Carboxamide Derivatives Are Potential Therapeutic AHR Ligands for Restoring IL-4 Mediated Repression of Epidermal Differentiation Proteins
Abstract
:1. Introduction
2. Results
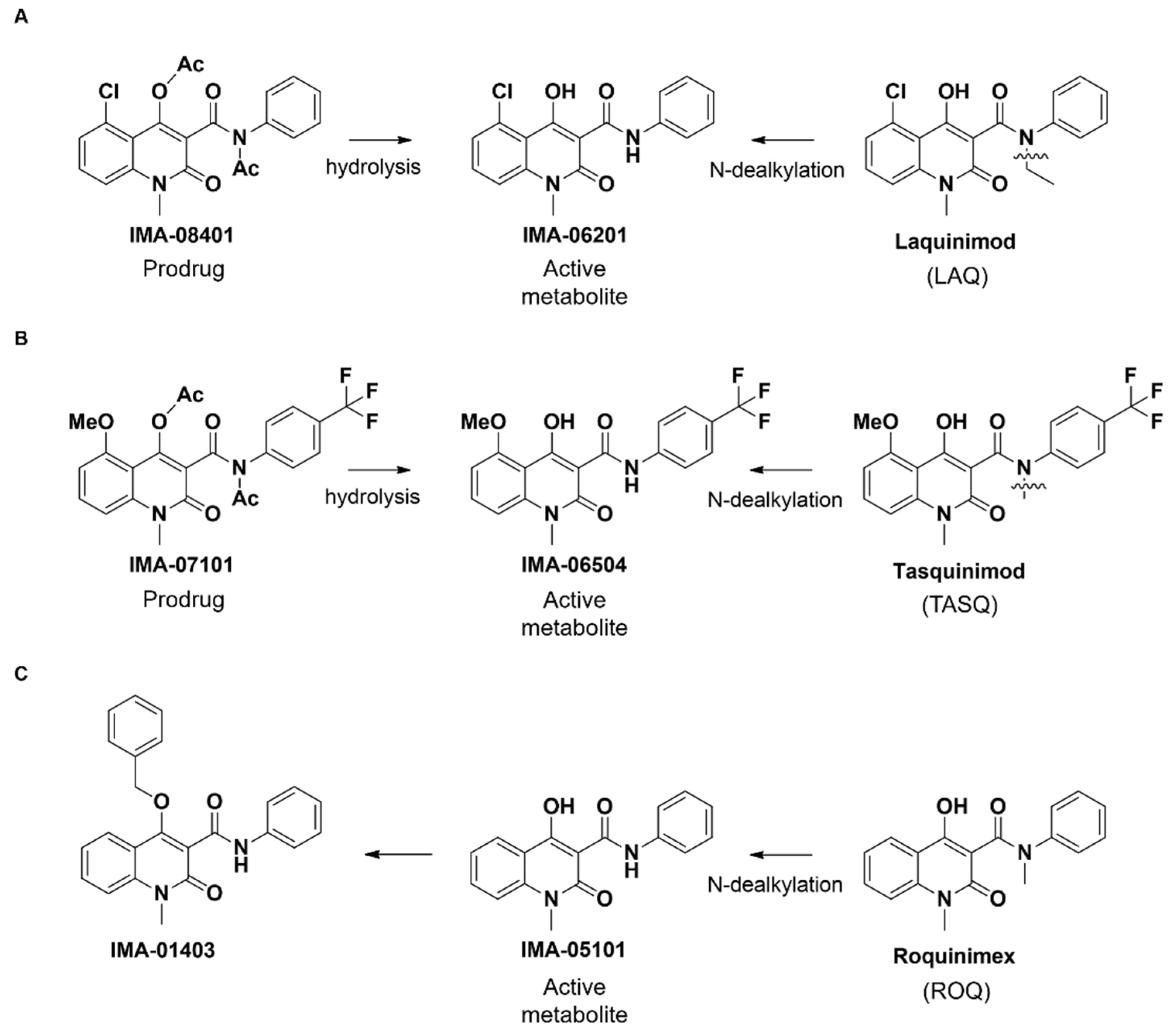
2.1. Structure of IMA-Compounds
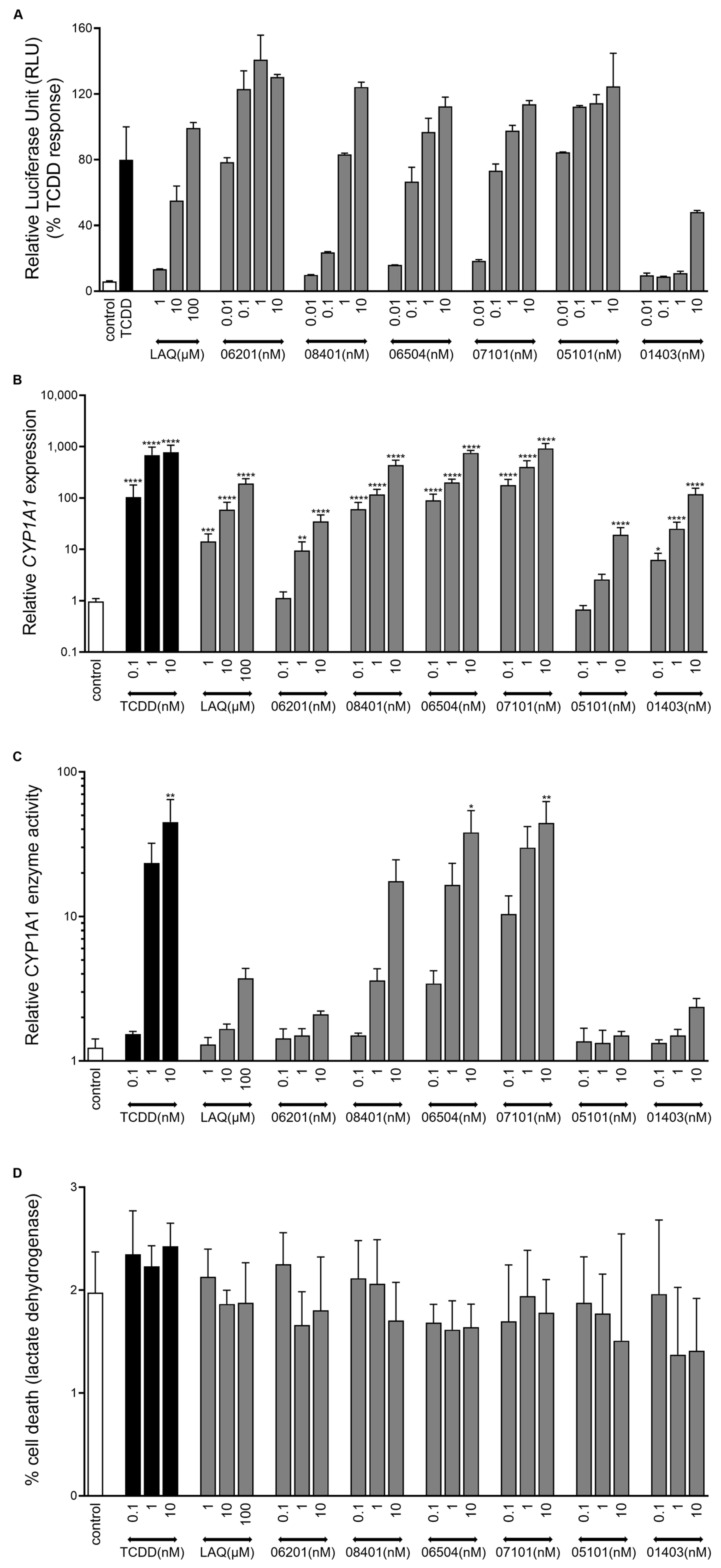
2.2. IMA-Compounds Induce AHR Activity in Reporter Cell Line and Primary Keratinocytes
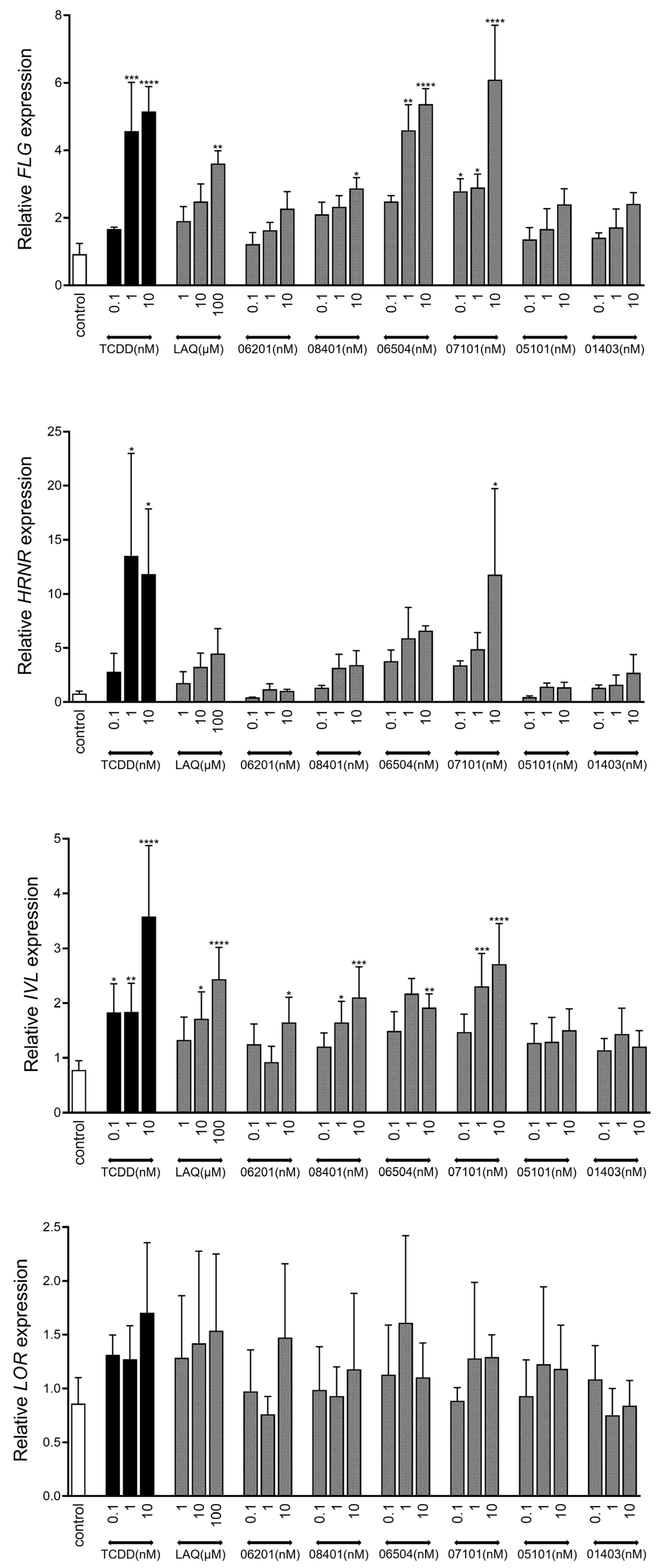
2.3. AHR-Mediated Expression of Epidermal Differentiation
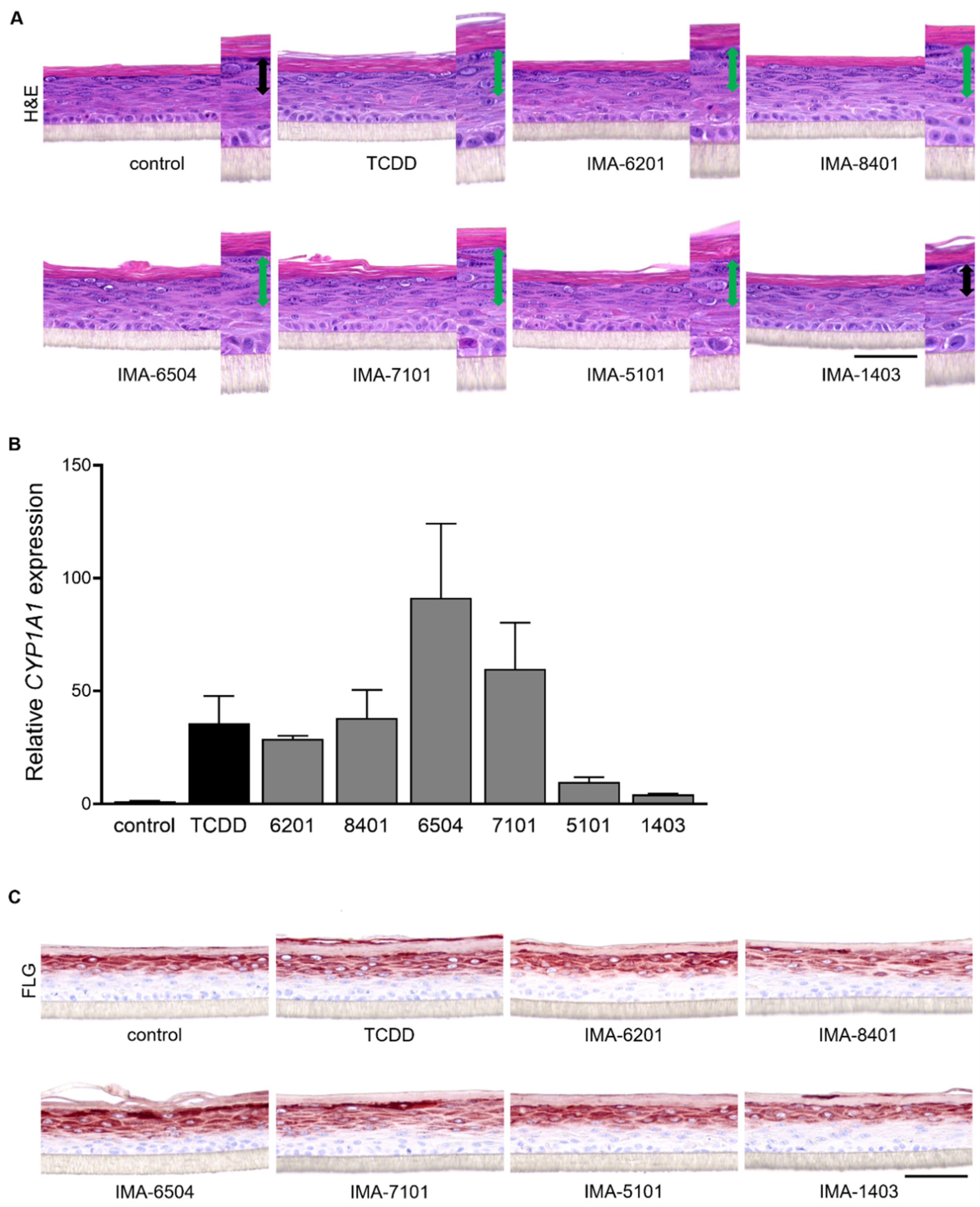
2.4. Therapeutic Effect of IMA-Compounds in Organotypic AD-like Epidermal Models
3. Discussion
4. Materials and Methods
4.1. Synthesis of IMA-Compounds
4.2. HepG2 (40/6) Luciferase Reporter Assay
4.3. Primary Keratinocyte Isolation
4.4. Monolayer Primary Keratinocyte Culture
4.5. Lactate Dehydrogenase (LDH) Assay
4.6. CYP1A1 Enzyme Activity Assay
4.7. Human Epidermal Equivalent (HEE) Culture (Normal Skin and Atopic Dermatitis Model)
4.8. Immunohistochemistry
4.9. RNA Isolation Real-Time Quantitative PCR (RT-qPCR)
4.10. Quantification of Differentiation Protein Expression in HEEs
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FLG | filaggrin |
| AD | atopic dermatitis |
| Pso | psoriasis |
| AHR | aryl hydrocarbon receptor |
| CYP1A1 | cytochrome P450 1A1 |
| TCDD | 2,3,7,8-tetrachlorodibenzodioxin |
| LAQ | laquinimod |
| TASQ | tasquinimod |
| ROQ | roquinimex |
| IMA | immunahr |
| IL-4 | interleukin-4 |
| HEE | human epidermal equivalent |
References
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; Terron-Kwiatkowski, A.; Hull, P.R.; O’Regan, G.M.; Clayton, T.H.; Watson, R.M.; Carrick, T.; Evans, A.T.; Liao, H.; Zhao, Y.; et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat. Genet. 2007, 39, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Kroboth, K.; Sandilands, A.; Campbell, L.E.; Pohler, E.; Kezic, S.; Cordell, H.J.; McLean, W.H.; Irvine, A.D. Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose-dependent effect. J. Invest. Dermatol. 2012, 132, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Knuppel, S.; Esparza-Gordillo, J.; Marenholz, I.; Holzhutter, H.G.; Bauerfeind, A.; Ruether, A.; Weidinger, S.; Lee, Y.A.; Rohde, K. Multi-locus stepwise regression: A haplotype-based algorithm for finding genetic associations applied to atopic dermatitis. BMC Med. Genet. 2012, 13, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trzeciak, M.; Wesserling, M.; Bandurski, T.; Glen, J.; Nowicki, R.; Pawelczyk, T. Association of a Single Nucleotide Polymorphism in a Late Cornified Envelope-like Proline-rich 1 Gene (LELP1) with Atopic Dermatitis. Acta Derm. Venereol. 2016, 96, 459–463. [Google Scholar] [CrossRef] [Green Version]
- Marenholz, I.; Rivera, V.A.; Esparza-Gordillo, J.; Bauerfeind, A.; Lee-Kirsch, M.A.; Ciechanowicz, A.; Kurek, M.; Piskackova, T.; Macek, M.; Lee, Y.A. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J. Invest. Dermatol. 2011, 131, 1644–1649. [Google Scholar] [CrossRef] [Green Version]
- van den Bogaard, E.H.; Bergboer, J.G.; Vonk-Bergers, M.; van Vlijmen-Willems, I.M.; Hato, S.V.; van der Valk, P.G.; Schroder, J.M.; Joosten, I.; Zeeuwen, P.L.; Schalkwijk, J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Invest. 2013, 123, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Smits, J.P.H.; Ederveen, T.H.A.; Rikken, G.; van den Brink, N.J.M.; van Vlijmen-Willems, I.; Boekhorst, J.; Kamsteeg, M.; Schalkwijk, J.; van Hijum, S.; Zeeuwen, P.; et al. Targeting the Cutaneous Microbiota in Atopic Dermatitis by Coal Tar via AHR-Dependent Induction of Antimicrobial Peptides. J. Invest. Dermatol. 2020, 140, 415–424.e410. [Google Scholar] [CrossRef]
- Loertscher, J.A.; Sattler, C.A.; Allen-Hoffmann, B.L. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters the differentiation pattern of human keratinocytes in organotypic culture. Toxicol. Appl. Pharmacol. 2001, 175, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Sutter, C.H.; Bodreddigari, S.; Campion, C.; Wible, R.S.; Sutter, T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases the expression of genes in the human epidermal differentiation complex and accelerates epidermal barrier formation. Toxicol. Sci. 2011, 124, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Fritsche, E.; Schafer, C.; Calles, C.; Bernsmann, T.; Bernshausen, T.; Wurm, M.; Hubenthal, U.; Cline, J.E.; Hajimiragha, H.; Schroeder, P.; et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. USA 2007, 104, 8851–8856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, U.H.; Lee, S.O.; Sridharan, G.; Lee, K.; Davidson, L.A.; Jayaraman, A.; Chapkin, R.S.; Alaniz, R.; Safe, S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol. Pharmacol. 2014, 85, 777–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.Q.; Chiu-Leung, L.C.; Lin, S.M.; Leung, L.K. The citrus flavonone hesperetin attenuates the nuclear translocation of aryl hydrocarbon receptor. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 210, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Uberoi, A.; Bartow-McKenney, C.; Zheng, Q.; Flowers, L.; Campbell, A.; Knight, S.A.B.; Chan, N.; Wei, M.; Lovins, V.; Bugayev, J.; et al. Commensal microbiota regulates skin barrier function and repair via signaling through the aryl hydrocarbon receptor. Cell. Host Microbe 2021, 29, 1235–1248.e1238. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaard, E.H.; Esser, C.; Perdew, G.H. The aryl hydrocarbon receptor at the forefront of host-microbe interactions in the skin: A perspective on current knowledge gaps and directions for future research and therapeutic applications. Exp. Dermatol. 2021, 30, 1477–1483. [Google Scholar] [CrossRef]
- Gialitakis, M.; Tolaini, M.; Li, Y.; Pardo, M.; Yu, L.; Toribio, A.; Choudhary, J.S.; Niakan, K.; Papayannopoulos, V.; Stockinger, B. Activation of the Aryl Hydrocarbon Receptor Interferes with Early Embryonic Development. Stem Cell Rep. 2017, 9, 1377–1386. [Google Scholar] [CrossRef] [Green Version]
- van den Bogaard, E.H.; Podolsky, M.A.; Smits, J.P.; Cui, X.; John, C.; Gowda, K.; Desai, D.; Amin, S.G.; Schalkwijk, J.; Perdew, G.H.; et al. Genetic and pharmacological analysis identifies a physiological role for the AHR in epidermal differentiation. J. Invest. Dermatol. 2015, 135, 1320–1328. [Google Scholar] [CrossRef] [Green Version]
- Haas, K.; Weighardt, H.; Deenen, R.; Kohrer, K.; Clausen, B.; Zahner, S.; Boukamp, P.; Bloch, W.; Krutmann, J.; Esser, C. Aryl Hydrocarbon Receptor in Keratinocytes Is Essential for Murine Skin Barrier Integrity. J. Invest. Dermatol. 2016, 136, 2260–2269. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Vazquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Roman, A.C.; Carvajal-Gonzalez, J.M.; Rico-Leo, E.M.; Fernandez-Salguero, P.M. Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J. Biol. Chem. 2009, 284, 25135–25148. [Google Scholar] [CrossRef] [Green Version]
- Kimura, E.; Kubo, K.I.; Endo, T.; Nakajima, K.; Kakeyama, M.; Tohyama, C. Excessive activation of AhR signaling disrupts neuronal migration in the hippocampal CA1 region in the developing mouse. J. Toxicol. Sci. 2017, 42, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N. The role of endogenous aryl hydrocarbon receptor signaling in cardiovascular physiology. J. Cardiovasc. Dis. Res. 2011, 2, 91–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furue, M.; Takahara, M.; Nakahara, T.; Uchi, H. Role of AhR/ARNT system in skin homeostasis. Arch. Dermatol. Res. 2014, 306, 769–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintana, F.J.; Sherr, D.H. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol. Rev. 2013, 65, 1148–1161. [Google Scholar] [CrossRef] [Green Version]
- Haarmann-Stemmann, T.; Esser, C.; Krutmann, J. The Janus-Faced Role of Aryl Hydrocarbon Receptor Signaling in the Skin: Consequences for Prevention and Treatment of Skin Disorders. J. Invest. Dermatol. 2015, 135, 2572–2576. [Google Scholar] [CrossRef] [Green Version]
- Denison, M.S.; Soshilov, A.A.; He, G.; DeGroot, D.E.; Zhao, B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 2011, 124, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof Is a Natural AhR Agonist that Resolves Skin Inflammation in Mice and Humans. J. Invest. Dermatol. 2017, 137, 2110–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peppers, J.; Paller, A.S.; Maeda-Chubachi, T.; Wu, S.; Robbins, K.; Gallagher, K.; Kraus, J.E. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2019, 80, 89–98.e83. [Google Scholar] [CrossRef]
- Boros, F.; Vecsei, L. Progress in the development of kynurenine and quinoline-3-carboxamide-derived drugs. Expert. Opin. Investig. Drugs 2020, 29, 1223–1247. [Google Scholar] [CrossRef]
- Kaye, J.; Piryatinsky, V.; Birnberg, T.; Hingaly, T.; Raymond, E.; Kashi, R.; Amit-Romach, E.; Caballero, I.S.; Towfic, F.; Ator, M.A.; et al. Laquinimod arrests experimental autoimmune encephalomyelitis by activating the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2016, 113, E6145–E6152. [Google Scholar] [CrossRef] [Green Version]
- Wegner, C.; Stadelmann, C.; Pfortner, R.; Raymond, E.; Feigelson, S.; Alon, R.; Timan, B.; Hayardeny, L.; Bruck, W. Laquinimod interferes with migratory capacity of T cells and reduces IL-17 levels, inflammatory demyelination and acute axonal damage in mice with experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2010, 227, 133–143. [Google Scholar] [CrossRef]
- Bruck, W.; Wegner, C. Insight into the mechanism of laquinimod action. J. Neurol. Sci. 2011, 306, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B. New use of quinoline-3-carboxamide compounds. WO 95/24196A1, 8 March 1995. [Google Scholar]
- Pettersson, L. 1,2-dihydro-4-hydroxy-2-oxo-quinoline-3-carboxanilides as AHR activators. WO 2012/050500, 19 April 2012. [Google Scholar]
- Mahiout, S.; Tagliabue, S.G.; Nasri, A.; Omoruyi, I.M.; Pettersson, L.; Bonati, L.; Pohjanvirta, R. In vitro toxicity and in silico docking analysis of two novel selective AH-receptor modulators. Toxicol. In Vitro 2018, 52, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Mahiout, S.; Linden, J.; Esteban, J.; Sanchez-Perez, I.; Sankari, S.; Pettersson, L.; Hakansson, H.; Pohjanvirta, R. Toxicological characterisation of two novel selective aryl hydrocarbon receptor modulators in Sprague-Dawley rats. Toxicol. Appl. Pharmacol. 2017, 326, 54–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, W.P.; Pray-Grant, M.; Tsai, J.C.; Perdew, G.H. Protein kinase C activity is required for aryl hydrocarbon receptor pathway-mediated signal transduction. Mol. Pharmacol. 1998, 53, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, L.H.; Sutter, C.H.; Leon Carrion, S.; Tran, Q.T.; Bodreddigari, S.; Kensicki, E.; Mohney, R.P.; Sutter, T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated production of reactive oxygen species is an essential step in the mechanism of action to accelerate human keratinocyte differentiation. Toxicol. Sci. 2013, 132, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Vu, Y.H.; Hashimoto-Hachiya, A.; Takemura, M.; Yumine, A.; Mitamura, Y.; Nakahara, T.; Furue, M.; Tsuji, G. IL-24 Negatively Regulates Keratinocyte Differentiation Induced by Tapinarof, an Aryl Hydrocarbon Receptor Modulator: Implication in the Treatment of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 9412. [Google Scholar] [CrossRef]
- de Wit, R.; Pawinsky, A.; Stoter, G.; van Oosterom, A.T.; Fossa, S.D.; Paridaens, R.; Svedberg, A.; de Mulder, P.H. EORTC phase II study of daily oral linomide in metastatic renal cell carcinoma patients with good prognostic factors. Eur. J. Cancer 1997, 33, 493–495. [Google Scholar] [CrossRef]
- Mackean, M.J.; Kerr, D.; Lesko, M.; Svedberg, A.; Hansson, F.; Jodrell, D.; Cassidy, J. A feasibility study of roquinimex (Linomide) and alpha interferon in patients with advanced malignant melanoma or renal carcinoma. Br. J. Cancer 1998, 78, 1620–1623. [Google Scholar] [CrossRef] [Green Version]
- Noseworthy, J.H.; Wolinsky, J.S.; Lublin, F.D.; Whitaker, J.N.; Linde, A.; Gjorstrup, P.; Sullivan, H.C. Linomide in relapsing and secondary progressive MS: Part I: Trial design and clinical results. North American Linomide Investigators. Neurology 2000, 54, 1726–1733. [Google Scholar] [CrossRef]
- Jonsson, S.; Andersson, G.; Fex, T.; Fristedt, T.; Hedlund, G.; Jansson, K.; Abramo, L.; Fritzson, I.; Pekarski, O.; Runstrom, A.; et al. Synthesis and biological evaluation of new 1,2-dihydro-4-hydroxy-2-oxo-3-quinolinecarboxamides for treatment of autoimmune disorders: Structure-activity relationship. J. Med. Chem. 2004, 47, 2075–2088. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.T.; Pili, R.; Qian, D.Z.; Dalrymple, S.L.; Garrison, J.B.; Kyprianou, N.; Bjork, A.; Olsson, A.; Leanderson, T. Identification of ABR-215050 as lead second generation quinoline-3-carboxamide anti-angiogenic agent for the treatment of prostate cancer. Prostate 2006, 66, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Haggiag, S.; Ruggieri, S.; Gasperini, C. Efficacy and safety of laquinimod in multiple sclerosis: Current status. Ther. Adv. Neurol. Disord. 2013, 6, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polman, C.; Barkhof, F.; Sandberg-Wollheim, M.; Linde, A.; Nordle, O.; Nederman, T.; Laquinimod in Relapsing, M.S.S.G. Treatment with laquinimod reduces development of active MRI lesions in relapsing MS. Neurology 2005, 64, 987–991. [Google Scholar] [CrossRef]
- Rouhi, F.; Mohammadpour, Z.; Noureini, S.K.; Abbastabar, H.; Harirchian, M.H.; Bitarafan, S. The effects and side effects of laquinimod for the treatment of multiple sclerosis patients: A systematic review and meta-analysis of clinical trials. Eur. J. Clin. Pharmacol. 2020, 76, 611–622. [Google Scholar] [CrossRef]
- Gong, P.; Liu, H.; Liu, X.; Zhou, G.; Liu, M.; Yang, X.; Xiong, W.; Wang, Q.; Ma, J.; Ren, Z.; et al. Efficacy of tasquinimod in men with metastatic castration-resistant prostate cancer: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e13204. [Google Scholar] [CrossRef]
- Williamson, S.C.; Hartley, A.E.; Heer, R. A review of tasquinimod in the treatment of advanced prostate cancer. Drug Des. Devel. Ther. 2013, 7, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Bock, K.W. From TCDD-mediated toxicity to searches of physiologic AHR functions. Biochem. Pharmacol. 2018, 155, 419–424. [Google Scholar] [CrossRef]
- Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Cytochrome P450 CYP1A1: Wider roles in cancer progression and prevention. BMC Cancer 2009, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Kyoreva, M.; Li, Y.; Hoosenally, M.; Hardman-Smart, J.; Morrison, K.; Tosi, I.; Tolaini, M.; Barinaga, G.; Stockinger, B.; Mrowietz, U.; et al. CYP1A1 Enzymatic Activity Influences Skin Inflammation Via Regulation of the AHR Pathway. J. Invest. Dermatol. 2021, 141, 1553–1563.e1553. [Google Scholar] [CrossRef]
- van den Bogaard, E.H.; Perdew, G.H. The Enigma of AHR Activation in the Skin: Interplay among Ligands, Metabolism, and Bioavailability. J. Invest. Dermatol. 2021, 141, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Stein Gold, L.; Rubenstein, D.S.; Tallman, A.M.; Armstrong, A. Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J. Am. Acad. Dermatol. 2021, 84, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, M.P.; Jendoubi, F.; Hegazy, S.; Bouznad, A.; Tauber, M.; Bulai-Livideanu, C.; Paul, C. Tapinarof-induced folliculitis: The paradigm of activation of the aryl hydrocarbon signaling pathway. J. Am. Acad. Dermatol. 2021, 85, e37–e38. [Google Scholar] [CrossRef]
- Kalmes, M.; Hennen, J.; Clemens, J.; Blomeke, B. Impact of aryl hydrocarbon receptor (AhR) knockdown on cell cycle progression in human HaCaT keratinocytes. Biol. Chem. 2011, 392, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.G.; Gold, L.S.; Strober, B.; Papp, K.A.; Armstrong, A.W.; Bagel, J.; Kircik, L.; Ehst, B.; Hong, H.C.; Soung, J.; et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N. Engl. J. Med. 2021, 385, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Rikken, G.; Niehues, H.; van den Bogaard, E.H. Organotypic 3D Skin Models: Human Epidermal Equivalent Cultures from Primary Keratinocytes and Immortalized Keratinocyte Cell Lines. Methods Mol. Biol. 2020, 2154, 45–61. [Google Scholar] [CrossRef]
- Nygaard, U.H.; Niehues, H.; Rikken, G.; Rodijk-Olthuis, D.; Schalkwijk, J.; van den Bogaard, E.H. Antibiotics in cell culture: Friend or foe? Suppression of keratinocyte growth and differentiation in monolayer cultures and 3D skin models. Exp. Dermatol. 2015, 24, 964–965. [Google Scholar] [CrossRef] [Green Version]
- Kamsteeg, M.; Bergers, M.; de Boer, R.; Zeeuwen, P.L.; Hato, S.V.; Schalkwijk, J.; Tjabringa, G.S. Type 2 helper T-cell cytokines induce morphologic and molecular characteristics of atopic dermatitis in human skin equivalent. Am. J. Pathol. 2011, 178, 2091–2099. [Google Scholar] [CrossRef] [Green Version]
- Niehues, H.; Schalkwijk, J.; van Vlijmen-Willems, I.; Rodijk-Olthuis, D.; van Rossum, M.M.; Wladykowski, E.; Brandner, J.M.; van den Bogaard, E.H.J.; Zeeuwen, P. Epidermal equivalents of filaggrin null keratinocytes do not show impaired skin barrier function. J. Allergy Clin. Immunol. 2017, 139, 1979–1981.e1913. [Google Scholar] [CrossRef] [Green Version]
- Niehues, H.; Rikken, G.; van Vlijmen-Willems, I.M.J.J.; Rodijk-Olthuis, D.; van Erp, P.E.J.; Zeeuwen, P.L.J.M.; Schalkwijk, J.; van den Bogaard, E.H. Identification of Keratinocyte Mitogens: Implications for Hyperproliferation in Psoriasis and Atopic Dermatitis. JID Innov. 2022, 2, 100066. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- McQuin, C.; Goodman, A.; Chernyshev, V.; Kamentsky, L.; Cimini, B.A.; Karhohs, K.W.; Doan, M.; Ding, L.; Rafelski, S.M.; Thirstrup, D.; et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 2018, 16, e2005970. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rikken, G.; van den Brink, N.J.M.; van Vlijmen-Willems, I.M.J.J.; van Erp, P.E.J.; Pettersson, L.; Smits, J.P.H.; van den Bogaard, E.H. Carboxamide Derivatives Are Potential Therapeutic AHR Ligands for Restoring IL-4 Mediated Repression of Epidermal Differentiation Proteins. Int. J. Mol. Sci. 2022, 23, 1773. https://doi.org/10.3390/ijms23031773
Rikken G, van den Brink NJM, van Vlijmen-Willems IMJJ, van Erp PEJ, Pettersson L, Smits JPH, van den Bogaard EH. Carboxamide Derivatives Are Potential Therapeutic AHR Ligands for Restoring IL-4 Mediated Repression of Epidermal Differentiation Proteins. International Journal of Molecular Sciences. 2022; 23(3):1773. https://doi.org/10.3390/ijms23031773
Chicago/Turabian StyleRikken, Gijs, Noa J. M. van den Brink, Ivonne M. J. J. van Vlijmen-Willems, Piet E. J. van Erp, Lars Pettersson, Jos P. H. Smits, and Ellen H. van den Bogaard. 2022. "Carboxamide Derivatives Are Potential Therapeutic AHR Ligands for Restoring IL-4 Mediated Repression of Epidermal Differentiation Proteins" International Journal of Molecular Sciences 23, no. 3: 1773. https://doi.org/10.3390/ijms23031773





