Develop Artificial Antibodies
6.8. Other Approaches
7. Recent Advances in Clinical Studies with Nanoparticles in Tumor Therapeutics
8. Therapeutic vs. Diagnostic Nanoparticles
9. Restrictions on the Use of Nanoparticles in Medicine
10. Future Perspective
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rhodes, K.R.; Green, J.J. Nanoscale artificial antigen presenting cells for cancer immunotherapy. Mol. Immunol. 2018, 98, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Nie, G. Multifunctional biomolecule nanostructures for cancer therapy. Nat. Rev. Mater. 2021, 6, 766–783. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.F.M.; Smyth, L.; Wang, J.T.-W.; Costa, P.M.; Ratnasothy, K.; Diebold, S.S.; Lombardi, G.; Al-Jamal, K.T. Dual stimulation of antigen presenting cells using carbon nanotube-based vaccine delivery system for cancer immunotherapy. Biomaterials 2016, 104, 310–322. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Bao, W.; Tian, F.; Lyu, C.; Liu, B.; Li, B.; Zhang, L.; Liu, X.; Li, F.; Li, D.; Gao, X. Experimental and theoretical explorations of nanocarriers’ multistep delivery performance for rational design and anticancer prediction. Sci. Adv. 2021, 7, eaba2458. [Google Scholar] [CrossRef]
- Sweeney, E.E.; Balakrishnan, P.B.; Powell, A.B.; Bowen, A.; Sarabia, I.; Burga, R.A.; Jones, R.B.; Bosque, A.; Cruz, C.R.Y.; Fernandes, R. PLGA nanodepots co-encapsulating prostratin and anti-CD25 enhance primary natural killer cell antiviral and antitumor function. Nano Res. 2020, 13, 736–744. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, S.; Chatterjee, S.; Das, J.; Sil, P.C. Nanoparticles as smart carriers for enhanced cancer immunotherapy. Front. Chem. 2020, 8, 1217. [Google Scholar] [CrossRef]
- Das, K.; Belnoue, E.; Rossi, M.; Hofer, T.; Danklmaier, S.; Nolden, T.; Schreiber, L.-M.; Angerer, K.; Kimpel, J.; Hoegler, S. A modular self-adjuvanting cancer vaccine combined with an oncolytic vaccine induces potent antitumor immunity. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Bu, J.; Nair, A.; Iida, M.; Jeong, W.; Poellmann, M.J.; Mudd, K.; Kubiatowicz, L.J.; Liu, E.W.; Wheeler, D.L.; Hong, S. An avidity-based PD-L1 antagonist using nanoparticle-antibody conjugates for enhanced immunotherapy. Nano Lett. 2020, 20, 4901–4909. [Google Scholar] [CrossRef]
- Simonetta, F.; Alam, I.S.; Lohmeyer, J.K.; Sahaf, B.; Good, Z.; Chen, W.; Xiao, Z.; Hirai, T.; Scheller, L.; Engels, P. Molecular imaging of chimeric antigen receptor T cells by ICOS-immunoPET. Clin. Cancer Res. 2021, 27, 1058–1068. [Google Scholar] [CrossRef]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Hemminki, O.; Dos Santos, J.M.; Hemminki, A. Oncolytic viruses for cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 1–15. [Google Scholar] [CrossRef]
- Park, W.; Heo, Y.-J.; Han, D.K. New opportunities for nanoparticles in cancer immunotherapy. Biomater. Res. 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, Á.; Sadrieh, N.; Dobrovolskaia, M.A. Minireview: Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Bay, B.H.; Leong, D.T. Gold nanoparticles induced endothelial leakiness depends on particle size and endothelial cell origin. ACS Nano 2017, 11, 5020–5030. [Google Scholar] [CrossRef]
- Tay, C.Y.; Setyawati, M.I.; Leong, D.T. Nanoparticle density: A critical biophysical regulator of endothelial permeability. ACS Nano 2017, 11, 2764–2772. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Peng, F.; Shi, X.; Leong, D.T. Targeting endothelial cell junctions with negatively charged gold nanoparticles. Chem. Mater. 2018, 30, 3759–3767. [Google Scholar] [CrossRef]
- Ge, H.; Wang, D.; Pan, Y.; Guo, Y.; Li, H.; Zhang, F.; Zhu, X.; Li, Y.; Zhang, C.; Huang, L. Sequence-dependent DNA functionalization of upconversion nanoparticles and their programmable assemblies. Angew. Chem. Int. Ed. 2020, 59, 8133–8137. [Google Scholar] [CrossRef]
- Ganbold, T.; Han, S.; Hasi, A.; Baigude, H. Receptor-mediated delivery of therapeutic RNA by peptide functionalized curdlan nanoparticles. Int. J. Biol. Macromol. 2019, 126, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Guo, M.; Han, Q.; Tian, Y.; Yuan, Y.; Wang, Z.; Qian, Y.; Wang, W. Synergetic tumor probes for facilitating therapeutic delivery by combined-functionalized peptide ligands. ACS Appl. Mater. Interfaces 2020, 92, 5650–5655. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Zhang, X. Aptamers as versatile ligands for biomedical and pharmaceutical applications. Int. J. Nanomed. 2020, 15, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing nanoparticles with cancer-targeting antibodies: A comparison of strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, S.; Zakharchenko, A.; Minko, S.; Kolpashchikov, D.; Katz, E. Towards nanomaterials for cancer theranostics: A system of DNA-modified magnetic nanoparticles for detection and suppression of rna marker in cancer cells. Magnetochemistry 2019, 5, 24. [Google Scholar] [CrossRef]
- Das, M.; Shen, L.; Liu, Q.; Goodwin, T.J.; Huang, L. Nanoparticle delivery of RIG-I agonist enables effective and safe adjuvant therapy in pancreatic cancer. Mol. Ther. 2019, 27, 507–517. [Google Scholar] [CrossRef] [PubMed]
- González-Ballesteros, N.; Diego-González, L.; Lastra-Valdor, M.; Rodríguez-Argüelles, M.C.; Grimaldi, M.; Cavazza, A.; Bigi, F.; Simón-Vázquez, R. Immunostimulant and biocompatible gold and silver nanoparticles synthesized using the: Ulva intestinalis L. aqueous extract. J. Mater. Chem. B 2019, 7, 4677–4691. [Google Scholar] [CrossRef]
- Song, S.; Jin, X.; Zhang, L.; Zhao, C.; Ding, Y.; Ang, Q.; Khaidav, O.; Shen, C. PEGegylated and CcD47-conjugated nanoellipsoidal artificial antigen-presenting cells minimize phagocytosis and augment anti-tumor T-cell responses. Int. J. Nanomed. 2019, 14, 2465–2483. [Google Scholar] [CrossRef]
- Kim, U.; Kim, C.-Y.; Lee, J.M.; Oh, H.; Ryu, B.; Kim, J.; Park, J.-H. Phloretin inhibits the human prostate cancer cells through the generation of reactive oxygen species. Pathol. Oncol. Res. 2020, 26, 977–984. [Google Scholar] [CrossRef]
- Druck, T.; Cheung, D.G.; Park, D.; Trapasso, F.; Pichiorri, F.; Gaspari, M.; Palumbo, T.; Aqeilan, R.I.; Gaudio, E.; Okumura, H. Fhit–Fdxr interaction in the mitochondria: Modulation of reactive oxygen species generation and apoptosis in cancer cells. Cell Death Dis. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific Targeting Cancer Cells with Nanoparticles and Drug Delivery in Cancer Therapy. Semin. Cancer Biol. 2021, 69, 166–177. [Google Scholar] [CrossRef]
- Buttacavoli, M.; Albanese, N.N.; Di Cara, G.; Alduina, R.; Faleri, C.; Gallo, M.; Pizzolanti, G.; Gallo, G.; Feo, S.; Baldi, F. Anticancer activity of biogenerated silver nanoparticles: An integrated proteomic investigation. Oncotarget 2018, 9, 9685. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Z.; Liu, X.; Agarwal, P.; Zhao, S.; Conroy, D.W.; Ji, G.; Yu, J.; Jaroniec, C.P.; Liu, Z. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Grueso, M.J.L.; Valero, R.M.T.; Carmona, H.B.; Ruiz, D.J.L.; Peinado, J.; McDonagh, B.; Aguilar, R.R.; Ruiz, J.A.B.; Peña, C.A.P. Peroxiredoxin 6 down-regulation induces metabolic remodeling and cell cycle arrest in HepG2 cells. Antioxidants 2019, 8, 505. [Google Scholar] [CrossRef]
- Khan, S.; Ansari, A.A.; Khan, A.A.; Abdulla, M.; Al-Obaid, O.; Ahmad, R. In vitro evaluation of cytotoxicity, possible alteration of apoptotic regulatory proteins, and antibacterial activity of synthesized copper oxide nanoparticles. Colloid. Surf. B Biointerfaces 2017, 153, 320–326. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Goltyaev, M.V.; Mal’tseva, V.N.; Turovsky, E.A.; Sarimov, R.M.; Simakin, A.V.; Gudkov, S.V. Mechanisms of the cytotoxic effect of selenium nanoparticles in different human cancer cell lines. Int. J. Mol. Sci. 2021, 22, 7798. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Varlamova, E.G. Mechanism of Ca2+-dependent pro-apoptotic action of selenium nanoparticles, mediated by activation of Cx43 hemichannels. Biology 2021, 10, 743. [Google Scholar] [CrossRef]
- Zhao, X.; Takabayashi, F.; Ibuki, Y. Coexposure to silver nanoparticles and ultraviolet a synergistically enhances the phosphorylation of histone H2AX. J. Photochem. Photobiol. B Biol. 2016, 162, 213–222. [Google Scholar] [CrossRef]
- Bhowmik, T.; Gomes, A. Down–regulation of cyclin–dependent kinase-4 and MAPK through estrogen receptor mediated cell cycle arrest in human breast cancer induced by gold nanoparticle tagged toxin protein NKCT1. Chem. Biol. Interact. 2017, 268, 119–128. [Google Scholar] [CrossRef]
- Huai, Y.; Zhang, Y.; Xiong, X.; Das, S.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles sensitize pancreatic cancer cells to gemcitabine. Cell Stress 2019, 3, 267–279. [Google Scholar] [CrossRef]
- Mi, Y.; Shao, Z.; Vang, J.; Kaidar-Person, O.; Wang, A.Z. Application of nanotechnology to cancer radiotherapy. Cancer Nanotechnol. 2016, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guryev, E.L.; Volodina, N.O.; Shilyagina, N.Y.; Gudkov, S.V.; Balalaeva, I.V.; Volovetskiy, A.B.; Lyubeshkin, A.V.; Sen, A.V.; Ermilov, S.A.; Vodeneev, V.A.; et al. Radioactive (90Y) upconversion nanoparticles conjugated with recombinant targeted toxin for synergistic nanotheranostics of cancer. Proc. Natl. Acad. Sci. USA 2018, 115, 9690–9695. [Google Scholar] [CrossRef]
- Laprise-Pelletier, M.; Lagueux, J.; Côté, M.; LaGrange, T.; Fortin, M. Low-dose prostate cancer brachytherapy with radioactive palladium–Gold nanoparticles. Adv. Healthc. Mater. 2017, 6, 1601120. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Xu, S.; Hu, W.; Xun, X.; Zheng, L.; Su, M. Tumor targeted, stealthy and degradable bismuth nanoparticles for enhanced X-ray radiation therapy of breast cancer. Biomaterials 2018, 154, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Delgadillo, R.; García-Cuéllar, C.M.; Sánchez-Pérez, Y.; Pineda-Aguilar, N.; Martínez-Martínez, M.A.; Rangel-Padilla, E.E.; Nakagoshi-Cepeda, S.E.; Solís-Soto, J.M.; Sánchez-Nájera, R.I.; Nakagoshi-Cepeda, M.A.A. In vitro evaluation of the antitumor effect of bismuth lipophilic nanoparticles (BisBAL NPs) on breast cancer cells. Int. J. Nanomed. 2018, 13, 6089. [Google Scholar] [CrossRef]
- Winter, H.; Brown, A.L.; Goforth, A.M. Bismuth-based nano-and microparticles in X-ray contrast, radiation therapy, and radiation shielding applications. Bismuth Adv. Appl. Defects Charact. 2018, 71, 1121–1141. [Google Scholar]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Tony, B.L. Phototherapy in cancer prevention and treatment. J. Cancer Prev. Curr. Res. 2017, 7, 22–24. [Google Scholar]
- Rivera-Rodriguez, A.; Chiu-Lam, A.; Morozov, V.M.; Ishov, A.M.; Rinaldi, C. Magnetic nanoparticle hyperthermia potentiates paclitaxel activity in sensitive and resistant breast cancer cells. Int. J. Nanomed. 2018, 13, 4771. [Google Scholar] [CrossRef]
- Sohail, A.; Ahmad, Z.; Bég, O.A.; Arshad, S.; Sherin, L. A review on hyperthermia via nanoparticle-mediated therapy. Bull. Cancer 2017, 104, 452–461. [Google Scholar] [CrossRef]
- Legge, C.J.; Colley, H.E.; Lawson, M.A.; Rawlings, A.E. Targeted magnetic nanoparticle hyperthermia for the treatment of oral cancer. J. Oral Pathol. Med. 2019, 48, 803–809. [Google Scholar] [CrossRef]
- Heidari, M.; Sattarahmady, N.; Azarpira, N.; Heli, H.; Mehdizadeh, A.R.; Zare, T. Photothermal cancer therapy by gold-ferrite nanocomposite and near-infrared laser in animal model. Lasers Med. Sci. 2016, 31, 221–227. [Google Scholar] [CrossRef]
- Ma, Y.C.; Zhu, Y.H.; Tang, X.F.; Hang, L.F.; Jiang, W.; Li, M.; Khan, M.I.; You, Y.Z.; Wang, Y.C. Au nanoparticles with enzyme-mimicking activity-ornamented ZIF-8 for highly efficient photodynamic therapy. Biomater. Sci. 2019, 7, 2740–2748. [Google Scholar] [CrossRef]
- Kaur, P.; Aliru, M.L.; Chadha, A.S.; Asea, A.; Krishnan, S. Hyperthermia using nanoparticles–promises and pitfalls. Int. J. Hyperth. 2016, 32, 76–88. [Google Scholar] [CrossRef]
- Bagheri, S.; Yasemi, M.; Safaie-Qamsari, E.; Rashidiani, J.; Abkar, M.; Hassani, M.; Mirhosseini, S.A.; Kooshki, H. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 462–471. [Google Scholar] [CrossRef]
- Afzal, M.; Alharbi, K.S.; Alruwaili, N.K.; Al-Abassi, F.A.; Al-Malki, A.A.L.; Kazmi, I.; Kumar, V.; Kamal, M.A.; Nadeem, M.S.; Aslam, M. Nanomedicine in Treatment of Breast Cancer–A Challenge to Conventional Therapy. Semin. Cancer Biol. 2021, 69, 279–292. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Lin, J.-N.; Hsieh, J.-T.; Chou, S.-C.; Lai, C.-H.; Yun, E.-J.; Lo, U.-G.; Pong, R.-C.; Lin, J.-H.; Lin, Y.-H. Nanoparticle targeting CD44-positive cancer cells for site-specific drug delivery in prostate cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 30722–30734. [Google Scholar] [CrossRef]
- Cheng, F.-F.; Sun, P.; Xiong, W.-W.; Zhang, Y.; Zhang, Q.; Yao, W.; Cao, Y.; Zhang, L. Multifunctional titanium phosphate nanoparticles for site-specific drug delivery and real-time therapeutic efficacy evaluation. Analyst 2019, 144, 3103–3110. [Google Scholar] [CrossRef]
- Bisht, G.; Rayamajhi, S.; Biplab, K.C.; Paudel, S.N.; Karna, D.; Shrestha, B.G. Synthesis, characterization, and study of in vitro cytotoxicity of ZnO-Fe3O4 magnetic composite nanoparticles in human breast cancer cell line (MDA-MB-231) and mouse fibroblast (NIH 3T3). Nanoscale Res. Lett. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Tai, W.; Mahato, R.; Cheng, K. The role of HER2 in cancer therapy and targeted drug delivery. J. Control. Release 2010, 146, 264–275. [Google Scholar] [CrossRef]
- Kaplan, A.; Kutlu, H.M.; Ciftci, G.A. Fe3O4 nanopowders: Genomic and apoptotic evaluations on A549 lung adenocarcinoma cell line. Nutr. Cancer 2020, 72, 708–721. [Google Scholar] [CrossRef]
- Nebu, J.; Devi, J.S.A.; Aparna, R.S.; Abha, K.; Sony, G. Erlotinib conjugated gold nanocluster enveloped magnetic iron oxide nanoparticles–A targeted probe for imaging pancreatic cancer cells. Sens. Actuators B Chem. 2018, 257, 1035–1043. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Hong, W.; Chen, Z.; Peng, P.; Yuan, S.; Qu, J.; Xiao, M.; Xu, L. Glucose oxidase and polydopamine functionalized iron oxide nanoparticles: Combination of the photothermal effect and reactive oxygen species generation for dual-modality selective cancer therapy. J. Mater. Chem. B 2019, 7, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Roy, A.; Barui, A.K.; Alabbasi, M.M.A.; Kuncha, M.; Sistla, R.; Sreedhar, B.; Patra, C.R. Anti-angiogenic vanadium pentoxide nanoparticles for the treatment of melanoma and their in vivo toxicity study. Nanoscale 2020, 12, 7604–7621. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, S.; Hu, M.; Zhang, H.; Duan, D.; He, J.; Hong, J.; Lv, R.; Choi, H.S.; Yan, X.; et al. Peroxidase-like nanozymes induce a novel form of cell death and inhibit tumor growth in vivo. Adv. Funct. Mater. 2020, 30, 2000647. [Google Scholar] [CrossRef]
- Chen, M.; Deng, G.; He, Y.; Li, X.; Liu, W.; Wang, W.; Zhou, Z.; Yang, H.; Yang, S. Ultrasound-enhanced generation of reactive oxygen species for mri-guided tumor therapy by the Fe@Fe3O4-based peroxidase-mimicking nanozyme. ACS Appl. Bio Mater. 2020, 3, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Jiang, S.; Ding, M.; Sun, S.; Ma, Y.; Younis, M.R.; He, G.; Wang, J.; Lin, J.; Cao, Z. Ultrasmall rhodium nanozyme with RONS scavenging and photothermal activities for anti-inflammation and antitumor theranostics of colon diseases. Nano Lett. 2020, 20, 3079–3089. [Google Scholar] [CrossRef]
- Wu, F.; Zhu, J.; Li, G.; Wang, J.; Veeraraghavan, V.P.; Krishna Mohan, S.; Zhang, Q. Biologically synthesized green gold nanoparticles from Siberian ginseng induce growth-inhibitory effect on melanoma cells (B16). Artif. Cells Nanomed. Biotechnol. 2019, 47, 3297–3305. [Google Scholar] [CrossRef]
- Khiavi, A.A. PEGylated gold nanoparticles-ribonuclease induced oxidative stress and apoptosis in colorectal cancer cells. BioImpacts 2019, 10, 27–36. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hsieh, D.-S.; Huang, K.-J.; Chan, Y.-L.; Hong, P.-D.; Yeh, M.-K.; Wu, C.-J. Improving anticancer efficacy of (–)-epigallocatechin-3-gallate gold nanoparticles in murine B16F10 melanoma cells. Drug Des. Devel. Ther. 2014, 8, 459. [Google Scholar]
- Jiang, T.; Zhang, B.; Zhang, L.; Wu, X.; Li, H.; Shen, S.; Luo, Z.; Liu, X.; Hu, Y.; Pang, Z. Biomimetic nanoparticles delivered hedgehog pathway inhibitor to modify tumour microenvironment and improved chemotherapy for pancreatic carcinoma. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1088–1101. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, J.; Yan, S.; Tao, Z.; Wang, C.; Huang, M.; Zhang, X. PEGylated zinc oxide nanoparticles induce apoptosis in pancreatic cancer cells through reactive oxygen species. IET Nanobiotechnol. 2019, 13, 80–84. [Google Scholar] [CrossRef]
- Shen, C.; James, S.A.; de Jonge, M.D.; Turney, T.W.; Wright, P.F.A.; Feltis, B.N. Relating cytotoxicity, zinc ions, and reactive oxygen in ZnO nanoparticle–exposed human immune cells. Toxicol. Sci. 2013, 136, 120–130. [Google Scholar] [CrossRef]
- Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Al-Massarani, S.M.; Saquib, Q.; Wahab, R.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Anticancer potential of green synthesized silver nanoparticles using extract of Nepeta deflersiana against human cervical cancer cells (HeLA). Bioinorg. Chem. Appl. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Dobrucka, R.; Romaniuk-Drapała, A.; Kaczmarek, M. Evaluation of biological synthesized platinum nanoparticles using Ononidis radix extract on the cell lung carcinoma A549. Biomed. Microdevices 2019, 21, 1–10. [Google Scholar] [CrossRef]
- Fujiwara, R.; Luo, Y.; Sasaki, T.; Fujii, K.; Ohmori, H.; Kuniyasu, H. Cancer therapeutic effects of titanium dioxide nanoparticles are associated with oxidative stress and cytokine induction. Pathobiology 2015, 82, 243–251. [Google Scholar] [CrossRef]
- Murugan, C.; Murugan, N.; Sundramoorthy, A.K.; Sundaramurthy, A. Nanoceria decorated flower-like molybdenum sulphide nanoflakes: An efficient nanozyme for tumour selective ROS generation and photo thermal therapy. Chem. Commun. 2019, 55, 8017–8020. [Google Scholar] [CrossRef]
- Nejdl, L.; Kudr, J.; Moulick, A.; Hegerova, D.; Ruttkay-Nedecky, B.; Gumulec, J.; Cihalova, K.; Smerkova, K.; Dostalova, S.; Krizkova, S.; et al. Platinum nanoparticles induce damage to DNA and inhibit DNA replication. PLoS ONE 2017, 12, e0180798. [Google Scholar] [CrossRef]
- Nourmohammadi, E.; Khoshdel-Sarkarizi, H.; Nedaeinia, R.; Sadeghnia, H.R.; Hasanzadeh, L.; Darroudi, M.; Kazemi oskuee, R. Evaluation of anticancer effects of cerium oxide nanoparticles on mouse fibrosarcoma cell line. J. Cell. Physiol. 2019, 234, 4987–4996. [Google Scholar] [CrossRef] [PubMed]
- Parvathya, S.; Venkatramanb, B.R. In vitro antibacterial and anticancer potential of CeO2 nanoparticles prepared by co-precipitation and green synthesis method. J. Nanosci. Curr. Res. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Kadhem, H.A.; Ibraheem, S.A.; Jabir, M.S.; Kadhim, A.A. ZainAg-doping regulates the cytotoxicity of TiO2 nanoparticles via oxidative stress in human cancer cellsin, Zinc Oxide nanoparticles induce apoptosis in human breast cancer cells via caspase-8 and P53 pathway. Nano Biomed. Eng. 2019, 11, 35–43. [Google Scholar] [CrossRef]
- Ahamed, M.; Khan, M.A.M.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Ag-doping regulates the cytotoxicity of TiO2 nanoparticles via oxidative stress in human cancer cells. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Sponchioni, M.; Morbidelli, M.; Moscatelli, D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: The checkpoints on the road from the synthesis to clinical translation. Nanoscale 2018, 10, 22701–22719. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, L.; Morin, E.E.; Liu, M.; Schwendeman, A. Survey of clinical translation of cancer nanomedicines—Lessons learned from successes and failures. Acc. Chem. Res. 2019, 52, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Rani, R.; Agarwal, V. Liposome and their applications in cancer therapy. Braz. Arch. Biol. Technol. 2016, 59, 59. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Rommasi, F.; Esfandiari, N. Liposomal nanomedicine: Applications for drug delivery in cancer therapy. Nanoscale Res. Lett. 2021, 16, 1–20. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy–an update from drug design perspective. Drug Des. Devel. Ther. 2017, 11, 599. [Google Scholar] [CrossRef]
- Lakshmi, B.A.; Reddy, A.S.; Sangubotla, R.; Hong, J.W.; Kim, S. Ruthenium (II)-curcumin liposome nanoparticles: Synthesis, characterization, and their effects against cervical cancer. Colloids Surf. B Biointerfaces 2021, 204, 111773. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Ceña, V.; Rodrigues, J.; Tomas, H.; Majoral, J.-P. Engineered non-invasive functionalized dendrimer/dendron-entrapped/complexed gold nanoparticles as a novel class of theranostic (radio) pharmaceuticals in cancer therapy. J. Control. Release 2021, 332, 346–366. [Google Scholar] [CrossRef]
- Gul, A.R.; Shaheen, F.; Rafique, R.; Bal, J.; Waseem, S.; Park, T.J. Grass-mediated biogenic synthesis of silver nanoparticles and their drug delivery evaluation: A biocompatible anti-cancer therapy. Chem. Eng. J. 2021, 407, 127202. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Turovsky, E.A.; Blinova, E.V. Therapeutic potential and main methods of obtaining selenium nanoparticles. Int. J. Mol. Sci. 2021, 22, 10808. [Google Scholar] [CrossRef]
- Lorkowski, M.E.; Atukorale, P.U.; Ghaghada, K.B.; Karathanasis, E. Stimuli-responsive iron oxide nanotheranostics: A versatile and powerful approach for cancer therapy. Adv. Healthc. Mater. 2021, 10, 2001044. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control Release 2021, 10, 127–147. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomedicine 2018, 13, 2921. [Google Scholar] [CrossRef]
- Persano, F.; Gigli, G.; Leporatti, S. Lipid-polymer hybrid nanoparticles in cancer therapy: Current overview and future directions. Nano Express 2021, 2, 012006–012022. [Google Scholar] [CrossRef]
- Jana, D.; Jia, S.; Bindra, A.K.; Xing, P.; Ding, D.; Zhao, Y. Clearable black phosphorus nanoconjugate for targeted cancer phototheranostics. ACS Appl. Mater. Interfaces 2020, 12, 18342–18351. [Google Scholar] [CrossRef]
- Payne, M.; Ellis, P.; Dunlop, D.; Ranson, M.; Danson, S.; Schacter, L.; Talbot, D. DHA-paclitaxel (Taxoprexin) as first-line treatment in patients with stage IIIB or IV non-small cell lung cancer: Report of a phase II open-label multicenter trial. J. Thorac. Oncol. 2006, 1, 984–990. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Fymat, A.L. Magnetic resonance imaging modalities with contrast enhancing nanomaterials. Curr. Trends Clin. Med. Imaging 2017, 1, 11–14. [Google Scholar]
- Alshammari, T.M. Drug safety: The concept, inception and its importance in patients’ health. Saudi Pharm. J. 2016, 24, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2021, 12, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Cross, A. Endotoxin: Back to the future. Crit. Care Med. 2016, 44, 450–454. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Sui, X.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Recent advances in drug delivery systems for enhancing drug penetration into tumors. Drug Deliv. 2020, 27, 1474–1490. [Google Scholar] [CrossRef]
- Murugan, K.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. Parameters and characteristics governing cellular internalization and trans-barrier trafficking of nanostructures. Int. J. Nanomed. 2015, 10, 2191. [Google Scholar]
- Wang, S.; Zhang, F.; Yu, G.; Wang, Z.; Jacobson, O.; Ma, Y.; Tian, R.; Deng, H.; Yang, W.; Chen, Z.-Y. Zwitterionic-to-cationic charge conversion polyprodrug nanomedicine for enhanced drug delivery. Theranostics 2020, 10, 6629–6637. [Google Scholar] [CrossRef]
- Du, Y.; Wang, S.; Zhang, T.; He, D.; Tu, J.; Shen, Y. Enhanced cytotoxicity of a redox-sensitive hyaluronic acid-based nanomedicine toward different oncocytes via various internalization mechanisms. Drug Deliv. 2020, 27, 128–136. [Google Scholar] [CrossRef]
- Abasian, P.; Shakibi, S.; Maniati, M.S.; Nouri Khorasani, S.; Khalili, S. Targeted delivery, drug release strategies, and toxicity study of polymeric drug nanocarriers. Polym. Adv. Technol. 2021, 32, 931–944. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Tiboni, M.; Coppari, S.; Casettari, L.; Guescini, M.; Colomba, M.; Fraternale, D.; Gorassini, A.; Verardo, G.; Ramakrishna, S.; Guidi, L. Prunus spinosa extract loaded in biomimetic nanoparticles evokes in vitro anti-inflammatory and wound healing exosoActivities. Nanomaterials 2021, 11, 36. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Y.; Feng, N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: A review. Mater. Sci. Eng. C 2020, 106, 110298. [Google Scholar] [CrossRef]
- Goswitz, V.C.; Peter Sawicki, Z. Cancer therapy based on a mechanism of action for controlling the immune system and the resulting patent portfolio. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013, 7, 1–10. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal. Transduct. Target. Ther. 2018, 3, 1–19. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Feldman, L.; Seckler, A.; Wilson, A. Trends in risks associated with new drug development: Success rates for investigational drugs. Clin. Pharmacol. Ther. 2010, 87, 272–277. [Google Scholar] [CrossRef]
- Matias, M.; Pinho, J.O.; Penetra, M.J.; Campos, G.; Reis, C.P.; Gaspar, M.M. The challenging melanoma landscape: From early drug discovery to clinical approval. Cells 2021, 10, 3088–3128. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef]
- Cheow, W.S.; Hadinoto, K. Factors affecting drug encapsulation and stability of lipid–polymer hybrid nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 214–220. [Google Scholar] [CrossRef]
- Hafeez, M.N.; Celia, C.; Petrikaite, V. Challenges towards targeted drug delivery in cancer nanomedicines. Processes 2021, 9, 1527. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 2021, 16, 1–21. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef]
- Sanna, V.; Pala, N.; Sechi, M. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar]
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty years of cancer nanomedicine: Success, frustration, and hope. Cancers 2019, 11, 1855. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, J.; García, I.; Liz-Marzán, L.M. Cellular uptake of nanoparticles versus small molecules: A matter of size. Acc. Chem. Res. 2018, 51, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V. Facilitating the translation of nanomedicines to a clinical product: Challenges and opportunities. Drug Discov. Today 2018, 23, 974–991. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, C.; Wang, Y.; Koivisto, O.; Zhou, J.; Shu, Y.; Zhang, H. Nanotechnology-based delivery of CRISPR/Cas9 for cancer treatment. Adv. Drug Deliv. Rev. 2021, 176, 113891. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Ouyang, K.; Xu, X.; Xu, L.; Wen, C.; Zhou, X.; Qin, Z.; Xu, Z.; Sun, W.; Liang, Y. Nanoparticle delivery of CRISPR/Cas9 for genome editing. Front. Genet. 2021, 12, 673286. [Google Scholar] [CrossRef]
- Guo, P.; Yang, J.; Huang, J.; Auguste, D.T.; Moses, M.A. Therapeutic genome editing of triple-negative breast tumors using a noncationic and deformable nanolipogel. Proc. Natl. Acad. Sci. USA 2019, 116, 18295–18303. [Google Scholar] [CrossRef]
- Finn, J.D.; Smith, A.R.; Patel, M.C.; Shaw, L.; Youniss, M.R.; van Heteren, J.; Dirstine, T.; Ciullo, C.; Lescarbeau, R.; Seitzer, J. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 2018, 22, 2227–2235. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, K.; Wang, C.; Zhang, Z.; Zheng, C.; Zhao, Y.; Zheng, Y.; Liu, C.; An, Y.; Shi, L. Multistage delivery nanoparticle facilitates efficient CRISPR/dCas9 activation and tumor growth suppression in vivo. Adv. Sci. 2019, 6, 1801423. [Google Scholar] [CrossRef]
- Shi, J.; Yang, X.; Li, Y.; Wang, D.; Liu, W.; Zhang, Z.; Liu, J.; Zhang, K. MicroRNA-responsive release of Cas9/sgRNA from DNA nanoflower for cytosolic protein delivery and enhanced genome editing. Biomaterials 2020, 256, 120221. [Google Scholar] [CrossRef]
- Mout, R.; Rotello, V.M. Cytosolic and nuclear delivery of CRISPR/Cas9-ribonucleoprotein for gene editing using arginine functionalized gold nanoparticles. Bio-Protocol 2017, 7, 20. [Google Scholar] [CrossRef]
- Hou, S.; Zhao, L.; Shen, Q.; Yu, J.; Ng, C.; Kong, X.; Wu, D.; Song, M.; Shi, X.; Xu, X. Polymer nanofiber-embedded microchips for detection, isolation, and molecular analysis of single circulating melanoma cells. Angew. Chem. 2013, 125, 3463–3467. [Google Scholar] [CrossRef]
- Jan, Y.J.; Yoon, J.; Chen, J.-F.; Teng, P.-C.; Yao, N.; Cheng, S.; Lozano, A.; Chu, G.C.Y.; Chung, H.; Lu, Y.-T. A circulating tumor cell-RNA assay for assessment of androgen receptor signaling inhibitor sensitivity in metastatic castration-resistant prostate cancer. Theranostics 2019, 9, 2812. [Google Scholar] [CrossRef]
- Gao, H.; Sun, X.; Rao, Y. PROTAC technology: Opportunities and challenges. ACS Med. Chem. Lett. 2020, 11, 237–240. [Google Scholar] [CrossRef]
- Donati, B.; Lorenzini, E.; Ciarrocchi, A. BRD4 and Cancer: Going beyond transcriptional regulation. Mol. Cancer 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Saraswat, A.; Patki, M.; Fu, Y.; Barot, S.; Dukhande, V.V.; Patel, K. Nanoformulation of PROteolysis TArgeting chimera targeting ‘undruggable’c-Myc for the treatment of pancreatic cancer. Nanomedicine 2020, 15, 1761–1777. [Google Scholar] [CrossRef]
- Wang, Y.; Han, L.; Liu, F.; Yang, F.; Jiang, X.; Sun, H.; Feng, F.; Xue, J.; Liu, W. Targeted degradation of anaplastic lymphoma kinase by gold nanoparticle-based multi-headed proteolysis targeting chimeras. Colloids Surf. B Biointerfaces 2020, 188, 110795–110804. [Google Scholar] [CrossRef]
- Cimas, F.J.; Niza, E.; Juan, A.; Noblejas-López, M.d.M.; Bravo, I.; Lara-Sanchez, A.; Alonso-Moreno, C.; Ocaña, A. Controlled delivery of BET-PROTACs: In vitro evaluation of mz1-loaded polymeric antibody conjugated nanoparticles in breast cancer. Pharmaceutics 2020, 12, 986. [Google Scholar] [CrossRef]
- Han, Y. Current status of proton therapy techniques for lung cancer. Radiat. Oncol. J. 2019, 37, 232–248. [Google Scholar] [CrossRef]
- Peukert, D.; Kempson, I.; Douglass, M.; Bezak, E. Gold nanoparticle enhanced proton therapy: Monte Carlo modeling of reactive species’ distributions around a gold nanoparticle and the effects of nanoparticle proximity and clustering. Int. J. Mol. Sci. 2019, 20, 4280. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Plummer, R.; Stock, J.K.; Greenhalgh, T.A.; Ataman, O.; Kelly, S.; Clay, R.; Adams, R.A.; Baird, R.D.; Billingham, L. Clinical development of new drug–radiotherapy combinations. Nat. Rev. Clin. Oncol. 2016, 13, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; De Kock, M.; Engelbrecht, M.; Miles, X.; Slabbert, J.; Vandevoorde, C. Radiosensitization effect of gold nanoparticles in proton therapy. Front. Public Health 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Peukert, D.; Kempson, I.; Douglass, M.; Bezak, E. Gold nanoparticle enhanced proton therapy: A Monte Carlo simulation of the effects of proton energy, nanoparticle size, coating material, and coating thickness on dose and radiolysis yield. Med. Phys. 2020, 47, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Calugaru, V.; Jouffroy, T.; Rodriguez, J.; Hoffmann, C.; Dodger, B.; Moreno, V.; Calvo, E. A phase 1 trial of NBTXR3 nanoparticles activated by intensity-modulated radiation therapy (IMRT) in the treatment of advanced-stage head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 2017, 35, 6080–6085. [Google Scholar] [CrossRef]
- Chen, T.; Ren, L.; Liu, X.; Zhou, M.; Li, L.; Xu, J.; Zhu, X. DNA nanotechnology for cancer diagnosis and therapy. Int. J. Mol. Sci. 2018, 19, 1671. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.M.F.A.; Lai, W.-F.; Akhtar, M.F.; Saleem, A.; Ahmed, S.A.; Xia, X.-H. DNA nanotechnology as a tool to develop molecular tension probes for bio-sensing and bio-imaging applications: An up-to-date review. Nano Struct. Nano Objects 2020, 23, 100523–100533. [Google Scholar] [CrossRef]
- Liu, Y.; Blanchfield, L.; Ma, V.P.-Y.; Andargachew, R.; Galior, K.; Liu, Z.; Evavold, B.; Salaita, K. DNA-based nanoparticle tension sensors reveal that T-cell receptors transmit defined pN forces to their antigens for enhanced fidelity. Proc. Natl. Acad. Sci. USA 2016, 113, 5610–5615. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Ding, J.; Liu, J. Fluorescent sensors using DNA-functionalized graphene oxide. Anal. Bioanal. Chem. 2014, 406, 6885–6902. [Google Scholar] [CrossRef]
- De Maria Marchiano, R.; Di Sante, G.; Piro, G.; Carbone, C.; Tortora, G.; Boldrini, L.; Pietragalla, A.; Daniele, G.; Tredicine, M.; Cesario, A. Translational research in the era of precision medicine: Where we are and where we will go. J. Pers. Med. 2021, 11, 216. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W. Protein catenation enhances both the stability and activity of folded structural domains. Angew. Chem. 2017, 129, 14173–14177. [Google Scholar] [CrossRef]
- Wu, W.-H.; Bai, X.; Shao, Y.; Yang, C.; Wei, J.; Wei, W.; Zhang, W.-B. Higher order protein catenation leads to an artificial antibody with enhanced affinity and in vivo stability. J. Am. Chem. Soc. 2021, 143, 18029–18040. [Google Scholar] [CrossRef]
- Luo, M.-J.; Palmieri, M.; Riffkin, C.D.; Sakthianandeswaren, A.; Djajawi, T.M.; Hirokawa, Y.; Shuttleworth, V.; Segal, D.H.; White, C.A.; Nhu, D. Defining the susceptibility of colorectal cancers to BH3-mimetic compounds. Cell Death Dis. 2020, 11, 1–4. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Wang, X.-Y.; Wei, Q.-Y.; Xu, Y.-M.; Lau, A.T.Y. Potency and selectivity of SMAC/DIABLO mimetics in solid tumor therapy. Cells 2020, 9, 1012. [Google Scholar] [CrossRef]
- Goldsmith, S.J. Targeted radionuclide therapy: A Historical and Personal Review. In Seminars in Nuclear Medicine; Elsevier: Amsterdam, The Netherlands, 2020; Volume 50, pp. 87–97. [Google Scholar]
- Chan, T.G.; O’Neill, E.; Habjan, C.; Cornelissen, B. Combination strategies to improve targeted radionuclide therapy. J. Nucl. Med. 2020, 61, 1544–1552. [Google Scholar] [CrossRef]
- Schuerle, S.; Soleimany, A.P.; Yeh, T.; Anand, G.M.; Häberli, M.; Fleming, H.E.; Mirkhani, N.; Qiu, F.; Hauert, S.; Wang, X. Synthetic and living micropropellers for convection-enhanced nanoparticle transport. Sci. Adv. 2019, 5, eaav4803. [Google Scholar] [CrossRef]
- Ebner, D.K.; Frank, S.J.; Inaniwa, T.; Yamada, S.; Shirai, T. The Emerging Potential of Multi-Ion Radiotherapy. Front. Oncol. 2021, 11, 27. [Google Scholar] [CrossRef]
- Wurz, G.T.; Kao, C.-J.; Wolf, M.; DeGregorio, M.W. Tecemotide: An antigen-specific cancer immunotherapy. Hum. Vaccin. Immunother. 2014, 10, 3383–3393. [Google Scholar] [CrossRef]
- Gargett, T.; Abbas, M.N.; Rolan, P.; Price, J.D.; Gosling, K.M.; Ferrante, A.; Ruszkiewicz, A.; Atmosukarto, I.I.C.; Altin, J.; Parish, C.R.; et al. Phase I trial of Lipovaxin-MM, a novel dendritic cell-targeted liposomal vaccine for malignant melanoma. Cancer Immunol. Immunother. 2018, 67, 1461–1472. [Google Scholar] [CrossRef]
- Young, C.; Schluep, T.; Hwang, J.; Eliasof, S. CRLX101 (formerly IT-101)-A novel nanopharmaceutical of camptothecin in clinical development. Curr. Bioact. Compd. 2011, 7, 8–14. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kageyama, S.; Miyahara, Y.; Okayama, T.; Kokura, S.; Wang, L.; Sato, E.; Yagita, H.; Itoh, Y.; Shiku, H. Safety and antibody immune response of CHP-NY-ESO-1 vaccine combined with poly-ICLC in advanced or recurrent esophageal cancer patients. Cancer Immunol. Immunother. 2021, 70, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.A.; Brufsky, A.; Coleman, R.E.; Conte, P.F.; Cortes, J.; Glück, S.; Nabholtz, J.-M.A.; O’Shaughnessy, J.; Beck, R.M.; Ko, A. Phase II/III weekly nab-paclitaxel plus gemcitabine or carboplatin versus gemcitabine/carboplatin as first-line treatment of patients with metastatic triple-negative breast cancer (the tnAcity study): Study protocol for a randomized controlled trial. Trials 2015, 16, 1–10. [Google Scholar] [CrossRef][Green Version]
- Weng, X.; Huang, X.; Li, H.; Lin, S.; Rao, X.; Guo, X.; Huang, P. First-line treatment with atezolizumab plus nab-paclitaxel for advanced triple-negative breast cancer: A cost-effectiveness analysis. Am. J. Clin. Oncol. 2020, 43, 340–348. [Google Scholar] [CrossRef]
- Ogino, M.; Fujii, T.; Koibuchi, Y.; Nakazawa, Y.; Takata, D.; Shirabe, K. Phase II study of nab-paclitaxel plus cyclophosphamide plus trastuzumab neoadjuvant chemotherapy in early HER-2-positive breast cancer. Anticancer Res. 2021, 41, 3899–3904. [Google Scholar] [CrossRef]
- Canetta, E. Current and future advancements of raman spectroscopy techniques in cancer nanomedicine. Int. J. Mol. Sci. 2021, 22, 13141. [Google Scholar] [CrossRef]
- Maiti, K.K.; Samanta, A.; Vendrell, M.; Soh, K.-S.; Olivo, M.; Chang, Y.-T. Multiplex cancer cell detection by SERS nanotags with cyanine and triphenylmethine Raman reporters. Chem. Commun. 2011, 47, 3514–3516. [Google Scholar] [CrossRef]
- Krishnan, T.; Wang, H.-N.; Vo-Dinh, T. Smartphone-based device for colorimetric detection of MicroRNA biomarkers using nanoparticle-based assay. Sensors 2021, 21, 8044. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Liang, R.; Ding, D.; Zheng, X.; Zhu, X.; Hu, S.; Wei, H.; Wei, B. A Smart multifunctional nanoparticle for enhanced near-infrared image-guided photothermal therapy against gastric cancer. Int. J. Nanomed. 2021, 16, 2897. [Google Scholar] [CrossRef]
- Tang, C.; He, Z.; Liu, H.; Xu, Y.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnol. 2020, 18, 1–19. [Google Scholar] [CrossRef]
- Schneider-Futschik, E.K.; Reyes-Ortega, F. Advantages and disadvantages of using magnetic nanoparticles for the treatment of complicated ocular disorders. Pharmaceutics 2021, 13, 1157. [Google Scholar] [CrossRef]
- Wang, C.; Lu, J.; Zhou, L.; Li, J.; Xu, J.; Li, W.; Zhang, L.; Zhong, X.; Wang, T. Effects of long-term exposure to zinc oxide nanoparticles on development, zinc metabolism and biodistribution of minerals (Zn, Fe, Cu, Mn) in mice. PLoS ONE 2016, 11, e0164434–e0164448. [Google Scholar] [CrossRef] [PubMed]
- Yada, R.; Maenaka, K.; Miyamoto, S.; Okada, G.; Sasakura, A.; Ashida, M.; Adachi, M.; Sato, T.; Wang, T.; Akasaka, H. Real-time in vivo dosimetry system based on an optical fiber-coupled microsized photostimulable phosphor for stereotactic body radiation therapy. Med. Phys. 2020, 47, 5235–5249. [Google Scholar] [CrossRef] [PubMed]




| Type of Nanoparticles/Nano-Conjugates | Cell Lines | Mechanism of Action | Reference |
|---|---|---|---|
| DNA-modified magnetic NPs | MCF-7 | Suppression of RNA marker | [25] |
| Au, Ag NPs | Human peripheral blood mononuclear cells (hPBMCs) | Compliment activation, cytokine production | [27] |
| Gold NP-tagged toxin | MCF-7 | Down-regulation of CDK-4 and MAPK | [39] |
| Au@ZIF-8 NPs | EMT-6 murine breast cancer cell | ROS generation | [53] |
| Fe3O4@AuNC@erlotinib | PANC-1 | Selective targeting of overexpressed EGFR | [62] |
| GOx and PDA functionalized iron oxide NPs | MDA-MB-231, MCF-10A and 4T1 | Photothermal therapy and ROS-mediated damage | [63] |
| V2O5 | B16F10, A549, and PANC1 | ROS-induced apoptosis | [64] |
| Fe3O4 | HepG2 | ATP-citrate lyase-dependent RAS signaling | [65] |
| Fe@Fe3O4@heparin | 4T1 breast tumor cell line, HUVEC cell | ROS generation | [66] |
| PEGylated rhodium nanodots | CT-26 colon tumor | Down-regulation of TNF-α and IL-6 | [67] |
| Au NPs | B16 melanoma cell | Up-regulation of Caspase 3, Caspase 9, Bid, Bax and down-regulation of BCl2 | [68] |
| Au NPs-PEG-RNase A conjugate | SW-480 | ROS generation | [69] |
| Au NPs | B16 F10 melanoma cell | Mitochondrial pathway-mediated apoptosis | [70] |
| RBC membrane-coated PLGA NPs | Pancreatic ductal adenocarcinoma | Tumor microenvironment modulation | [71] |
| PEGylated ZnO NPs | PANC1 | ROS-induced apoptosis | [72] |
| ZnO NPs | Human acute monocytic leukemia cell line (THP-1) | Mitochondrial membrane damage and elevated ROS concentration | [73] |
| Ag NPs | HeLa | SubG1 arrest and apoptotic/necrotic cell death | [74] |
| Pt NPs | A549 | Induction of apoptosis and cell cycle arrest | [75] |
| TiO2 NPs | LL2 mouse lung cancer cell line | Oxidative stress and cytokine induction | [76] |
| MoS2 nanoflakes | MDA-MB-231 | Selective ROS generation and photo thermal therapy | [77] |
| Pt NPs | Human foreskin fibroblast cell | Damage to DNA and inhibition of DNA replication | [78] |
| CeO2 NPs | Mouse fibrosarcoma cell line | ROS-induced apoptosis | [79] |
| CeO2 NPs | A549 | ROS-mediated apoptosis | [80] |
| ZnO NPs | MCF-7 | Up-regulation of caspase-8 and p53 | [81] |
| TiO2 NPs | HepG2, A549, MCF-7 and IMR-90 | Oxidative stress | [82] |
| Challenges | Reference |
|---|---|
| The long process of drug development | [114] |
| Years required for pre-clinical and clinical research on higher animals and humans | [115] |
| Hassles in obtaining regulatory approval to release the drug in the market | [116] |
| Failure to effectively load the drug inside the nanoparticles | [117] |
| Instability of the formulation | [118] |
| Issues with biocompatibility and toxicity | [119] |
| Insufficient residence time in the body | [120] |
| Failure of the drug formulation to selectively accumulate on the target | [121] |
| Failure in loading, internalization, and drug release | [122] |
| Incomplete biodegradation and elimination | [123] |
| Challenges in cellular uptake | [124] |
| Failure to translate the in vitro results to in vivo studies | [125] |
| Nanodrug | Conventional Drug | Cancer Type | Clinical Trials.gov Identifier |
|---|---|---|---|
| Paclitaxel Nab | 5-Fluorouracil, Epirubicin, Cyclophosphamide (FEC) | Breast cancer | NCT00110695 |
| Carboplatin, Erlotinib hydrochloride | NSCLC | NCT01928160 | |
| Phenelzine sulfate | Metastatic breast cancer | NCT03505528 | |
| Doxorubicin hydrochloride, Cyclophosphamide, Filgrastim, Trastuzumab | Estrogen receptor-positive Breast cancer HER2-positive breast cancer | NCT00407888 | |
| Bevacizumab, Gemcitabine hydrochloride | Breast cancer | NCT00623233 | |
| Carboplatin, Erlotinib hydrochloride | NSCLC | NCT00661193 | |
| Sargramostim | Brenner tumor, Fallopian tube cancer, Ovarian clear cell cystadenocarcinoma, Ovarian epithelial cancer | NCT00466960 | |
| PIPAC | Peritoneal carcinomatosis, Ovarian cancer, Breast cancer, Stomach cancer, Pancreatic cancer | NCT03304210 | |
| Carboplatin, Herceptin® | Breast cancer | NCT00093145 | |
| Ceritinib, Cisplatin, Gemcitabine hydrochloride | Advanced malignant solid neoplasm, ALK positive lung cancer, Metastatic pancreatic adenocarcinoma, Stages III and IV of pancreatic cancer | NCT02227940 | |
| Azacitidine (Vidaza) | Advanced or metastatic Breast cancer | NCT00748553 | |
| Etrumadenant, IPI-549, Pegylated liposomal doxorubicin (PLD) | Triple-negative breast cancer, Ovarian cancer | NCT03719326 | |
| Mifepristone | Male breast cancer, Recurrent breast cancer | NCT01493310 | |
| Cetuximab, IMRT (Intensity-modulated radiation therapy) | Head and neck cancer | NCT00736619 | |
| Cetuximab, Cisplatin | Head and neck cancer | NCT00833261 | |
| Leucovorin calcium, Irinotecan hydrochloride, Fluorouracil | Adenocarcinoma, Cholangiocarcinoma, Gallbladder carcinoma, Gastric adenocarcinoma, Malignant gastrointestinal neoplasm, Metastatic pancreatic adenocarcinoma, Pancreatic adenocarcinoma, Stage III Ampulla of vater cancer, Stage III Pancreatic cancer, Stage IIIA Gallbladder cancer, Stage IIIA Gastric cancer, Stage IIIB Gallbladder cancer, Stage IIIB Gastric cancer, Stage IV Ampulla of vater cancer, Stage IV Gallbladder cancer, Stage IV Gastric cancer, Stage IV Pancreatic cancer | NCT02333188 | |
| Imiquimod | Male breast cancer, Recurrent breast cancer, Skin metastases, Stage IV breast cancer | NCT00821964 | |
| Lapatinib | Neoplasms, breast cancer | NCT00650910 | |
| Pembrolizumab, Epirubicin, Cyclophosphamide | Malignant neoplasm of breast | NCT03289819 | |
| Alisertib | Adenocarcinoma, Pancreatic neoplasms | NCT01677559 | |
| Lapatinib | Bladder cancer, Brain and central nervous system tumors, Breast cancer, Esophageal cancer, Extragonadal germ cell tumor, Gastric cancer, Lung cancer, Ovarian cancer, Prostate cancer | NCT00313599 | |
| Doxorubicin, Cyclophosphamide, Carboplatin, Trastuzumab, Bevacizumab | Breast cancer | NCT00254592 | |
| BBI608, Gemcitabine, Oxaliplatin, Leucovorin, Irinotecan, Fluorouracil, MM-398 | Metastatic pancreatic adenocarcinoma | NCT02231723 | |
| Gemcitabine, Capecitabine | Pancreatic neoplasms, Pancreatic cancer, Adenocarcinoma | NCT01161186 | |
| CORT125134 | Solid tumors | NCT02762981 | |
| Pembrolizumab | Metastatic urothelial carcinoma | NCT03464734 | |
| Bevacizumab, Carboplatin, Temozolomide | Melanoma (skin) | NCT00626405 | |
| Docetaxel, Ixabepilone, Paricalcitol | Breast cancer | NCT00637897 | |
| Paclical®, Taxol® | Epithelial ovarian cancer, Primary peritoneal cancer, Fallopian tube cancer | NCT00989131 | |
| NC-6004 (NP-cisplatin) | Gemcitabine | Solid tumors | NCT02240238 |
| CRLX101 (cyclodextrin-based polymer) | Camptothecin | NSCLC, Primary peritoneal cancer | NCT01380769 |
| CPC634 (CriPec®) | Docetaxel | Ovarian cancer | NCT03742713 |
| AGuIX | Polysiloxane gadolinium-chelates based nanoparticles | Brain metastases | NCT02820454 |
| Docetaxel-PNP | Taxotere | Solid tumors | NCT02274610 |
| VYXEOS | Cytarabine, daunorubicin | Acute myeloid leukemia | NCT04920500 |
| ONPATTRO | Patisiran | Transthyretin amyloidosis | NCT03862807 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. https://doi.org/10.3390/ijms23031685
Mundekkad D, Cho WC. Nanoparticles in Clinical Translation for Cancer Therapy. International Journal of Molecular Sciences. 2022; 23(3):1685. https://doi.org/10.3390/ijms23031685
Chicago/Turabian StyleMundekkad, Deepa, and William C. Cho. 2022. "Nanoparticles in Clinical Translation for Cancer Therapy" International Journal of Molecular Sciences 23, no. 3: 1685. https://doi.org/10.3390/ijms23031685
APA StyleMundekkad, D., & Cho, W. C. (2022). Nanoparticles in Clinical Translation for Cancer Therapy. International Journal of Molecular Sciences, 23(3), 1685. https://doi.org/10.3390/ijms23031685







