Calebin A, a Compound of Turmeric, Down-Regulates Inflammation in Tenocytes by NF-κB/Scleraxis Signaling
Abstract
:1. Introduction
2. Results
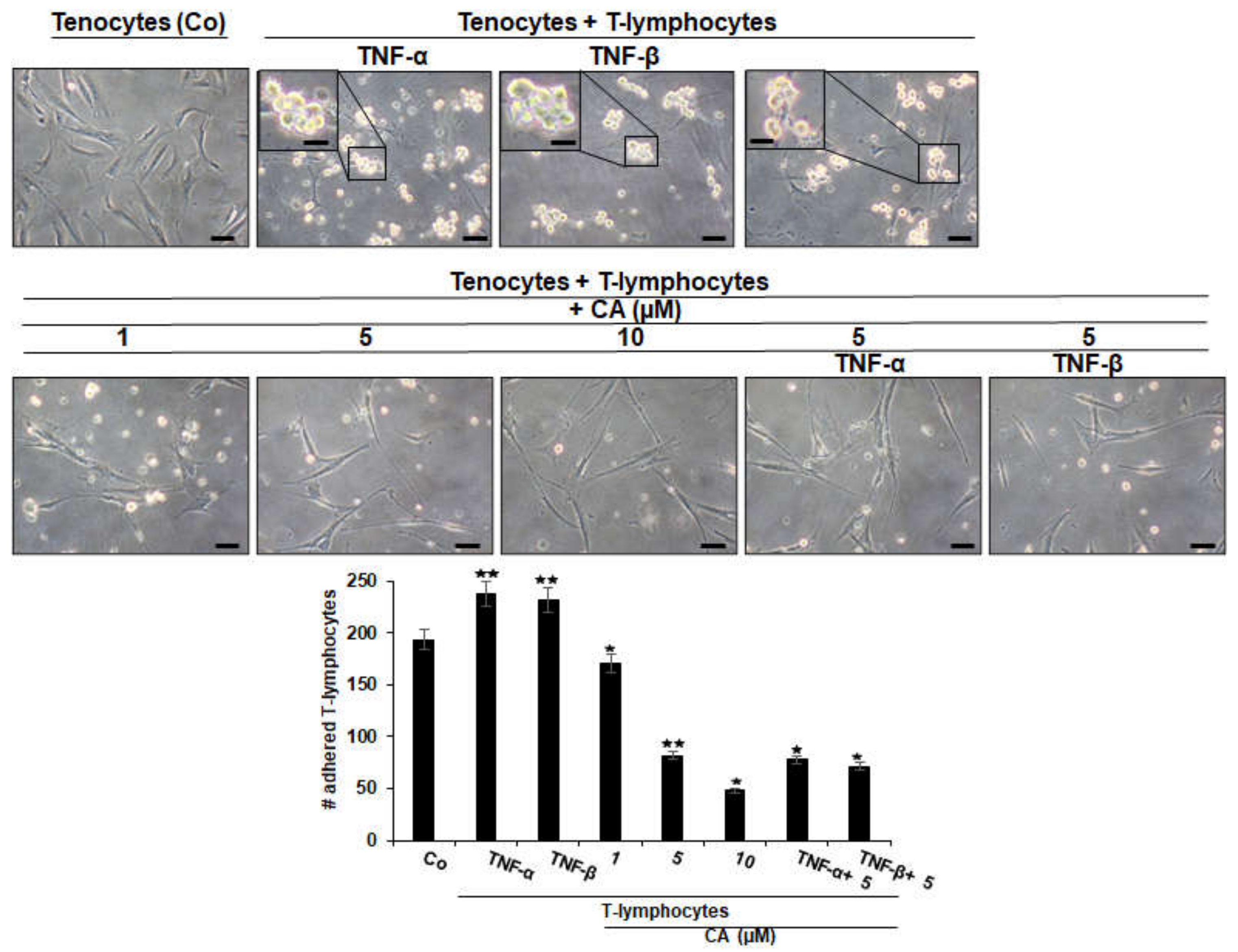
2.1. Calebin A Suppresses TNF-β- Similar to TNF-α-Mediated Adhesiveness of T-Lymphocytes to Tenocytes
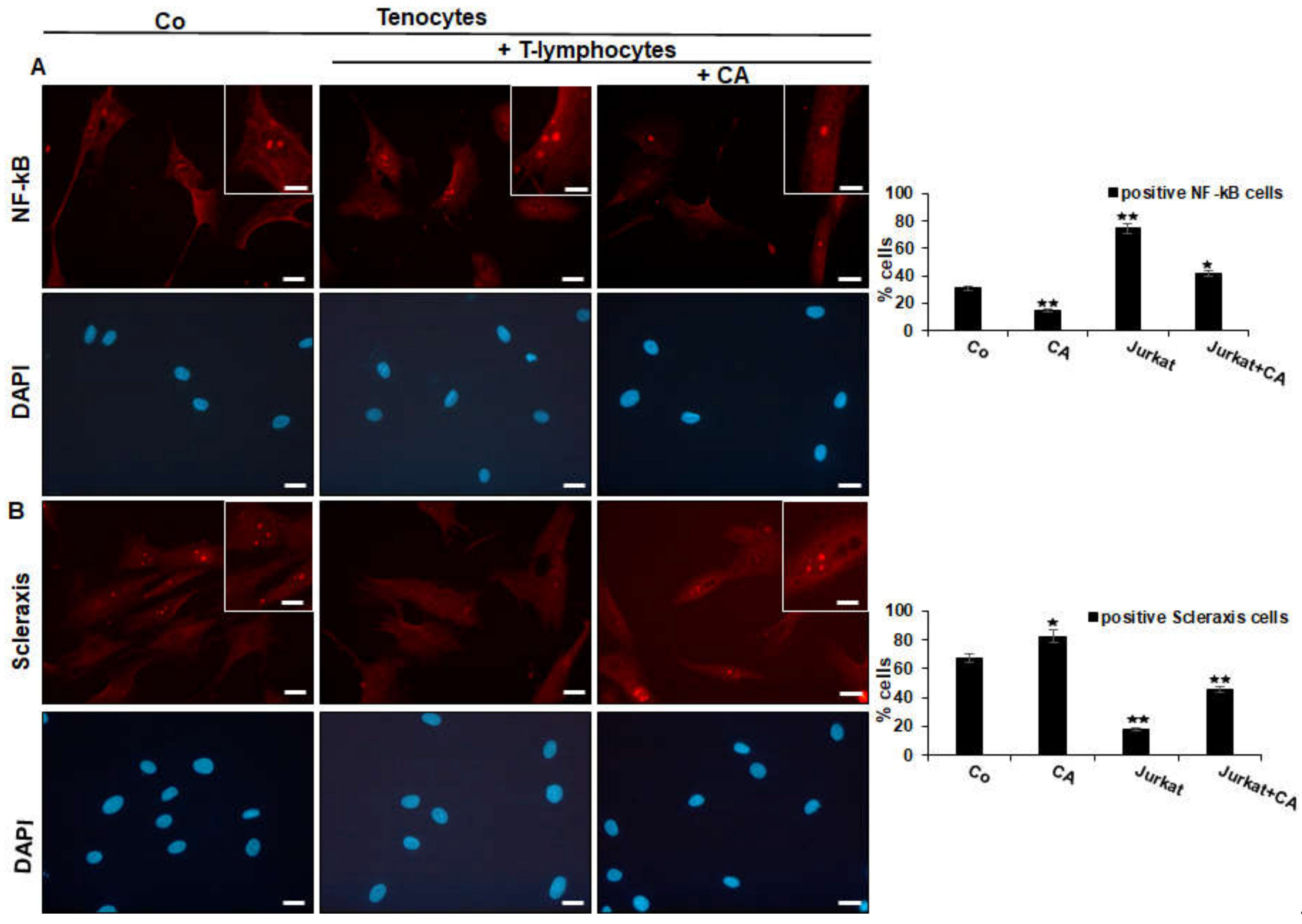
2.2. Calebin A Suppresses T-Lymphocyte-Induced NF-κB Phosphorylation/Nuclear Translocation and Down-Regulation of Scleraxis
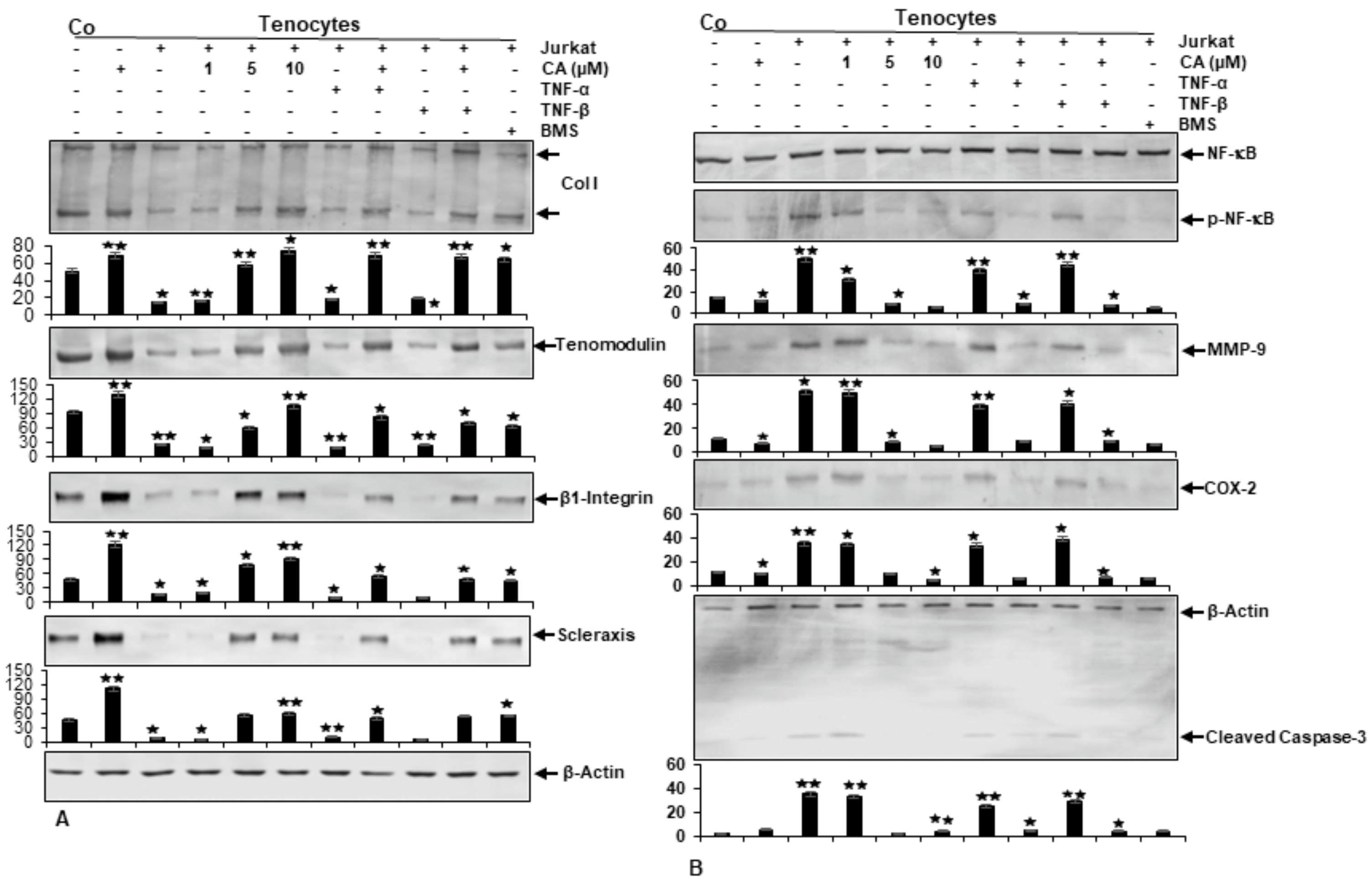
2.3. Calebin A Suppresses T-Lymphocyte-, TNF-β-, or TNF-α-Promoted ECM Degradation and Signaling Gene Expression, Similar to IKK Inhibitor (BMS-345541) in Tenocytes
2.4. Calebin A Suppresses T-Lymphocyte-, TNF-β-, or TNF-α-Promoted NF-κB Activation and NF-κB-Regulated Pro-Inflammatory and Matrix-Degrading Gene Products, Similar to IKK Inhibitor (BMS-345541), in Monolayer Tenocyte Cultures
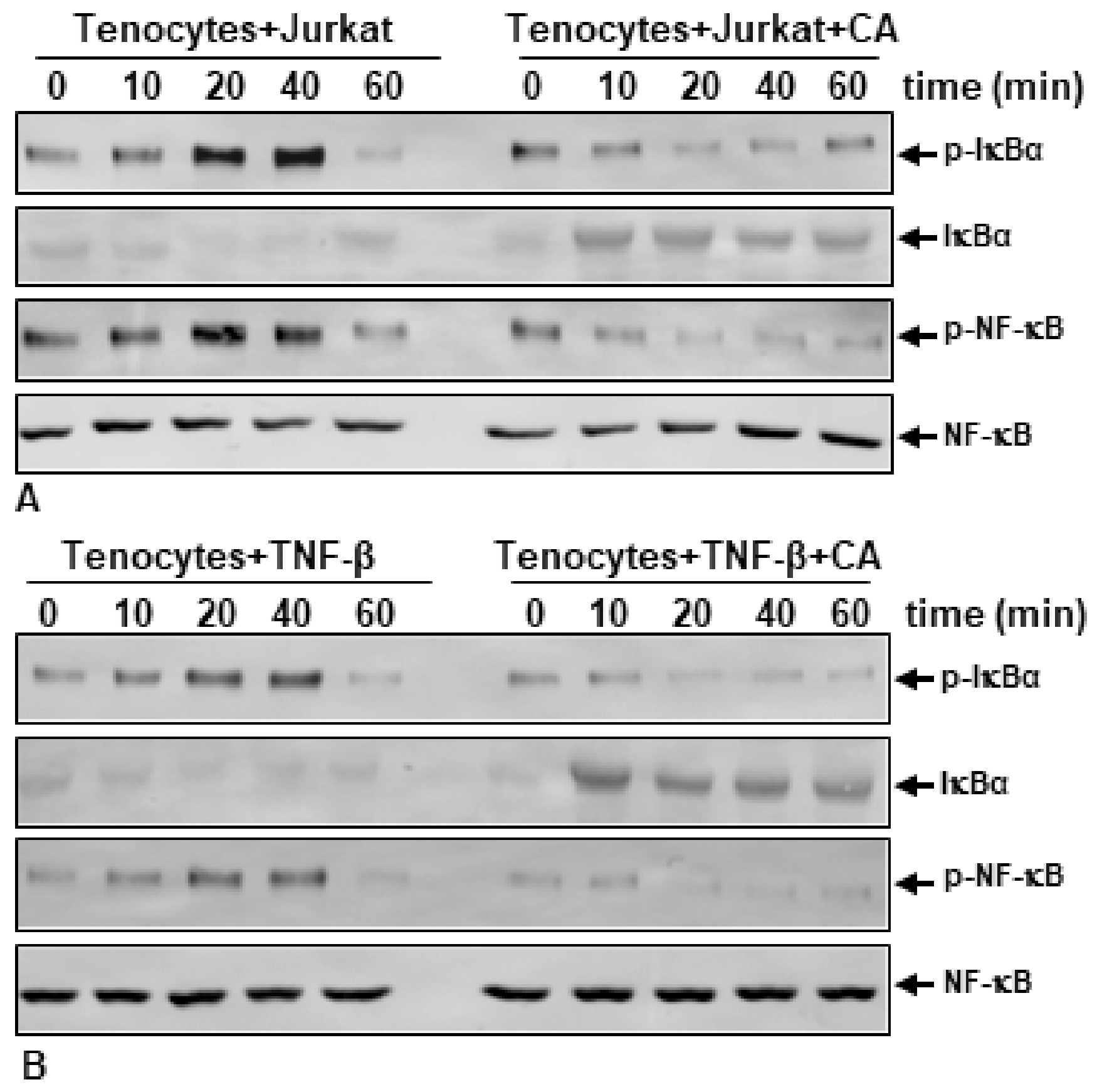
2.5. Calebin A Suppresses T-Lymphocyte- or TNF-β-Promoted Phosphorylation and Degradation of IκB-α and Phosphorylation of NF-κB in a Time-Dependent Manner in Monolayer Tenocyte Cultures
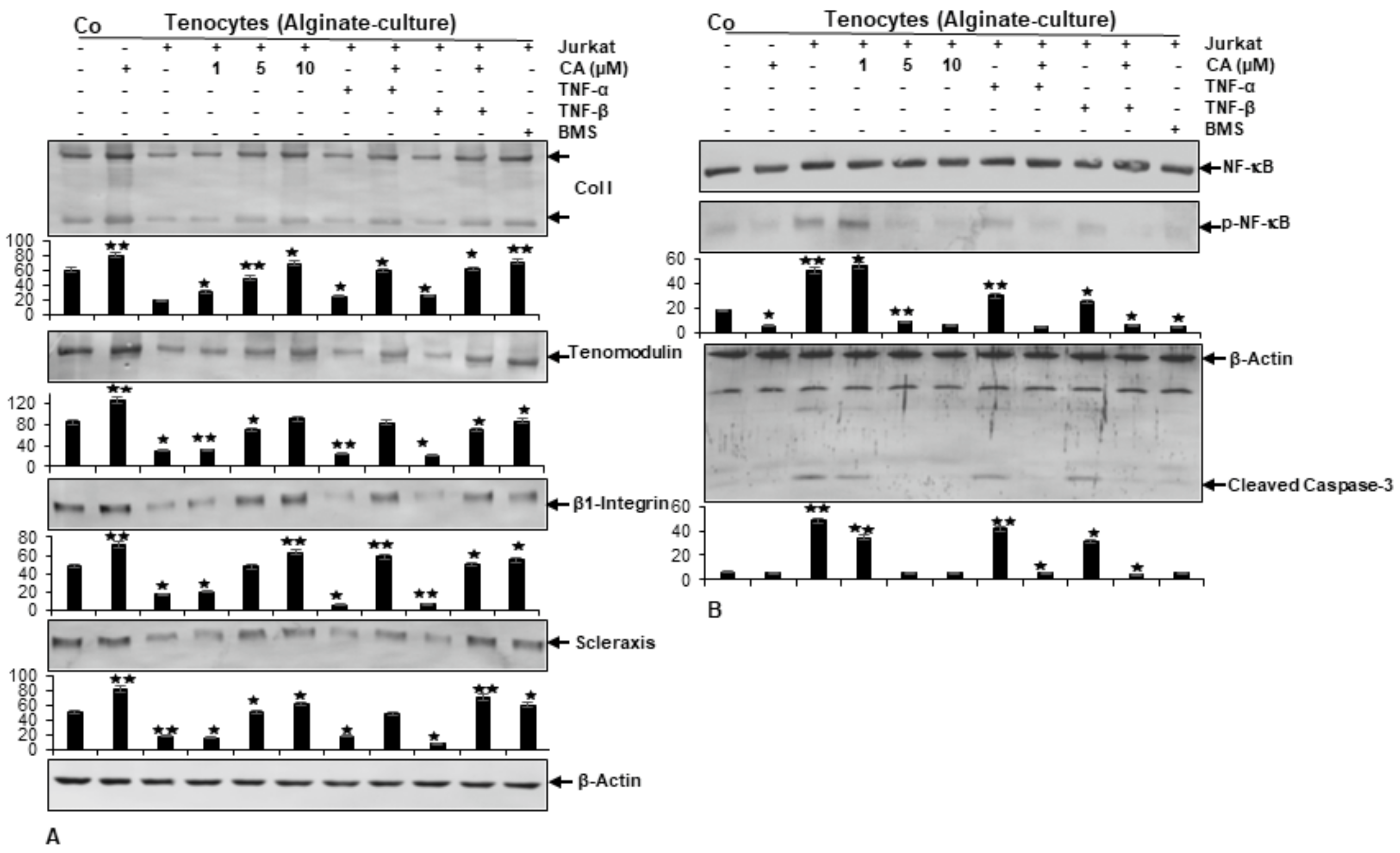
2.6. Calebin A Prevents Tendinitis Microenvironment-Triggered Degradation of Extracellular Matrix, β1-Integrin, and Scleraxis Similarly to a Targeted IKK Inhibitor (BMS-345541) in Alginate-Cultured Tenocytes
2.7. Calebin A Suppresses Tendinitis Microenvironment-Promoted Elevation of NF-κB, NF-κB-Regulated Matrix-Degrading and Apoptotic Proteins Similarly to a Targeted IKK Inhibitor (BMS-345541) in Alginate-Cultured Tenocytes
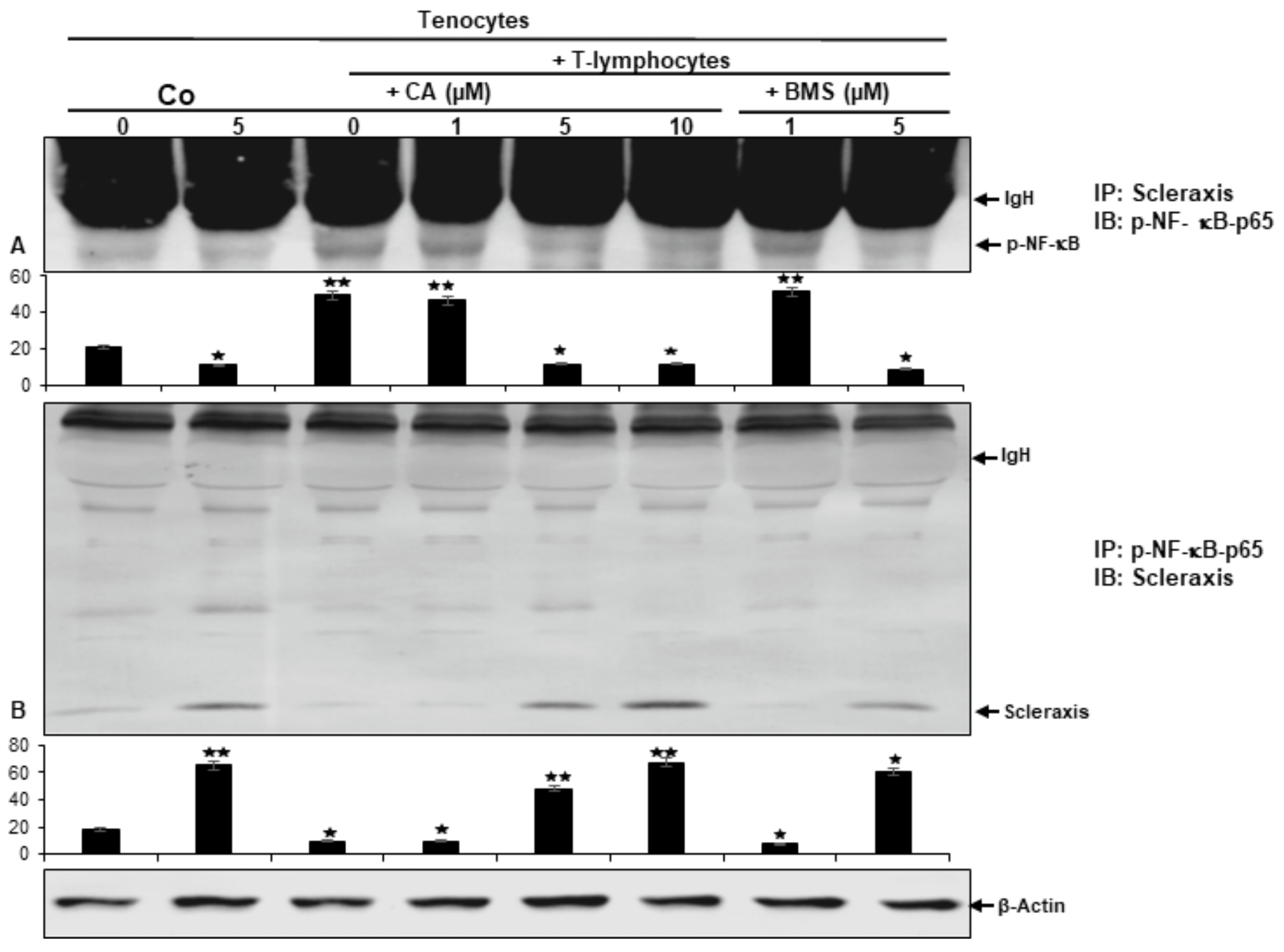
2.8. Calebin A Suppresses T-Lymphocyte-Promoted p-NF-κB-p65 Association with Scleraxis, Comparable to IKK Inhibitor (BMS-345541) in a Multicellular Tendinitis Microenvironment
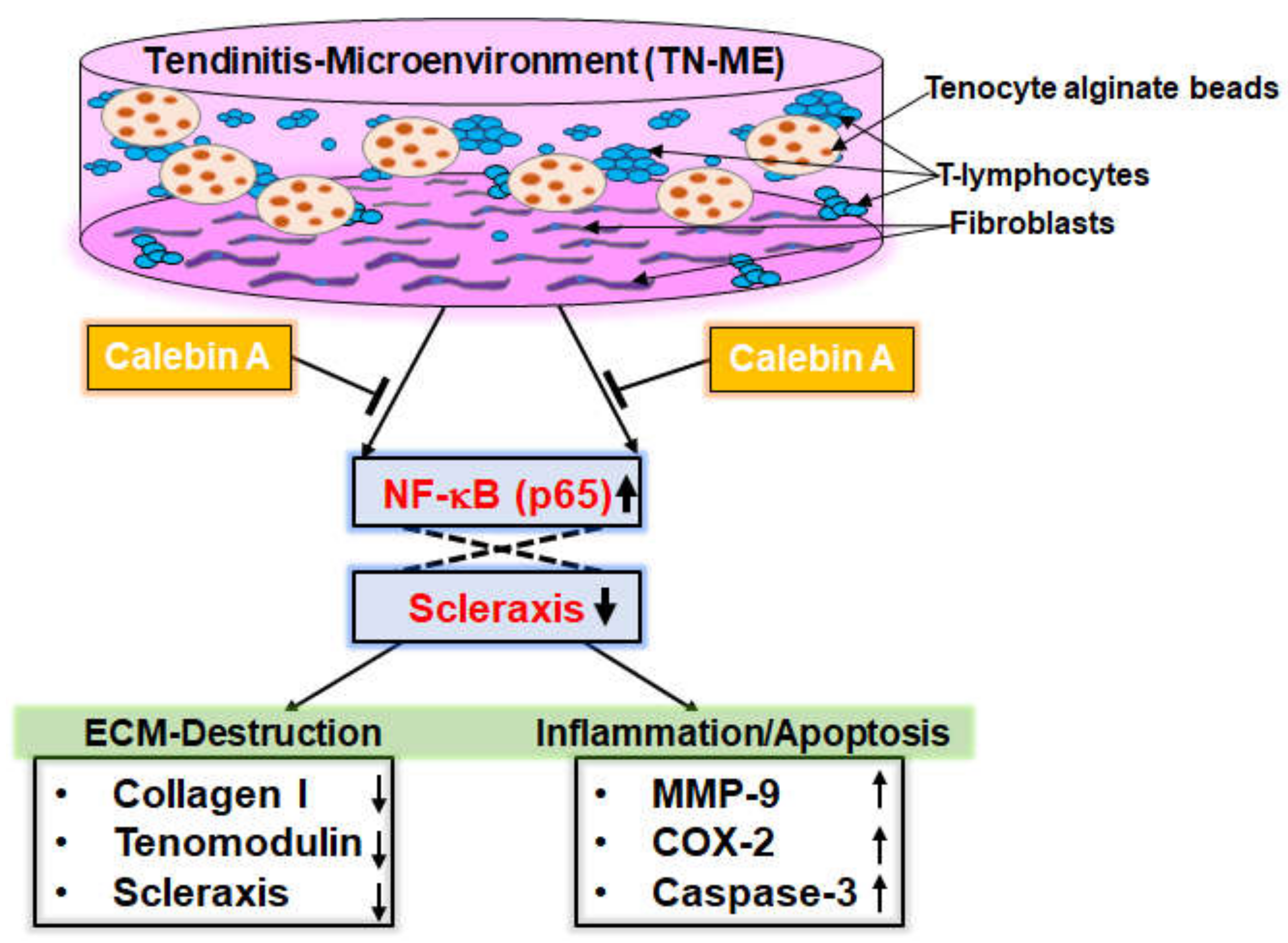
3. Discussion
4. Materials and Methods
4.1. Antibodies, Cytokines and Reagents
4.2. Cell Lines and Cell Culture Conditions
4.3. Experimental Set-Up
4.4. Adhesion Assay of Tenocytes with T-Lymphocytes
4.5. Western Blot Analysis
4.6. Immunofluorescence Analysis
4.7. Immunoprecipitation
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephenson, A.L.; Wu, W.; Cortes, D.; Rochon, P.A. Tendon Injury and Fluoroquinolone Use: A Systematic Review. Drug Saf. 2013, 36, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Sendzik, J.; Shakibaei, M.; Schäfer-Korting, M.; Lode, H.; Stahlmann, R. Synergistic effects of dexamethasone and quinolones on human-derived tendon cells. Int. J. Antimicrob. Agents 2010, 35, 366–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andarawis-Puri, N.; Flatow, E.L.; Soslowsky, L.J. Tendon basic science: Development, repair, regeneration, and healing. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2015, 33, 780–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childress, M.A.; Beutler, A. Management of chronic tendon injuries. Am. Fam. Physician 2013, 87, 486–490. [Google Scholar] [PubMed]
- D’Addona, A.; Maffulli, N.; Formisano, S.; Rosa, D. Inflammation in tendinopathy. Surg. J. R. Coll. Surg. Edinb. Irel. 2017, 15, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Beaubois, K.; Hecquet, C.; Houcine, O.; Hayem, G.; Adolphe, M. Culture and characterization of juvenile rabbit tenocytes. Cell Biol. Toxicol. 1997, 13, 103–113. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Midiri, F.; Mauri, G.; Aliprandi, A.; Catapano, M.; Pescatori, L.C.; Monaco, C.G.; Gitto, S.; et al. Rotator cuff calcific tendinopathy: From diagnosis to treatment. Acta Bio-Med. Atenei Parm. 2018, 89, 186–196. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Süleyman, H.; Demircan, B.; Karagöz, Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. PR 2007, 59, 247–258. [Google Scholar]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Riley, G.P.; Cox, M.; Harrall, R.L.; Clements, S.; Hazleman, B.L. Inhibition of tendon cell proliferation and matrix glycosaminoglycan synthesis by non-steroidal anti-inflammatory drugs in vitro. J. Hand Surg. 2001, 26, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Mikolyzk, D.K.; Wei, A.S.; Tonino, P.; Marra, G.; Williams, D.A.; Himes, R.D.; Wezeman, F.H.; Callaci, J.J. Effect of corticosteroids on the biomechanical strength of rat rotator cuff tendon. J. Bone Jt. Surgery. Am. Vol. 2009, 91, 1172–1180. [Google Scholar] [CrossRef]
- Tillander, B.; Franzén, L.E.; Karlsson, M.H.; Norlin, R. Effect of steroid injections on the rotator cuff: An experimental study in rats. J. Shoulder Elb. Surg. 1999, 8, 271–274. [Google Scholar] [CrossRef]
- Akpinar, S.; Hersekli, M.A.; Demirors, H.; Tandogan, R.N.; Kayaselcuk, F. Effects of methylprednisolone and betamethasone injections on the rotator cuff: An experimental study in rats. Adv. Ther. 2002, 19, 194–201. [Google Scholar] [CrossRef]
- Rolf, C.; Movin, T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int. 1997, 18, 565–569. [Google Scholar] [CrossRef]
- Dakin, S.G.; Newton, J.; Martinez, F.O.; Hedley, R.; Gwilym, S.; Jones, N.; Reid, H.A.B.; Wood, S.; Wells, G.; Appleton, L.; et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br. J. Sports Med. 2018, 52, 359–367. [Google Scholar] [CrossRef]
- Florit, D.; Pedret, C.; Casals, M.; Malliaras, P.; Sugimoto, D.; Rodas, G. Incidence of Tendinopathy in Team Sports in a Multidisciplinary Sports Club Over 8 Seasons. J. Sports Sci. Med. 2019, 18, 780–788. [Google Scholar]
- Minghelli, B.; Cadete, J. Epidemiology of musculoskeletal injuries in tennis players: Risk factors. J. Sports Med. Phys. Fit. 2019, 59, 2045–2052. [Google Scholar] [CrossRef]
- Hayes, D.W., Jr.; Gilbertson, E.K.; Mandracchia, V.J.; Dolphin, T.F. Tendon pathology in the foot. The use of corticosteroid injection therapy. Clin. Podiatr. Med. Surg. 2000, 17, 723–735. [Google Scholar]
- Sendzik, J.; Lode, H.; Stahlmann, R. Quinolone-induced arthropathy: An update focusing on new mechanistic and clinical data. Int. J. Antimicrob. Agents 2009, 33, 194–200. [Google Scholar] [CrossRef]
- Van der Linden, P.D.; Sturkenboom, M.C.; Herings, R.M.; Leufkens, H.G.; Stricker, B.H. Fluoroquinolones and risk of Achilles tendon disorders: Case-control study. BMJ Clin. Res. Ed. 2002, 324, 1306–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Ramcharan, M.; Zhou, Z.; Leong, D.J.; Akinbiyi, T.; Majeska, R.J.; Sun, H.B. The Role of Scleraxis in Fate Determination of Mesenchymal Stem Cells for Tenocyte Differentiation. Sci. Rep. 2015, 5, 13149. [Google Scholar] [CrossRef] [Green Version]
- Best, K.T.; Loiselle, A.E. Scleraxis lineage cells contribute to organized bridging tissue during tendon healing and identify a subpopulation of resident tendon cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 8578–8587. [Google Scholar] [CrossRef]
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: Role of the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 2011, 286, 28556–28566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.H.; Guo, Q.; Li, B. Tendon biomechanics and mechanobiology—A minireview of basic concepts and recent advancements. J. Hand Ther. Off. J. Am. Soc. Hand Ther. 2012, 25, 133–140; quiz 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1032. [Google Scholar] [CrossRef]
- Loeser, R.F. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 39, 11–16. [Google Scholar] [CrossRef]
- Moser, M.; Legate, K.R.; Zent, R.; Fässler, R. The tail of integrins, talin, and kindlins. Science 2009, 324, 895–899. [Google Scholar] [CrossRef]
- Schwartz, M.A.; Ginsberg, M.H. Networks and crosstalk: Integrin signalling spreads. Nat. Cell Biol. 2002, 4, E65–E68. [Google Scholar] [CrossRef]
- Shakibaei, M.; Csaki, C.; Mobasheri, A. Diverse roles of integrin receptors in articular cartilage. Adv. Anat. Embryol. Cell Biol. 2008, 197, 1–60. [Google Scholar] [CrossRef]
- Mobasheri, A.; Carter, S.D.; Martín-Vasallo, P.; Shakibaei, M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol. Int. 2002, 26, 1–18. [Google Scholar] [CrossRef]
- Busch, F.; Mobasheri, A.; Shayan, P.; Lueders, C.; Stahlmann, R.; Shakibaei, M. Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes. J. Biol. Chem. 2012, 287, 38050–38063. [Google Scholar] [CrossRef] [Green Version]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.P.; Carmody, R.J. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.S.; Aggarwal, B.B. Transcription factor NF-kappaB: A sensor for smoke and stress signals. Ann. N. Y. Acad. Sci. 2005, 1056, 218–233. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Takada, Y. Pro-apototic and anti-apoptotic effects of tumor necrosis factor in tumor cells. Role of nuclear transcription factor NF-kappaB. Cancer Treat. Res. 2005, 126, 103–127. [Google Scholar] [CrossRef]
- Das, S.; Das, D.K. Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targets 2007, 6, 168–173. [Google Scholar] [CrossRef]
- Shakibaei, M.; Csaki, C.; Nebrich, S.; Mobasheri, A. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 2008, 76, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Güleç, A.; Türk, Y.; Aydin, B.K.; Erkoçak, Ö.F.; Safalı, S.; Ugurluoglu, C. Effect of curcumin on tendon healing: An experimental study in a rat model of Achilles tendon injury. Int. Orthop. 2018, 42, 1905–1910. [Google Scholar] [CrossRef]
- Huang, X.M.; Yang, Z.J.; Xie, Q.; Zhang, Z.K.; Zhang, H.; Ma, J.Y. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharmacother. 2019, 117, 109142. [Google Scholar] [CrossRef]
- Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-Curcuminoids from Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulations. Biomolecules 2019, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Kim, D.S. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimer’s disease. J. Nat. Prod. 2002, 65, 1227–1231. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Prasad, S.; Majeed, M.; Aggarwal, B.B. Calebin A downregulates osteoclastogenesis through suppression of RANKL signalling. Arch. Biochem. Biophys. 2016, 593, 80–89. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Buhrmann, C.; Popper, B.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Evidence That Calebin A, a Component of Curcuma Longa Suppresses NF-B Mediated Proliferation, Invasion and Metastasis of Human Colorectal Cancer Induced by TNF-β (Lymphotoxin). Nutrients 2019, 11, 2904. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, A.K.; Prasad, S.; Majeed, M.; Aggarwal, B.B. Calebin A, a novel component of turmeric, suppresses NF-κB regulated cell survival and inflammatory gene products leading to inhibition of cell growth and chemosensitization. Phytomedicine Int. J. Phytother. Phytopharm. 2017, 34, 171–181. [Google Scholar] [CrossRef]
- Buhrmann, C.; Shayan, P.; Banik, K.; Kunnumakkara, A.B.; Kubatka, P.; Koklesova, L.; Shakibaei, M. Targeting NF-κB Signaling by Calebin A, a Compound of Turmeric, in Multicellular Tumor Microenvironment: Potential Role of Apoptosis Induction in CRC Cells. Biomedicines 2020, 8, 236. [Google Scholar] [CrossRef]
- Chimen, M.; Apta, B.H.; McGettrick, H.M. Introduction: T Cell Trafficking in Inflammation and Immunity. Methods Mol. Biol. 2017, 1591, 73–84. [Google Scholar] [CrossRef]
- Burke, J.R.; Pattoli, M.A.; Gregor, K.R.; Brassil, P.J.; MacMaster, J.F.; McIntyre, K.W.; Yang, X.; Iotzova, V.S.; Clarke, W.; Strnad, J.; et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J. Biol. Chem. 2003, 278, 1450–1456. [Google Scholar] [CrossRef] [Green Version]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Reviews. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [Green Version]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Reviews. Dis. Primers 2021, 7, 1. [Google Scholar] [CrossRef]
- Favata, M.; Beredjiklian, P.K.; Zgonis, M.H.; Beason, D.P.; Crombleholme, T.M.; Jawad, A.F.; Soslowsky, L.J. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2006, 24, 2124–2132. [Google Scholar] [CrossRef]
- Titan, A.L.; Foster, D.S.; Chang, J.; Longaker, M.T. Flexor Tendon: Development, Healing, Adhesion Formation, and Contributing Growth Factors. Plast. Reconstr. Surg. 2019, 144, 639e–647e. [Google Scholar] [CrossRef]
- Lin, T.W.; Cardenas, L.; Soslowsky, L.J. Biomechanics of tendon injury and repair. J. Biomech. 2004, 37, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-β (lymphotoxin α) can activate the inflammatory environment in human chondrocytes. Arthritis Res. Ther. 2013, 15, R202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, A.C.; Shah, S.A.; Golman, M.; Song, L.; Li, X.; Kurtaliaj, I.; Akbar, M.; Millar, N.L.; Abu-Amer, Y.; Galatz, L.M.; et al. Targeting the NF-κB signaling pathway in chronic tendon disease. Sci. Transl. Med. 2019, 11, eaav4319. [Google Scholar] [CrossRef] [PubMed]
- Riley, G.P.; Curry, V.; DeGroot, J.; van El, B.; Verzijl, N.; Hazleman, B.L.; Bank, R.A. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. J. Int. Soc. Matrix Biol. 2002, 21, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Buhrmann, C.; Popper, B.; Aggarwal, B.B.; Shakibaei, M. Resveratrol downregulates inflammatory pathway activated by lymphotoxin α (TNF-β) in articular chondrocytes: Comparison with TNF-α. PLoS ONE 2017, 12, e0186993. [Google Scholar] [CrossRef]
- Madhu, K.; Chanda, K.; Saji, M.J. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: A randomized placebo-controlled trial. Inflammopharmacology 2013, 21, 129–136. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef]
- Buhrmann, C.; Brockmueller, A.; Mueller, A.L.; Shayan, P.; Shakibaei, M. Curcumin Attenuates Environment-Derived Osteoarthritis by Sox9/NF-kB Signaling Axis. Int. J. Mol. Sci. 2021, 22, 7645. [Google Scholar] [CrossRef]
- Zhuang, Z.; Ye, G.; Huang, B. Kaempferol Alleviates the Interleukin-1β-Induced Inflammation in Rat Osteoarthritis Chondrocytes via Suppression of NF-κB. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3925–3931. [Google Scholar] [CrossRef] [Green Version]
- Karousou, E.; Ronga, M.; Vigetti, D.; Passi, A.; Maffulli, N. Collagens, proteoglycans, MMP-2, MMP-9 and TIMPs in human achilles tendon rupture. Clin. Orthop. Relat. Res. 2008, 466, 1577–1582. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Caceres, M.D.; Yan, Z.; Schieker, M.; Nerlich, M.; Docheva, D. Tenomodulin regulates matrix remodeling of mouse tendon stem/progenitor cells in an ex vivo collagen I gel model. Biochem. Biophys. Res. Commun. 2019, 512, 691–697. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Schonk, M.M.; Kharaz, Y.A.; Comerford, E.; Mendias, C.L. Scleraxis is required for the growth of adult tendons in response to mechanical loading. JCI Insight 2020, 5, e138295. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, S.; Zheng, W.; Tu, B.; Liu, S.; Ruan, H.; Fan, C. RelA/p65 inhibition prevents tendon adhesion by modulating inflammation, cell proliferation, and apoptosis. Cell Death Dis. 2017, 8, e2710. [Google Scholar] [CrossRef]
- Dakin, S.G.; Martinez, F.O.; Yapp, C.; Wells, G.; Oppermann, U.; Dean, B.J.; Smith, R.D.; Wheway, K.; Watkins, B.; Roche, L.; et al. Inflammation activation and resolution in human tendon disease. Sci. Transl. Med. 2015, 7, 311ra173. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef] [Green Version]
- Shakibaei, M.; De Souza, P. Differentiation of mesenchymal limb bud cells to chondrocytes in alginate beads. Cell Biol. Int. 1997, 21, 75–86. [Google Scholar] [CrossRef]
- Busch, F.; Mobasheri, A.; Shayan, P.; Stahlmann, R.; Shakibaei, M. Sirt-1 is required for the inhibition of apoptosis and inflammatory responses in human tenocytes. J. Biol. Chem. 2012, 287, 25770–25781. [Google Scholar] [CrossRef] [Green Version]
- Sakabe, T.; Sakai, K.; Maeda, T.; Sunaga, A.; Furuta, N.; Schweitzer, R.; Sasaki, T.; Sakai, T. Transcription factor scleraxis vitally contributes to progenitor lineage direction in wound healing of adult tendon in mice. J. Biol. Chem. 2018, 293, 5766–5780. [Google Scholar] [CrossRef] [Green Version]
- Best, K.T.; Korcari, A.; Mora, K.E.; Nichols, A.E.; Muscat, S.N.; Knapp, E.; Buckley, M.R.; Loiselle, A.E. Scleraxis-lineage cell depletion improves tendon healing and disrupts adult tendon homeostasis. eLife 2021, 10, e62203. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, A.-L.; Brockmueller, A.; Kunnumakkara, A.B.; Shakibaei, M. Calebin A, a Compound of Turmeric, Down-Regulates Inflammation in Tenocytes by NF-κB/Scleraxis Signaling. Int. J. Mol. Sci. 2022, 23, 1695. https://doi.org/10.3390/ijms23031695
Mueller A-L, Brockmueller A, Kunnumakkara AB, Shakibaei M. Calebin A, a Compound of Turmeric, Down-Regulates Inflammation in Tenocytes by NF-κB/Scleraxis Signaling. International Journal of Molecular Sciences. 2022; 23(3):1695. https://doi.org/10.3390/ijms23031695
Chicago/Turabian StyleMueller, Anna-Lena, Aranka Brockmueller, Ajaikumar B. Kunnumakkara, and Mehdi Shakibaei. 2022. "Calebin A, a Compound of Turmeric, Down-Regulates Inflammation in Tenocytes by NF-κB/Scleraxis Signaling" International Journal of Molecular Sciences 23, no. 3: 1695. https://doi.org/10.3390/ijms23031695







