Relationship between Translational and Rotational Dynamics of Alkyltriethylammonium-Based Ionic Liquids
Abstract
:1. Introduction
2. Theory
3. Materials and Methods
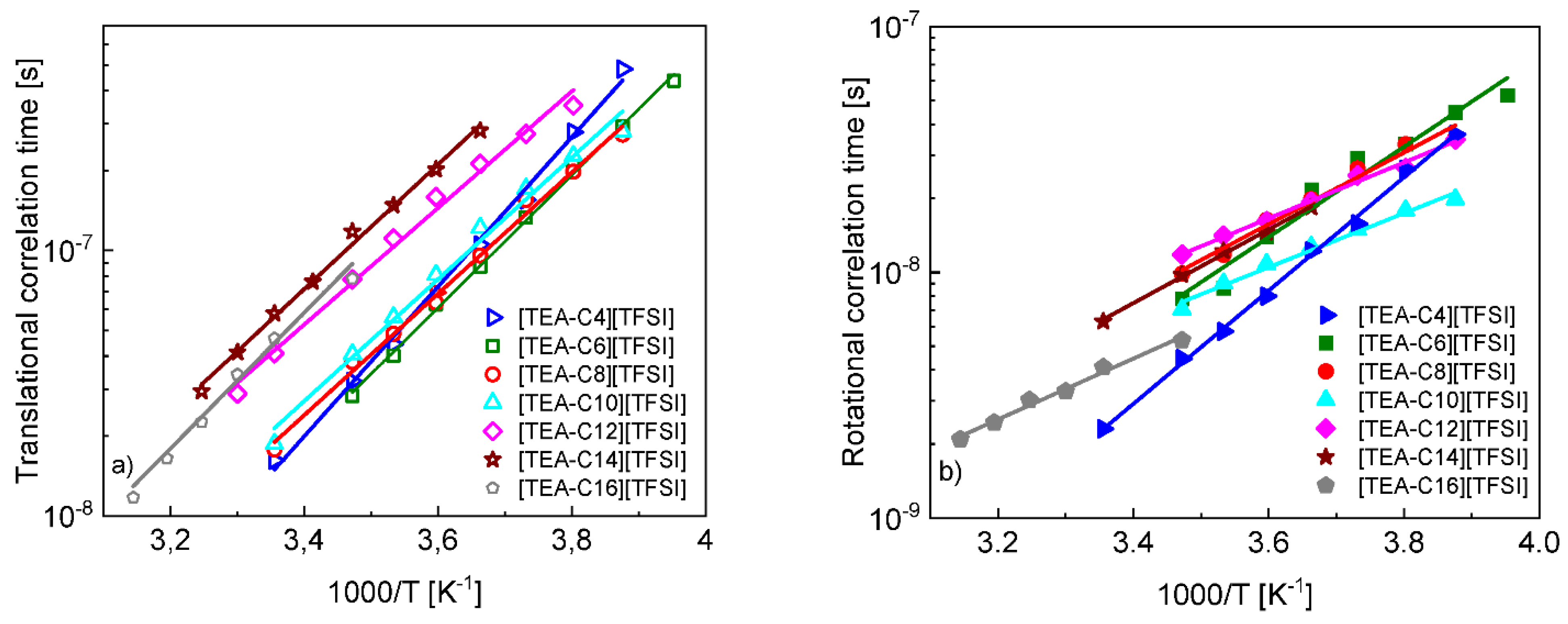
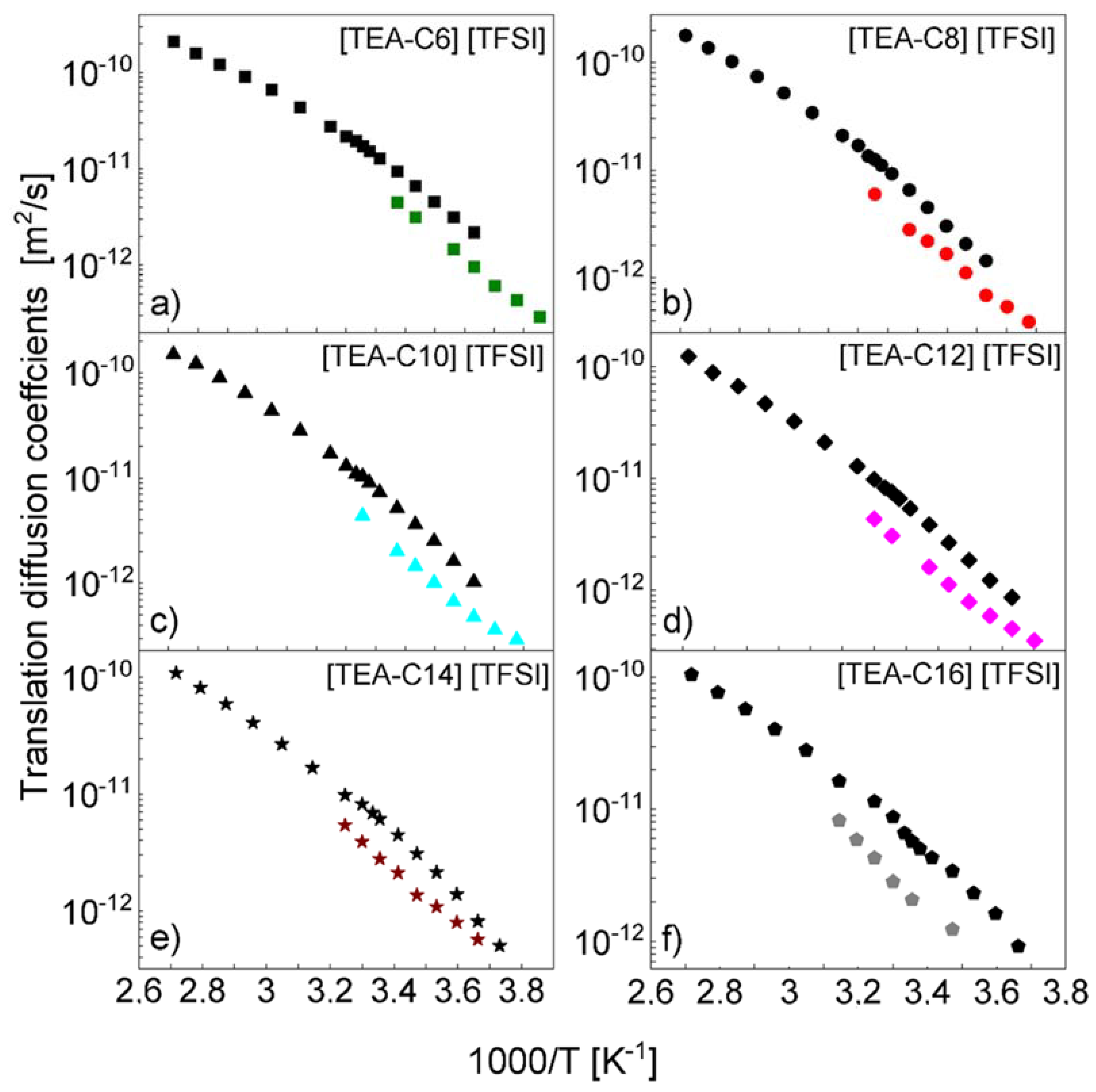
4. Results and Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
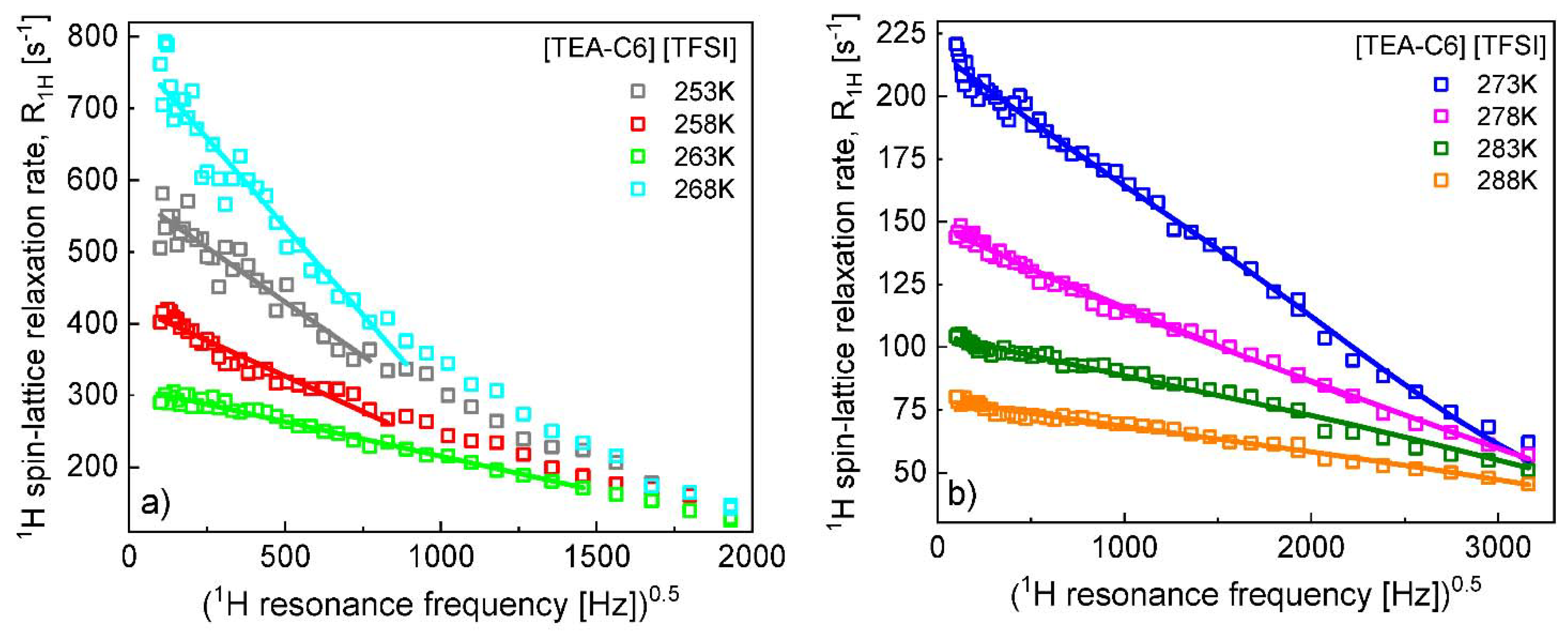

Appendix B
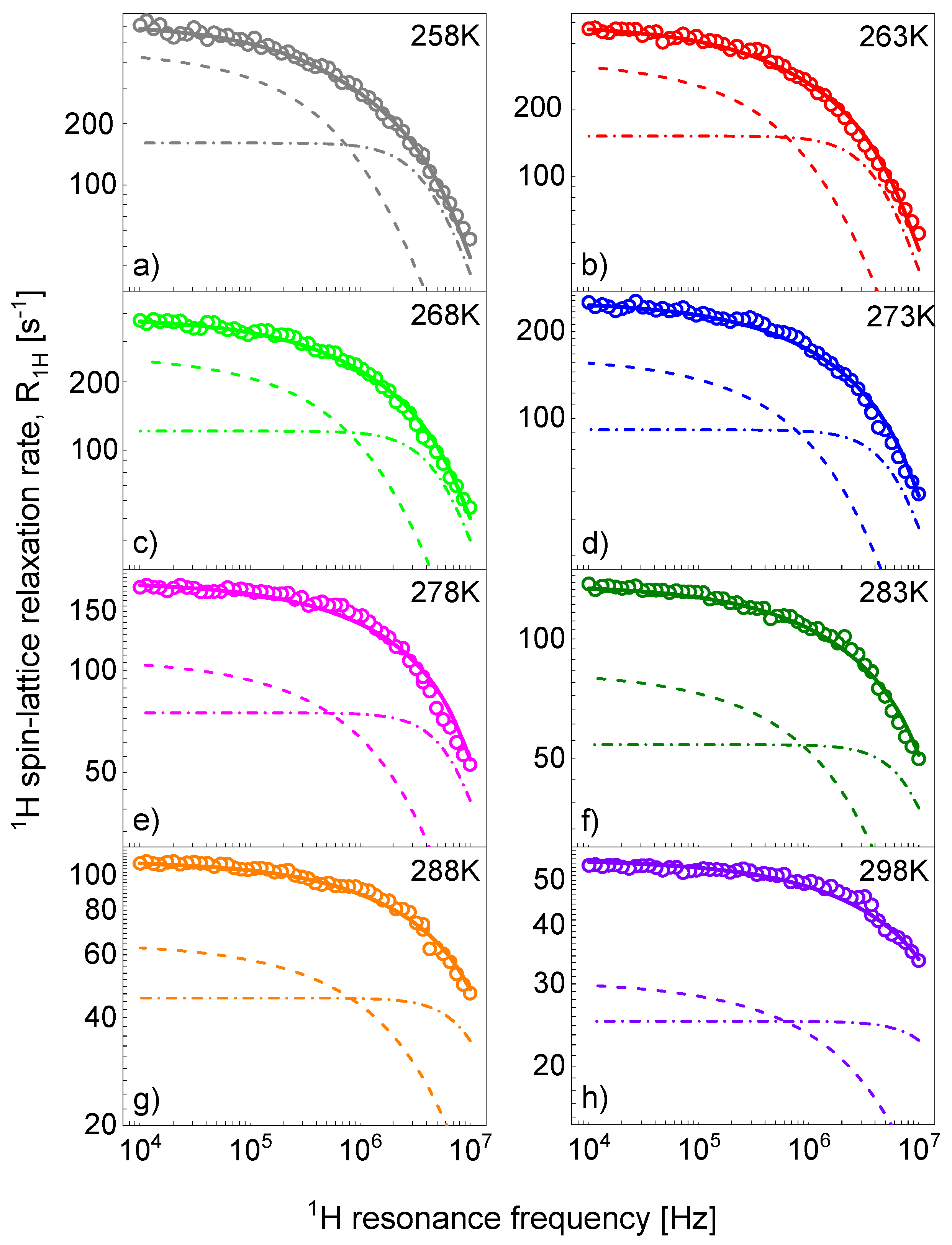
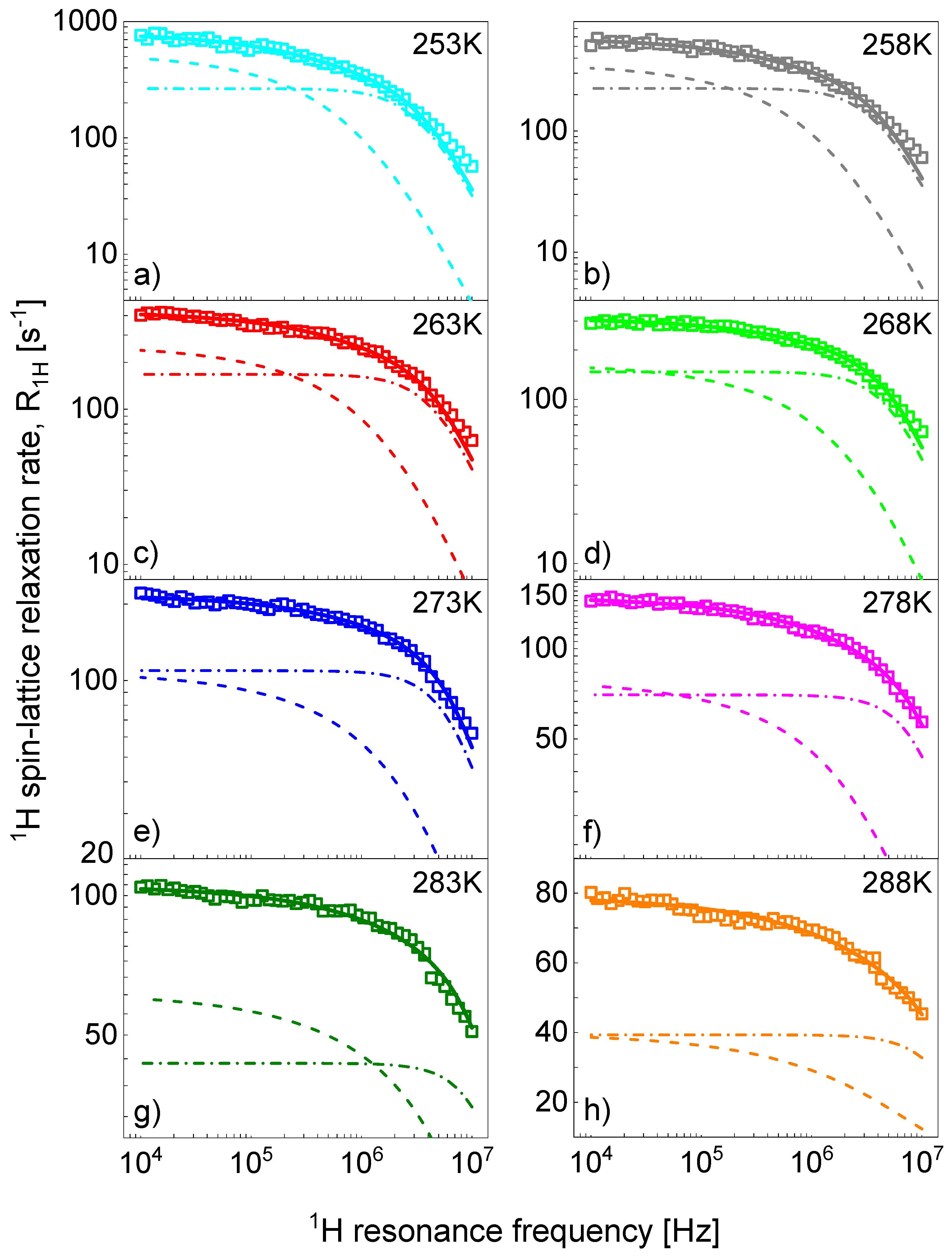
References
- Price, W.S. NMR Diffusometry. In Modern Magnetic Resonance; Springer: Dordrecht, The Netherlands, 2008; pp. 109–115. [Google Scholar]
- Price, W.S. NMR Studies of Translational Motion; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Slichter, C.P. Principles of Magnetic Resonance; Springer Series in Solid-State Sciences; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Kowalewski, J.; Mäler, L. Nuclear Spin Relaxation in Liquids: Theory, Experiments, and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–372. [Google Scholar]
- Kruk, D. Understanding spin dynamics; Pan Stanford Publishing: Singapore, 2015. [Google Scholar]
- Kruk, D. Essentials of the Theory of Spin Relaxation as Needed for Field-cycling NMR. In Field-Cycling NMR Relaxometry: Instrumentation, Model Theories and Applications; Kimmich, R., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 42–66. [Google Scholar]
- Canet, D. Introduction: General Theory of Nuclear Relaxation. Adv. Inorg. Chem. 2005, 57, 3–40. [Google Scholar] [CrossRef]
- Hwang, L.; Freed, J.H. Dynamic Effects of Pair Correlation Functions on Spin Relaxation by Translational Diffusion in Liquids. J. Chem. Phys. 1975, 63, 4017. [Google Scholar] [CrossRef]
- Fries, P.H.; Belorizky, E. Time Correlation Functions of Isotropic Intermolecular Site-Site Interactions in Liquids: Effects of the Site Eccentricity and of the Molecular Distribution. J. Phys. 1989, 50, 3347–3363. [Google Scholar] [CrossRef]
- Levitz, P.; Zinsmeister, M.; Davidson, P.; Constantin, D.; Poncelet, O. Intermittent Brownian Dynamics over a Rigid Strand: Heavily Tailed Relocation Statistics in a Simple Geometry. Phys. Rev. E 2008, 78, 030102. [Google Scholar] [CrossRef]
- Belorizky, E.; Fries, P.H.; Guillermo, A.; Poncelet, O. Almost Ideal 1D Water Diffusion in Imogolite Nanotubes Evidenced by NMR Relaxometry. Chemphyschem 2010, 11, 2021–2026. [Google Scholar] [CrossRef]
- Kimmich, R.; Anoardo, E. Field-Cycling NMR Relaxometry. Prog. Nucl. Magn. Reson. Spectrosc. 2004, 44, 257–320. [Google Scholar] [CrossRef]
- Fujara, F.; Kruk, D.; Privalov, A.F. Solid State Field-Cycling NMR Relaxometry: Instrumental Improvements and New Applications. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 82, 39–69. [Google Scholar] [CrossRef]
- Korb, J. Multiscale Nuclear Magnetic Relaxation Dispersion of Complex Liquids in Bulk and Confinement. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 104, 12–55. [Google Scholar] [CrossRef]
- Kruk, D.; Herrmann, A.; Rössler, E.A. Field-cycling NMR Relaxometry of Viscous Liquids and Polymers. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 63, 33–64. [Google Scholar] [CrossRef]
- Meier, R.; Kruk, D.; Bourdick, A.; Schneider, E.; Rössler, E.A. Inter- and Intramolecular Relaxation in Molecular Liquids by Field Cycling 1H NMR Relaxometry. Appl. Magn. Reson. 2012, 44, 153–168. [Google Scholar] [CrossRef]
- Meier, R.; Kruk, D.; Gmeiner, J.; Rössler, E.A. Intermolecular Relaxation in Glycerol as Revealed by Field Cycling 1H NMR Relaxometry Dilution Experiments. J. Chem. Phys. 2012, 136, 034508. [Google Scholar] [CrossRef] [PubMed]
- Kruk, D.; Meier, R.; Rössler, E.A. Translational and Rotational Diffusion of Glycerol by Means of Field Cycling 1H NMR Relaxometry. J. Phys. Chem. B 2011, 115, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Carignani, E.; Juszyńska-Gałązka, E.; Gałązka, M.; Forte, C.; Geppi, M.; Calucci, L. Translational and Rotational Diffusion of Three Glass Forming Alcohols by 1H Field Cycling NMR Relaxometry. J. Mol. Liq. 2021, 330, 115597. [Google Scholar] [CrossRef]
- Kimmich, R.; Fatkullin, N. Self-diffusion studies by intra- and intermolecular spinlattice relaxometry using field-cycling: Liquids, plastic crystals, porous media, and polymer segments. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 101, 18–50. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, V.; Golub, B.; Schröder, H.; Appelhagen, A.; Paschek, D.; Neymeyr, K.; Ludwig, R. Probing relaxation models by means of Fast Field-Cycling relaxometry, NMR spectroscopy and molecular dynamics simulations: Detailed insight into the translational and rotational dynamics of a protic ionic liquid. J. Mol. Liq. 2020, 319, 114207. [Google Scholar] [CrossRef]
- Kruk, D.; Meier, R.; Rachocki, A.; Korpała, A.; Singh, R.K.; Rössler, E.A. Determining diffusion coefficients of ionic liquids by means of field cycling nuclear magnetic resonance relaxometry. J. Chem. Phys. 2014, 140, 244509. [Google Scholar] [CrossRef] [PubMed]
- Kruk, D.; Wojciechowski, M.; Verma, Y.L.; Chaurasia, S.K.; Singh, R.K. Dynamical properties of EMIM-SCN confined in a SiO2 matrix by means of 1H NMR relaxometry. Phys. Chem. Chem. Phys. 2017, 19, 32605–32616. [Google Scholar] [CrossRef]
- Kruk, D.; Wojciechowski, M.; Brym, S.; Singh, R.K. Dynamics of ionic liquids in bulk and in confinement by means of 1H NMR relaxometry – BMIM-OcSO4 in an SiO2 matrix as an example. Phys. Chem. Chem. Phys. 2016, 18, 23184–23194. [Google Scholar] [CrossRef]
- Ordikhani, A.; Stapf, S.; Mattea, C. Nuclear magnetic relaxation and diffusion study of the ionic liquids 1-ethyl- and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide confined in porous glass. Magn. Reson. Chem. 2019, 57, 818–828. [Google Scholar] [CrossRef]
- Seyedlar, A.O.; Stapf, S.; Mattea, C. Dynamics of the ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethylsulphonyl)imide studied by nuclear magnetic resonance dispersion and diffusion. Phys. Chem. Chem. Phys. 2014, 17, 1653–1659. [Google Scholar] [CrossRef]
- Wencka, M.; Apih, T.; Korošec, R.C.; Jenczyk, J.; Jarek, M.; Szutkowski, K.; Jurga, S.; Dolinšek, J. Molecular dynamics of 1-ethyl-3-methylimidazolium triflate ionic liquid studied by 1 H and 19 F nuclear magnetic resonances. Phys. Chem. Chem. Phys. 2017, 19, 15368–15376. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, N.K.; Fraenza, C.C.; Greenbaum, S.G.; Ashby, D.; Dunn, B.S. NMR Relaxometry and Diffusometry Analysis of Dynamics in Ionic Liquids and Ionogels for Use in Lithium-Ion Batteries. J. Phys. Chem. B. 2020, 124, 6843–6856. [Google Scholar] [CrossRef]
- Pilar, K.; Rua, A.; Suarez, S.N.; Mallia, C.; Lai, S.; Jayakody, J.R.P.; Hatcher, J.L.; wishart, J.F.; Greenbaum, S. Investigation of Dynamics in BMIM TFSA Ionic Liquids through Variable Temperature and Pressure NMR Relaxometry and Diffusometry. J. Electrochem. Soc. 2017, 164, H5189. [Google Scholar] [CrossRef] [Green Version]
- Mladenova, B.Y.; Chumakova, N.A.; Pergushov, V.I.; Kokorin, A.I.; Grampp, G.; Kattnig, D.R. Rotational and Translational Diffusion of Spin Probes in Room-Temperature Ionic Liquids. J. Phys. Chem. B. 2012, 116, 12295–12305. [Google Scholar] [CrossRef] [PubMed]
- Stoesser, R.; Herrmann, W.; Zehl, A.; Laschewsky, A.; Strehmel, V. Microviscosity and Micropolarity Effects of Imidazolium Based Ionic Liquids Investigated by Spin Probes Their Diffusion and Spin Exchange. Z. für. Phys. Chemie 2006. [Google Scholar] [CrossRef]
- Stoesser, R.; Herrmann, W.; Zehl, A.; Strehmel, V.; Laschewsky, A. ESR Spin Probes in Ionic Liquids. ChemPhysChem 2006, 7, 1106–1111. [Google Scholar] [CrossRef]
- Kundu, K.; Kattnig, D.R.; Mladenova, B.Y.; Grampp, G.; Das, R. Electron Spin-Lattice Relaxation Mechanisms of Nitroxyl Radicals in Ionic Liquids and Conventional Organic Liquids: Temperature Dependence of a Thermally Activated Process. J. Phys. Chem. B 2015, 119, 4501–4511. [Google Scholar] [CrossRef]
- Mladenova, B.Y.; Kattnig, D.R.; Grampp, G. Room-Temperature Ionic Liquids Discerned Via Nitroxyl Spin Probe Dynamics. J. Phys. Chem. B 2011, 115, 8183–8198. [Google Scholar] [CrossRef]
- Kruk, D.; Masiewicz, E.; Lotarska, S.; Markiewicz, R.; Jurga, S. Correlated Dynamics in Ionic Liquids by Means of NMR Relaxometry: Butyltriethylammonium bis(Trifluoromethanesulfonyl)imide as an Example. Int. J. Mol. Sci. 2021, 22, 9117. [Google Scholar] [CrossRef]
- Kruk, D.; Meier, R.; Rössler, E.A. Nuclear Magnetic Resonance Relaxometry as a Method of Measuring Translational Diffusion Coefficients in Liquids. Phys. Rev. E 2012, 85, 020201. [Google Scholar] [CrossRef]
- Markiewicz, R.; Klimaszyk, A.; Jarek, M.; Taube, M.; Florczak, P.; Kempka, M.; Fojud, Z.; Jurga, S. Influence of Alkyl Chain Length on Thermal Properties, Structure, and Self-Diffusion Coefficients of Alkyltriethylammonium-Based Ionic Liquids. Int. J. Mol. Sci. 2021, 22, 5935. [Google Scholar] [CrossRef] [PubMed]

| Name; Abbreviation | Chemical Formula | Molecular Mass [g/mol] | Density [g/cm3] | |
|---|---|---|---|---|
| Triethylhexylammonium bis(trifluoromethanesulfonyl)imide; [TEA-C6] [TFSI] | C14H28F6N2O4S2 | 466.50 | 1.29 | 228.18 |
| Triethyloctylammonium bis(trifluoromethanesulfonyl)imide; [TEA-C8] [TFSI] | C16H32F6N2O4S2 | 494.56 | 1.25 | 231.83 |
| Decyltriethylammonium bis(trifluoromethanesulfonyl)imide; [TEA-C10] [TFSI] | C18H36F6N2O4S2 | 522.61 | 1.21 | - |
| Dodecyltriethylammonium bis(trifluoromethanesulfonyl)imide; [TEA-C12] [TFSI] | C20H40F6N2O4S2 | 550.66 | 1.17 | - |
| Triethyltetradecylammonium bis(trifluoromethanesulfonyl)imide; [TEA-C14] [TFSI] | C22H44F6N2O4S2 | 578.72 | 1.16 | 240.03 |
| Hexadecyltriethylammonium bis(trifluoromethanesulfonyl)imide [TEA-C16] [TFSI] | C24H48F6N2O4S2 | 606.77 | 1.13 | 261.69 |
| Temp. [K] | Rel. Error [%] | |||||
|---|---|---|---|---|---|---|
| TEA-C6 | = 9.96 × 108 Hz2, = 3.57 Å | |||||
| 253 | 2.92 × 10−13 | 4.16 × 10−13 | 5.26 × 10−8 | 10.4 | 4.36 × 10−7 | 8.3 |
| 258 | 4.35 × 10−13 | 5.76 × 10−13 | 4.46 × 10−8 | 8.9 | 2.93 × 10−7 | 6.6 |
| 263 | 6.11 × 10−13 | 7.54 × 10−13 | 3.32 × 10−8 | 8.4 | 2.09 × 10−7 | 6.3 |
| 268 | 9.55 × 10−13 | 1.23 × 10−12 | 2.92 × 10−8 | 6.5 | 1.33 × 10−7 | 4.6 |
| 273 | 1.47 × 10−12 | 1.85 × 10−12 | 2.17 × 10−8 | 4.4 | 8.67 × 10−8 | 4.0 |
| 278 | 2.04 × 10−12 | 2.62 × 10−12 | 1.39 × 10−8 | 2.0 | 6.25 × 10−8 | 4.5 |
| 283 | 3.17 × 10−12 | 4.06 × 10−12 | 8.62 × 10−9 | 3.9 | 4.02 × 10−8 | 4.7 |
| 288 | 4.50 × 10−12 | 5.22 × 10−12 | 7.80 × 10−9 | 3.3 | 2.83 × 10−8 | 3.6 |
| TEA-C8 | = 8.79 × 108 Hz2, =3.26 Å | |||||
| 258 | 3.89 × 10−13 | 4.74 × 10−13 | 3.50 × 10−8 | 14.6 | 2.73 × 10−7 | 7.8 |
| 263 | 5.36 × 10−13 | 7.00 × 10−13 | 3.31 × 10−8 | 12.2 | 1.98 × 10−7 | 6.0 |
| 268 | 6.87 × 10−13 | 9.02 × 10−13 | 2.64 × 10−8 | 8.3 | 1.55 × 10−7 | 5.9 |
| 273 | 1.11 × 10−12 | 1.54 × 10−12 | 1.98 × 10−8 | 8.0 | 9.57 × 10−8 | 4.8 |
| 278 | 1.67 × 10−12 | 2.28 × 10−12 | 1.63 × 10−8 | 11.5 | 6.36 × 10−8 | 3.9 |
| 283 | 2.19 × 10−12 | 2.76 × 10−12 | 1.18 × 10−8 | 3.7 | 4.85 × 10−8 | 4.1 |
| 288 | 2.80 × 10−12 | 3.50 × 10−12 | 9.87 × 10−9 | 3.6 | 3.80 × 10−8 | 3.9 |
| 298 | 5.97 × 10−12 | 7.71 × 10−12 | 5.42 × 10−9 | 4.7 | 1.78 × 10−8 | 3.3 |
| TEA-C10 | = 7.77 × 108 Hz2, =2.86 Å | |||||
| 258 | 2.91 × 10−13 | 3.20 × 10−13 | 1.98 × 10−8 | 16.2 | 2.81 × 10−7 | 14.2 |
| 263 | 3.61 × 10−13 | 4.70 × 10−13 | 1.79 × 10−8 | 13.9 | 2.27 × 10−7 | 12.7 |
| 268 | 4.78 × 10−13 | 6.34 × 10−13 | 1.49 × 10−8 | 6.2 | 1.71 × 10−7 | 11.5 |
| 273 | 6.70 × 10−13 | 9.20 × 10−13 | 1.27 × 10−8 | 5.3 | 1.22 × 10−7 | 9.6 |
| 278 | 1.01 × 10−12 | 1.44 × 10−12 | 1.08 × 10−8 | 5.6 | 8.10 × 10−8 | 7.5 |
| 283 | 1.46 × 10−12 | 2.00 × 10−12 | 9.02 × 10−9 | 8.3 | 5.60 × 10−8 | 6.2 |
| 288 | 2.01 × 10−12 | 2.69 × 10−12 | 7.06 × 10−9 | 7.7 | 4.07 × 10−8 | 5.8 |
| 298 | 4.37 × 10−12 | 5.49 × 10−12 | 4.76 × 10−9 | 7.9 | 1.87 × 10−8 | 3.9 |
| TEA-C12 | = 7.65 × 108 Hz2, =3.54 Å | |||||
| 263 | 3.56 × 10−13 | 5.34 × 10−13 | 3.14× 10−8 | 13.3 | 3.52× 10−7 | 11.2 |
| 268 | 4.55 × 10−13 | 6.58 × 10−13 | 2.48× 10−8 | 9.2 | 2.75× 10−7 | 11.1 |
| 273 | 5.91 × 10−13 | 8.10 × 10−13 | 1.94× 10−8 | 5.3 | 2.12× 10−7 | 10.9 |
| 278 | 7.87 × 10−13 | 1.13 × 10−12 | 1.61× 10−8 | 10.1 | 1.59× 10−7 | 9.9 |
| 283 | 1.13 × 10−12 | 1.62 × 10−12 | 1.41× 10−8 | 16.5 | 1.11× 10−7 | 7.8 |
| 288 | 1.61 × 10−12 | 2.36× 10−12 | 1.18× 10−8 | 11.4 | 7.78× 10−8 | 6.6 |
| 298 | 3.06 × 10−12 | 3.73× 10−12 | 6.51× 10−9 | 14.2 | 4.10× 10−8 | 6.3 |
| 303 | 4.34 × 10−12 | 4.33× 10−12 | 5.38× 10−9 | 15.7 | 2.89× 10−8 | 5.4 |
| TEA-C14 | = 7.50 × 108 Hz2, =4.02 Å | |||||
| 273 | 5.72 × 10−13 | 8.13 × 10−13 | 1.84 × 10−8 | 11.6 | 2.83 × 10−7 | 15.4 |
| 278 | 8.01 × 10−13 | 1.16 × 10−12 | 1.45 × 10−8 | 15.1 | 2.02 × 10−7 | 13.9 |
| 283 | 1.09 × 10−12 | 1.66 × 10−12 | 1.22 × 10−8 | 14.7 | 1.48 × 10−7 | 12.2 |
| 288 | 1.37 × 10−12 | 2.00 × 10−12 | 9.96 × 10−9 | 10.8 | 1.18 × 10−7 | 11.8 |
| 293 | 2.12 × 10−12 | 3.21 × 10−12 | 8.32 × 10−9 | 18.9 | 7.62 × 10−8 | 9.2 |
| 298 | 2.79 × 10−12 | 3.96 × 10−12 | 6.41 × 10−9 | 18.1 | 5.79 × 10−8 | 9.0 |
| 303 | 3.90 × 10−12 | 5.37 × 10−12 | 5.76 × 10−9 | 14.6 | 4.14 × 10−8 | 7.2 |
| 308 | 5.43 × 10−12 | 7.42 × 10−12 | 4.72 × 10−9 | 13.4 | 2.95 × 10−8 | 5.4 |
| TEA-C16 | = 6.50 × 108 Hz2, = 3.17Å | |||||
| 288 | 1.23 × 10−12 | 1.57 × 10−12 | 5.29 × 10−9 | 2.6 | 8.17 × 10−8 | 15.4 |
| 298 | 2.07 × 10−12 | 3.31 × 10−12 | 4.11 × 10−9 | 8.2 | 4.85 × 10−8 | 11.8 |
| 303 | 2.83 × 10−12 | 5.11 × 10−12 | 3.28 × 10−9 | 12.7 | 3.55 × 10−8 | 10.8 |
| 308 | 4.27 × 10−12 | 7.02 × 10−12 | 3.02 × 10−9 | 11.1 | 2.35 × 10−8 | 7.8 |
| 313 | 5.86 × 10−12 | 8.57 × 10−12 | 2.45 × 10−9 | 11.0 | 1.71 × 10−8 | 7.0 |
| 318 | 8.21 × 10−12 | 1.36 × 10−11 | 2.09 × 10−9 | 9.6 | 1.22 × 10−8 | 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk, D.; Masiewicz, E.; Lotarska, S.; Markiewicz, R.; Jurga, S. Relationship between Translational and Rotational Dynamics of Alkyltriethylammonium-Based Ionic Liquids. Int. J. Mol. Sci. 2022, 23, 1688. https://doi.org/10.3390/ijms23031688
Kruk D, Masiewicz E, Lotarska S, Markiewicz R, Jurga S. Relationship between Translational and Rotational Dynamics of Alkyltriethylammonium-Based Ionic Liquids. International Journal of Molecular Sciences. 2022; 23(3):1688. https://doi.org/10.3390/ijms23031688
Chicago/Turabian StyleKruk, Danuta, Elzbieta Masiewicz, Sylwia Lotarska, Roksana Markiewicz, and Stefan Jurga. 2022. "Relationship between Translational and Rotational Dynamics of Alkyltriethylammonium-Based Ionic Liquids" International Journal of Molecular Sciences 23, no. 3: 1688. https://doi.org/10.3390/ijms23031688






