Role of Combined Na2HPO4 and ZnCl2 in the Unprecedented Catalysis of the Sequential Pretreatment of Sustainable Agricultural and Agro-Industrial Wastes in Boosting Bioethanol Production
Abstract
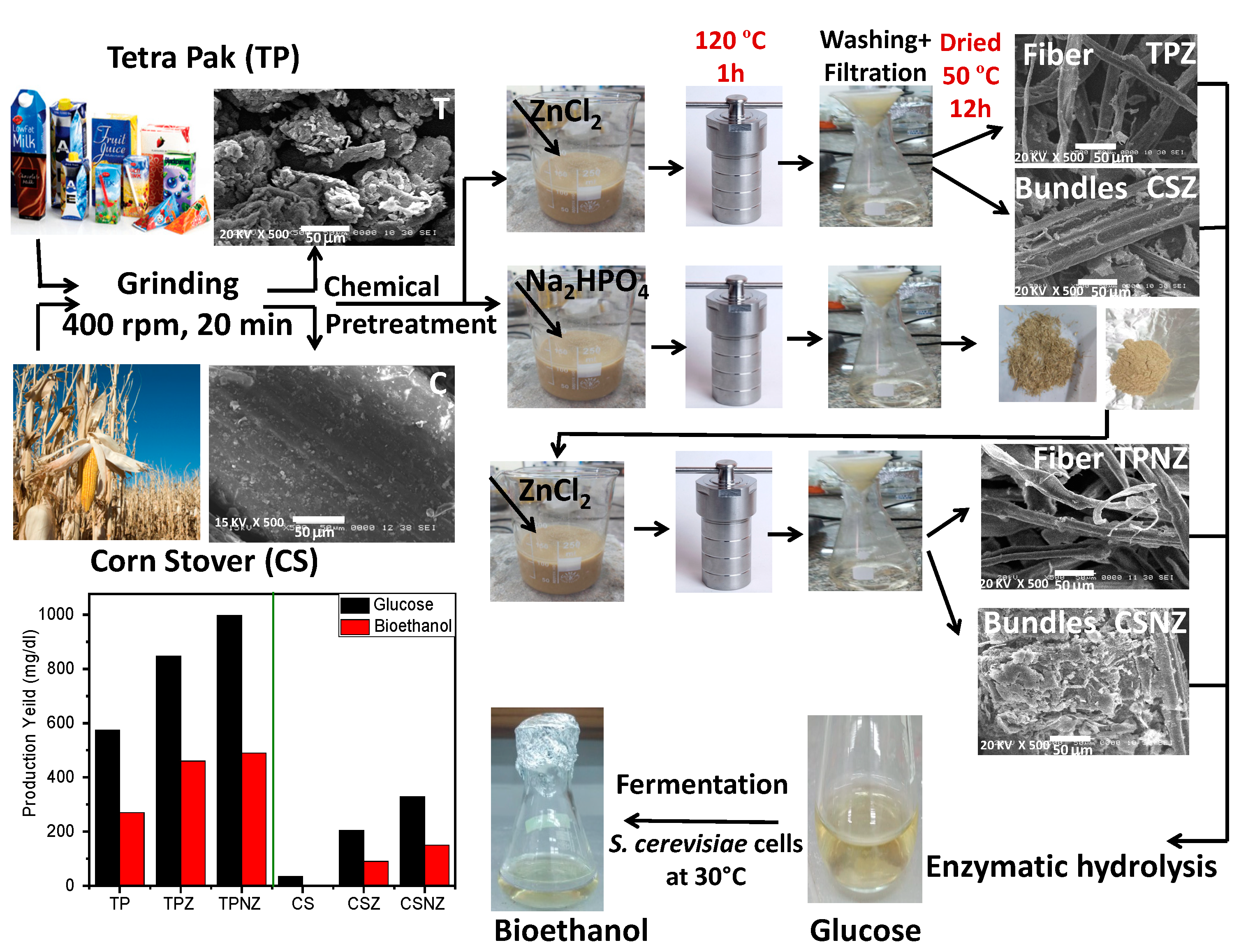
:1. Introduction
2. Results
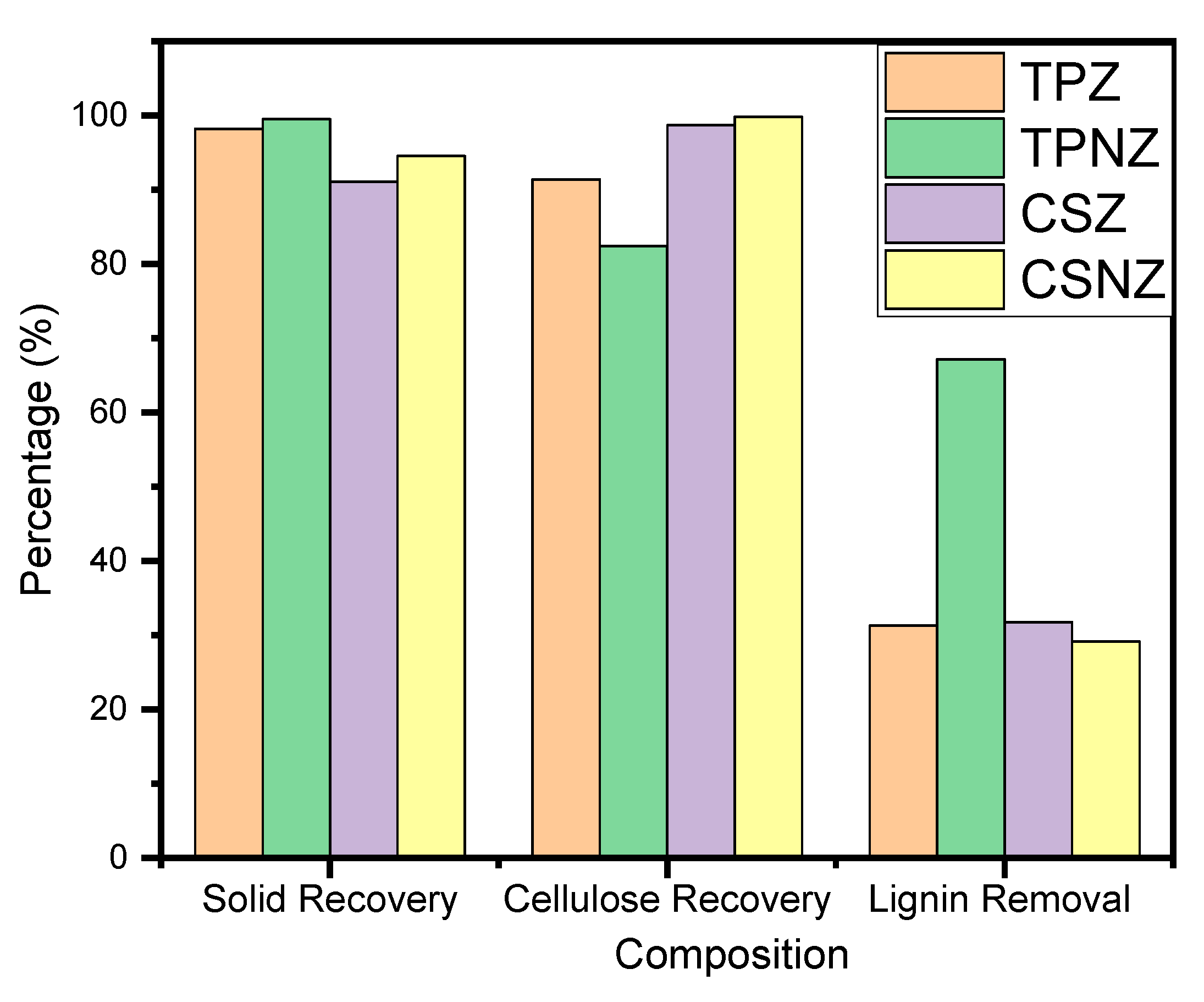
2.1. Correlation between Composition Variance and Enzymatic Hydrolysis of the Pretreated Samples
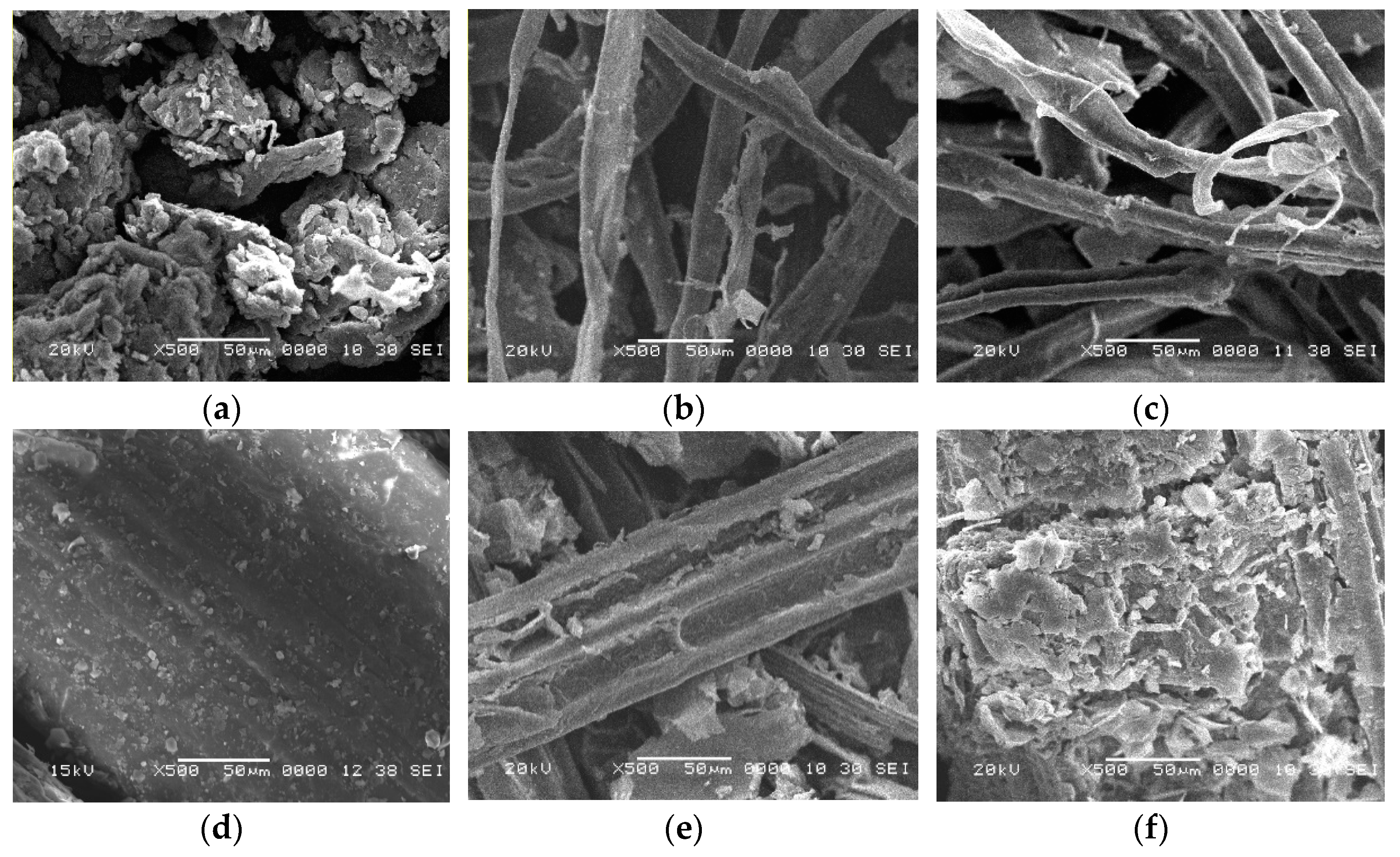
2.2. Surface Morphology
2.3. Chemical Structure
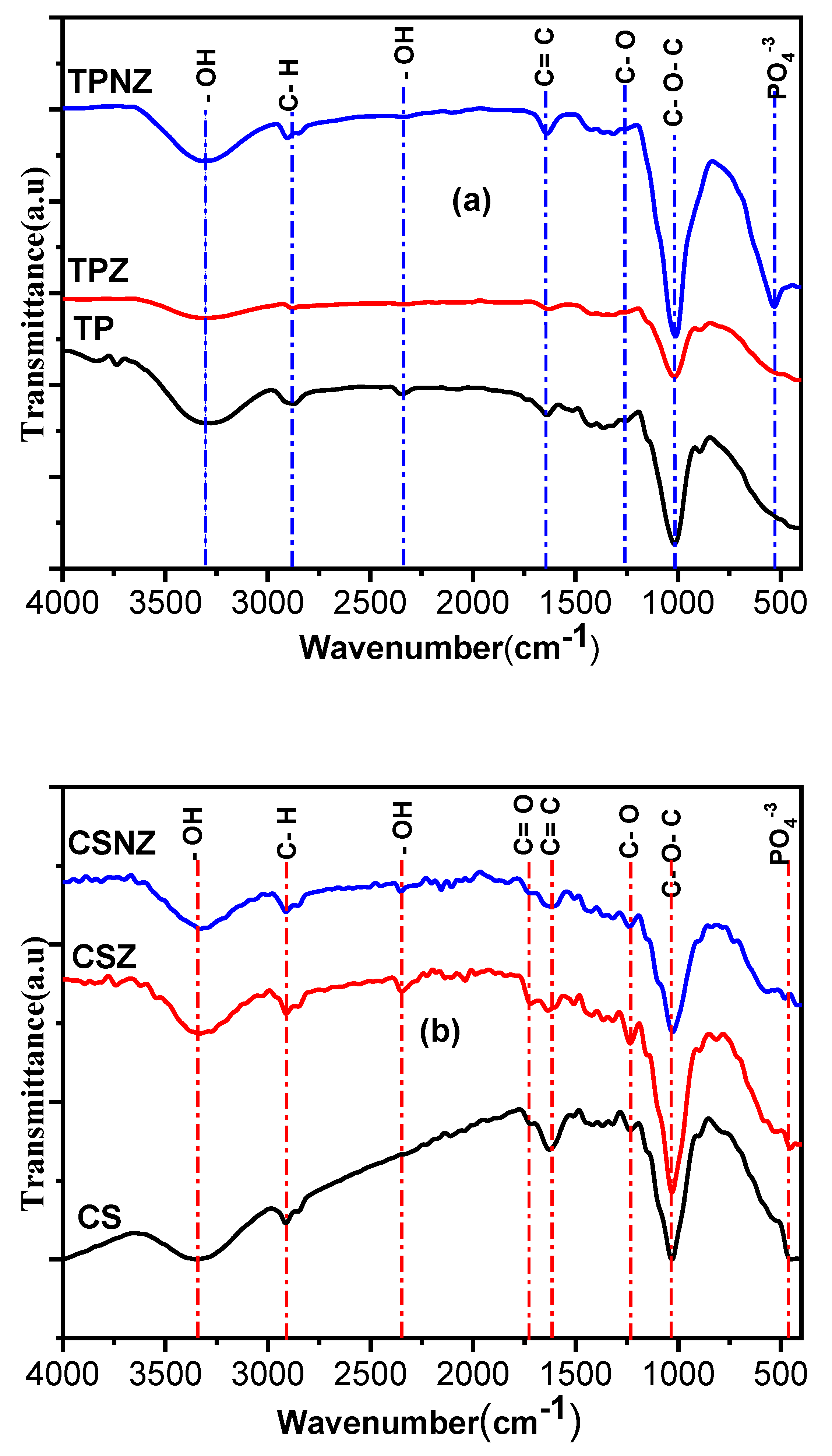
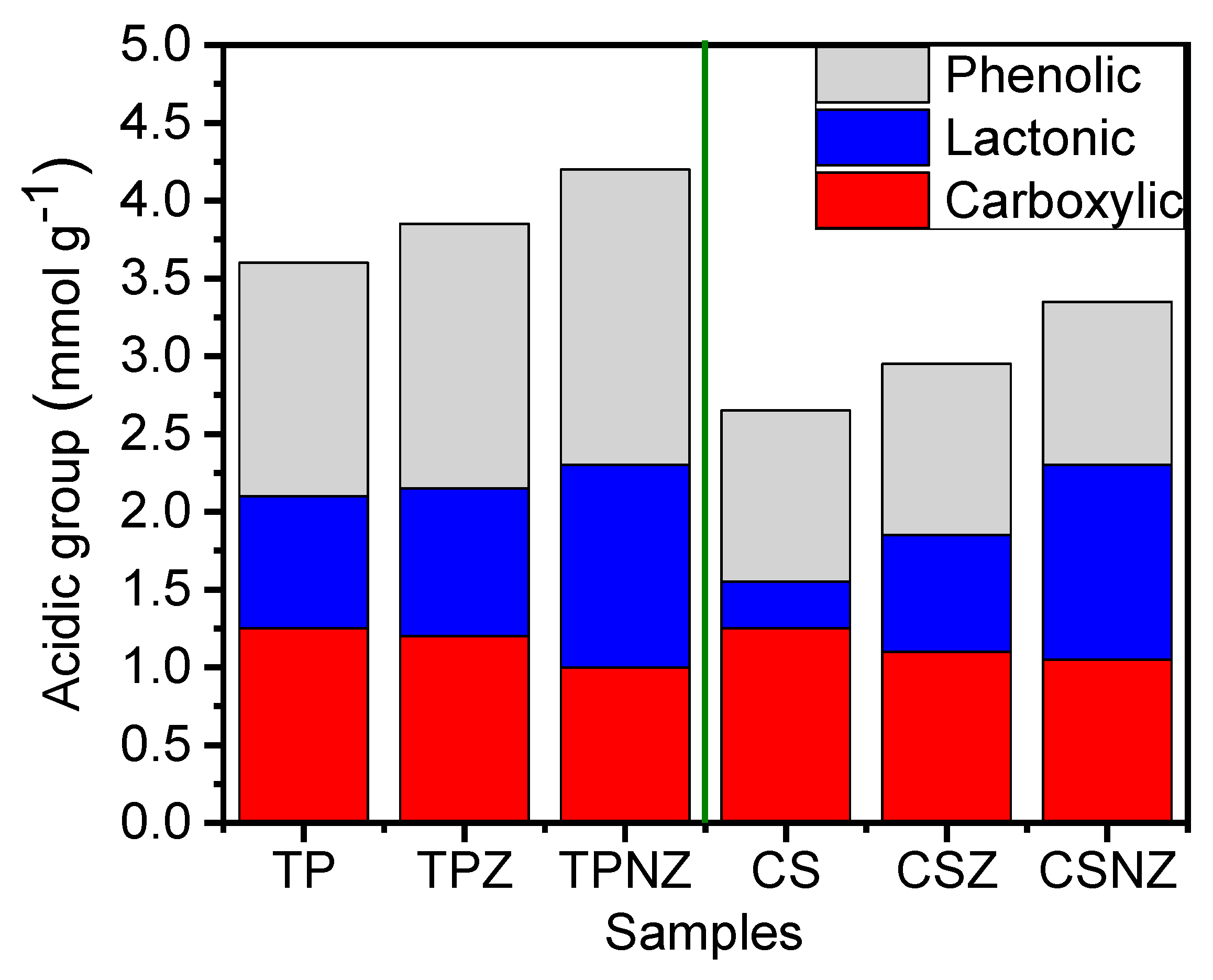
2.4. Surface Functional Groups
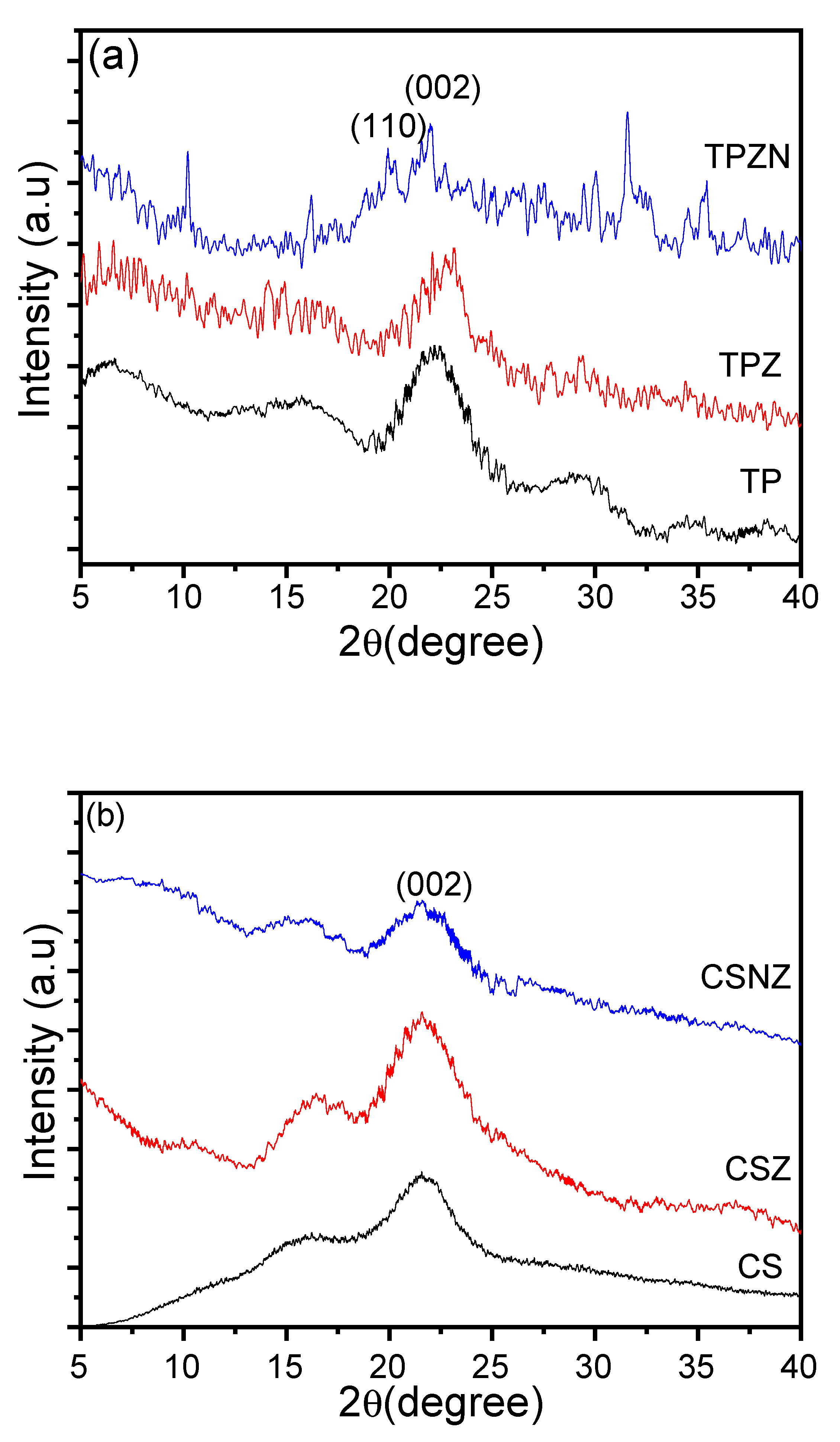
2.5. Crystal Structure
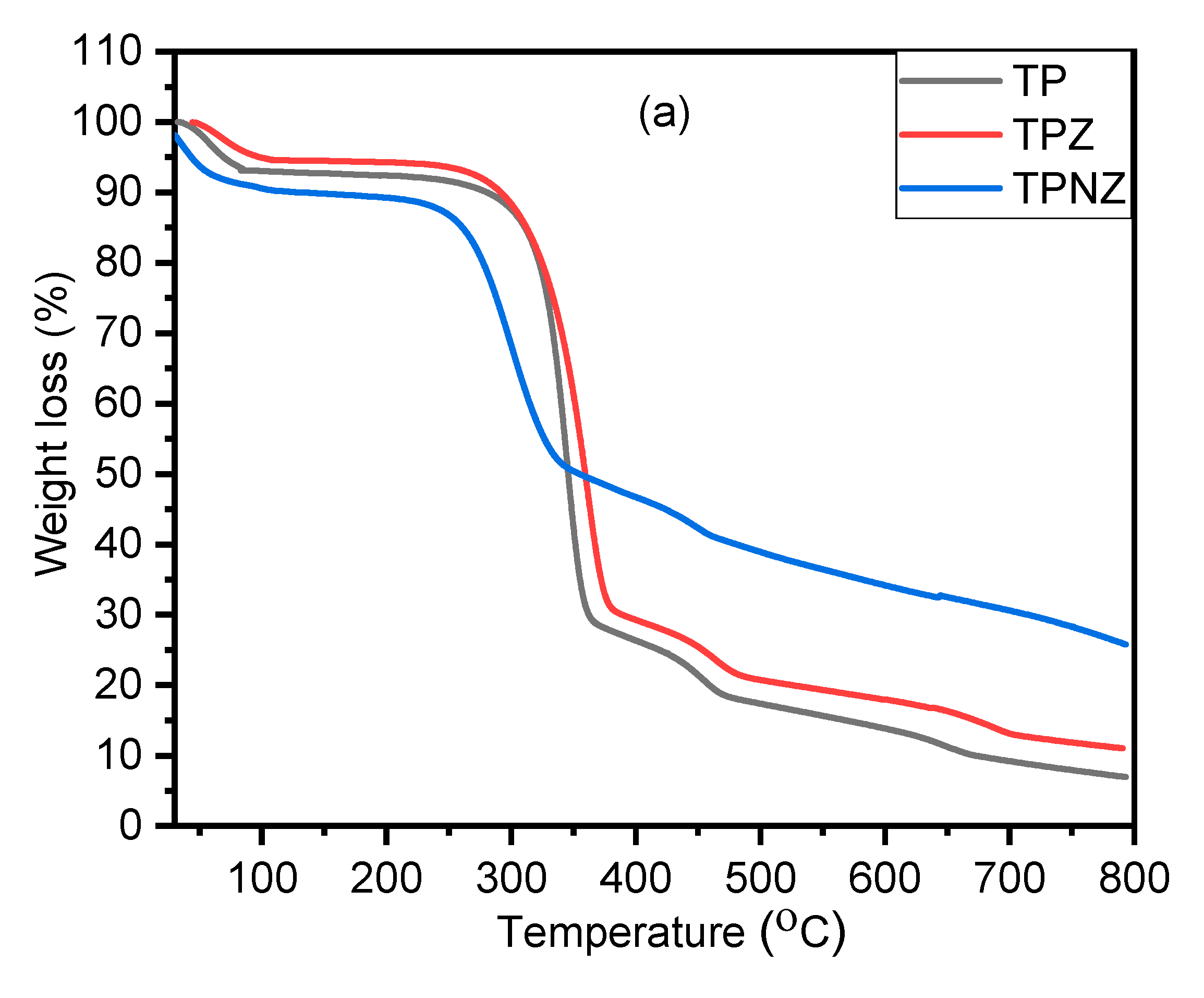
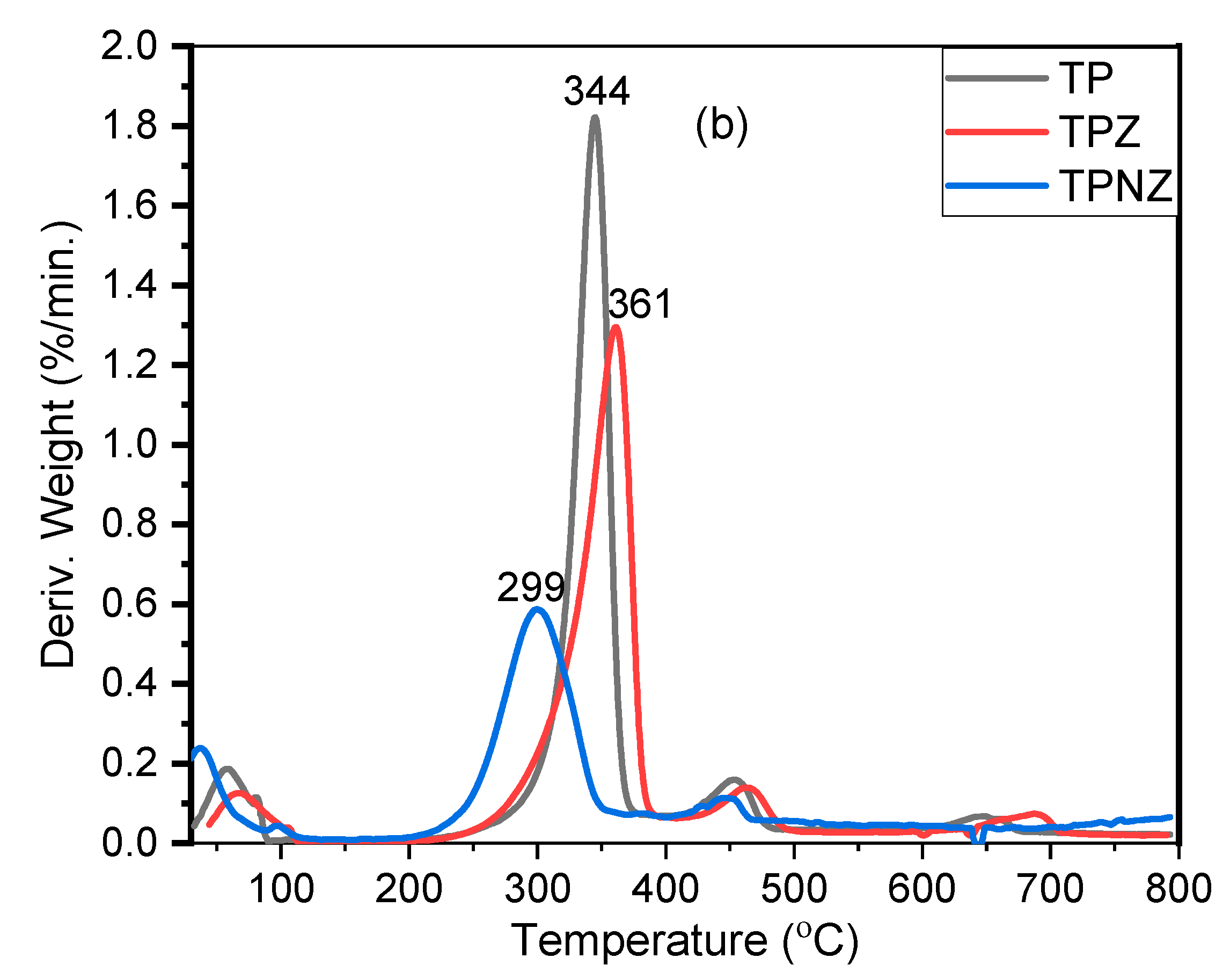
2.6. Thermal Stability
3. Discussions
3.1. Action Mechanism of Na2HPO4 and ZnCl2 Pretreatment Reactions
4. Materials and Methods
4.1. Sampling Materials
4.2. Pretreatment Process
4.2.1. Pretreatment Using ZnCl2
4.2.2. Pretreatment Using Modified ZnCl2
4.3. Enzyme Hydrolysis
4.4. Fermentation Process
4.4.1. Yeast Screening
4.4.2. Yeast Cultivation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S.; Das, D.; Kim, S.; Cho, B.; Kalia, V.; Lee, L. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew. Sustain. Energy Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Li, H.J.; Pu, Y.Q.; Kumar, R.; Ragauskas, A.J. Investigation of lignin deposition on cellulose during hydrothermal pretreatment, its effect on cellulose hydrolysis, and underlying mechanisms. Biotechnol. Bioeng. 2014, 111, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Liu, Y.; He, Q.; Liu, S.; Zhu, Y.; Tong, T.; Liu, B. Efficient adsorption of organic matters and ions by porous biochar aerogel as pre-treatment of ultrafiltration for shale gas wastewater reuse. Chem. Eng. J. Adv. 2020, 2, 100011. [Google Scholar] [CrossRef]
- Haydary, J.; Susa, D.; Dudáš, J. Pyrolysis of aseptic packages (tetrapak) in a laboratory screw type reactor and secondary thermal/catalytic tar decomposition. Waste Manag. 2013, 33, 1136–1141. [Google Scholar] [CrossRef]
- Tetra Pak. Tetra Pak in Figures (2021). Available online: www.tetrapak.com/mx/about/facts-figures (accessed on 9 December 2021).
- Tetra Pak. Post-Consumer Recycling (2019). Available online: https://www.tetrapak.com/us/sustainability/recycling (accessed on 9 December 2021).
- Patel, S.; Gupta, R.; Kalia, V.; Lee, J. Integrating anaerobic digestion of potato peels to methanol production by methanotrophs immobilized on banana leaves. Bioresour. Technol. 2021, 323, 124550. [Google Scholar] [CrossRef] [PubMed]
- McKendry, P. Energy production from biomass (Part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Hamad, H.; Bailón-García, E.; Morales-Torres, S.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado, F.J. Physicochemical properties of new cellulose-TiO2 composites for the removal of water pollutants: Developing specific interactions and performances by cellulose functionalization. J. Environ. Chem. Eng. 2018, 6, 5032–5041. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Hamdy, A.; Elhafez, S.A.; Hamad, H.; Ali, R. The interplay of autoclaving with oxalate as pretreatment technique in the view of bioethanol production based on corn stover. Polymers 2021, 13, 3762. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.; Mohammadi, A. Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Qing, Q.; Zhou, L.; Guo, Q.; Huang, M.; He, Y.; Wang, L.; Zhang, Y.A. combined sodium phosphate and sodium sulphide pretreatment for enhanced enzymatic digestibility and delignification of corn stover. Bioresour. Technol. 2016, 218, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.E.; Park, D.H.; Jeong, G.T. Effects of NH4Cl and MgCl2 on pretreatment and xylan hydrolysis of Miscanthus straw. Carbohydr. Polym. 2013, 92, 1321–1326. [Google Scholar] [CrossRef]
- Nakashima, K.; Ebi, Y.; Kubo, M.; Shibasaki-Kitakawa, N.; Yonemoto, T. Pretreatment combining ultrasound and sodium percarbonate under mild conditions for efficient degradation of corn stover. Ultrason. Sonochem. 2016, 29, 455–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Kumar, A. Process development for sodium carbonate pretreatment and enzymatic saccharification of rice straw for bioethanol production. Biomass Bioenergy 2020, 138, 105574. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.H.; Huang, T.; Jin, Y.C.; Song, J.L.; Chang, H.M.; Jameel, H. Comparison of sodium carbonate-oxygen and sodium-hydroxide oxygen pretreatments on the chemical composition and enzymatic saccharafication of wheat straw. Bioresour. Technol. 2014, 161, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hamad, H.; Castelo-Quibén, J.; Morales-Torres, S.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado, F.J. On the interactions and synergism between phases of carbon-phosphorus-titanium composites synthetized from cellulose for the removal of the orange-G dye. Materials 2018, 11, 1766. [Google Scholar] [CrossRef] [Green Version]
- Moodley, P.; Kana, E.B.G. Microwave-assisted inorganic salt pretreatment of sugarcane leaf waste: Effect on physiochemical structure and enzymatic saccharification. Bioresour. Technol. 2017, 235, 35–42. [Google Scholar] [CrossRef]
- Shivhare, A.; Kumar, A.; Srivastava, R. Metal phosphate catalysts to upgrade lignocellulose biomass into value-added chemicals and biofuels. Green Chem. 2021, 23, 3818–3841. [Google Scholar] [CrossRef]
- Loow, Y.L.; Wu, T.Y.; Tan, K.A.; Lim, Y.S.; Siow, L.F.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Recent advances in the application of inorganic salt pretreatment for transforming lignocellulosic biomass into reducing sugars. J. Agric. Food Chem. 2015, 63, 8349–8363. [Google Scholar] [CrossRef]
- Moodley, P.; Sukai, Y.S.; Kana, E.B.G. Progress in the development of alkali and metal salt catalysed lignocellulosic pretreatment regimes: Potential for bioethanol production. Bioresour. Technol. 2020, 310, 123372. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.W.; Kim, H.Y.; Park, N.; Lee, S.M.; Yeo, H.; Choi, I.G. Organosolv pretreatment of Liriodendron tulipifera and simultaneous saccharification and fermentation for bioethanol production. Biomass Bioenergy 2011, 35, 1833–1840. [Google Scholar] [CrossRef]
- Ibrahim, Q.; Arauzo, P.J.; Kruse, A. The effect of using different acids to catalyze the prehydrolysis stage on the organosolv delignification of beech wood in two-stage process. Renew. Energy 2020, 153, 1479–1487. [Google Scholar] [CrossRef]
- Burger, D.; Winter, A.; Subbiahdoss, G.; Oberlerchner, J.T.; Beaumont, M.; Tamada, Y.; Rosenau, T. Partial amorphization of cellulose through zinc chloride treatment: A facile and sustainable pathway to functional cellulose nanofibers with flame-retardant and catalytic properties. ACS Sustain. Chem. Eng. 2020, 8, 13576–13582. [Google Scholar] [CrossRef]
- Guan, W.; Tsang, C.; Lin, C.S.K.; Len, C.; Hu, H.; Liang, C. A review on high catalytic efficiency of solid acid catalysts for lignin valorization. Bioresour. Technol. 2020, 298, 122432. [Google Scholar] [CrossRef] [PubMed]
- Jugwanth, Y.; Sewsynker-Sukai, Y.; Kana, G. Valorization of sugarcane bagasse for bioethanol production through simultaneous saccharification and fermentation: Optimization and kinetic studies. Fuel 2020, 262, 116552. [Google Scholar] [CrossRef]
- Zhu, J.; Pan, X. Woody biomass pretreatment for cellulosic ethanol production: Technology and energy consumption evaluation. Bioresour. Technol. 2010, 101, 4992–5002. [Google Scholar] [CrossRef]
- Ahmed, M.; Rehman, M.; Teran-Hilares, R.; Khalid, S.; Han, J. Optimization of twin gear-based pretreatment of rice straw for bioethanol production. Energy Convers. Manag. 2017, 141, 120–125. [Google Scholar] [CrossRef]
- Prasad, S.; Malav, M.; Kumar, S.; Singh, A.; Pant, D.; Radhakrishnan, S. Enhancement of bio-ethanol production potential of wheat straw by reducing furfural and 5-hydroxymethylfurfural (HMF). Bioresour. Technol. 2018, 4, 50–56. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G.; Menka, A.; Koim-Puchowska, B. Microwave-assisted pretreatment of maize distillery stillage with the use of dilute sulfuric acid in the production of cellulosic ethanol. Bioresour. Technol. 2019, 278, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.E.; Park, D.; Jeong, G. Effects of inorganic salts on pretreatment of Miscanthus straw. Bioresour. Technol. 2013, 132, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of using green adsorbent of heavy metal removal fromaqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Pantelo, A.; Luigi, T.; Elkatory, M.R.; Ali, R.M.; El Nemr, A. Enhancement of biogas production via green ZnO nanoparticles: Experimental results of selected herbaceous crops. Chem. Eng. Commun. 2021, 208, 242–255. [Google Scholar] [CrossRef]
- El-Sayed, E.M.; Hamad, H.A.; Ali, R.M. Journey from ceramic waste to highly efficient toxic dye adsorption from aqueous solutions via one-pot synthesis of CaSO4 rod-shape with silica. J. Mater. Res. Technol. 2020, 6, 16051–16063. [Google Scholar] [CrossRef]
- Ali, R.M.; Elkatory, M.R.; Hamad, H. Highly active and stable magnetically recyclable CuFe2O4 as a heterogenous catalyst for efficient conversion of waste frying oil to biodiesel. Fuel 2020, 268, 117297. [Google Scholar] [CrossRef]
- Ali, M.R.; Elkatory, M.R.; Hassaan, M.A.; Amer, K.; El Geiheini, A. Highly crystalline heterogeneous catalyst synthesis from industrial waste for sustainable biodiesel production. Egypt. J. Chem. 2020, 63, 1161–1178. [Google Scholar] [CrossRef]
- Hamadi, A.; Yeddou-Mezenner, N.; Azeddine, L.; Ali, R.M.; Hamad, H. Upgrading of agro-industrial green biomass residues from chocolate industry for adsorption process: Diffusion and mechanistic insights. J. Food Sci. Technol. 2021, 58, 1081–1092. [Google Scholar] [CrossRef]
- Hassaan, M.; Elkatory, M.R.; Ali, R.M.; El Nemr, A. Photocatalytic degradation of reactive black 5 using photo-fenton and ZnO nanoparticles under UV irradiation. Egypt. J. Chem. 2020, 63, 1443–1459. [Google Scholar] [CrossRef]
- Kamel, D.A.; Farag, H.A.; Amin, N.K.; Zaatout, A.A.; Ali, R.M. Smart utilization of jatropha (Jatropha curcas Linnaeus) seeds for biodiesel production: Optimization and mechanism. Ind. Crop. Prod. 2018, 111, 407–413. [Google Scholar] [CrossRef]
- Hamad, A.A.; Hassouna, M.S.; Shalaby, T.I.; Elkady, M.F.; Abd Elkawi, M.A.; Hamad, H.A. Electrospun cellulose acetate nanofiber incorporated with hydroxyapatite for removal of heavy metals. Int. J. Biol. Macromol. 2020, 151, 1313. [Google Scholar] [CrossRef] [PubMed]
- Bassyouni, D.; Mohamed, M.E.; El-Ashtoukhy, E.; Abd El-Latif, M.; Zaatout, A.; Hamad, H. Fabrication and characterization of electrospun Fe3O4/o-MWCNTs/polyamide 6 hybrid nanofibrous membrane composite as an efficient and recoverable adsorbent for removal of Pb (II). Microchem. J. 2019, 149, 103998. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, X.; Ma, J.; Liu, X. Preparation and characterization of novel regenerated cellulose films via sol−gel technology. Ind. Eng. Chem. Res. 2013, 52, 17900–17906. [Google Scholar] [CrossRef]
- Eltarahony, M.; Abu-Serie, M.; Hamad, H.; Zaki, S.; Abd-El-Haleem, D. Unveiling the role of novel biogenic functionalized CuFe hybrid nanocomposites in boosting anticancer, antimicrobial and biosorption activities. Sci. Rep. 2021, 11, 7790. [Google Scholar] [CrossRef] [PubMed]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite—Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Pan, X.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: Relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef]
- Jaouadi, M.; Hbaieb, S.; Guedidi, H.; Reinert, L.; Amdouni, N.; Duclaux, L. Preparation and characterization of carbons from β-cyclodextrin dehydration and from olive pomace activation and their application for boron adsorption. J. Saudi Chem. Soc. 2017, 21, 822–829. [Google Scholar] [CrossRef] [Green Version]
- Diop, C.I.K.; Lavoie, J. Isolation of nanocrystalline cellulose: A technological route for valorizing recycled tetra pak aseptic multilayered food packaging wastes. Waste Biomass Valoriz. 2017, 8, 41–56. [Google Scholar] [CrossRef]
- Hamad, H.; Bailón-García, E.; Morales-Torres, S.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. Functionalized cellulose for the controlled synthesis of novel carbon–Ti nanocomposites: Physicochemical and photocatalytic properties. Nanomaterials 2020, 10, 729. [Google Scholar] [CrossRef] [Green Version]
- Raghavi, S.; Sindhu, R.; Binod, P.; Gnansounou, E.; Pandey, A. Development of a novel sequential pretreatment strategy for the production of bioethanol from sugarcane trash. Bioresour. Technol. 2016, 199, 202–210. [Google Scholar] [CrossRef]
- Santana, J.C.; Abud, A.K.S.; Junior, A.W.; Navickiene, S.; Romao, L.P.C. Optimization of an organosolv method using glycerol with iron catalysts for the pretreatment of water hyacinth. Biomass Bioenergy 2020, 133, 105454. [Google Scholar] [CrossRef]
- Kim, Y.; Yu, A.; Han, M.; Choib, G.; Chunga, B. Ethanosolv pretreatment of barley straw with iron (III) chloride for enzymatic saccharification. J. Chem. Technol. Biotechnol. 2010, 85, 1494–1498. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Boehm, H. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]

| Substrate | Analyzed Sample | Cellulose Content, % | Lignin Content, % |
|---|---|---|---|
| Tetra Pak (TP) | TP | 64 | 10 |
| TPZ | 59 | 7 | |
| TPNZ | 59 | 3.3 | |
| Corn Stover (CS) | CS | 36 | 12 |
| CSZ | 39 | 9 | |
| CSNZ | 38 | 8 |
| Feedstock | Pretreatment Conditions | Fermentation Conditions | Biorthanol Production (g/L) | References |
|---|---|---|---|---|
| Tetra Pak (TP) | ZnCl2 and Na2HPO4 (3% w/v), 120 °C, 60 min. | 30 °C/48 h | 5 | Our study |
| Corn stover | Oxalic acid (2% w/v), 120 °C, 60 min. | 50 °C/72 h | 3.6 | [11] |
| Sugarcane bagasse | 1.73 M ZnCl2, 1.36 M NaOH, 9.69% Sla, 121 °C for 60 min | 30 °C/15 h | 4.88 | [29] |
| Rice straw | [Emim][Oac]-DMSO | - | 1.68 | [30] |
| Eucalyptus | NaOH (2–6%), 50–90 °C | 37 °C/72 h | 3.4 | [31] |
| Wheat straw | H2SO4 (2%), 180 °C; 10 min | 30 °C/72 h | 0.44 | [32] |
| Maize | Microwave-assisted H2SO4, 50 °C; 20 min | 50 °C/24 h | 0.51 | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elyamny, S.; Hamdy, A.; Ali, R.; Hamad, H. Role of Combined Na2HPO4 and ZnCl2 in the Unprecedented Catalysis of the Sequential Pretreatment of Sustainable Agricultural and Agro-Industrial Wastes in Boosting Bioethanol Production. Int. J. Mol. Sci. 2022, 23, 1777. https://doi.org/10.3390/ijms23031777
Elyamny S, Hamdy A, Ali R, Hamad H. Role of Combined Na2HPO4 and ZnCl2 in the Unprecedented Catalysis of the Sequential Pretreatment of Sustainable Agricultural and Agro-Industrial Wastes in Boosting Bioethanol Production. International Journal of Molecular Sciences. 2022; 23(3):1777. https://doi.org/10.3390/ijms23031777
Chicago/Turabian StyleElyamny, Shaimaa, Ali Hamdy, Rehab Ali, and Hesham Hamad. 2022. "Role of Combined Na2HPO4 and ZnCl2 in the Unprecedented Catalysis of the Sequential Pretreatment of Sustainable Agricultural and Agro-Industrial Wastes in Boosting Bioethanol Production" International Journal of Molecular Sciences 23, no. 3: 1777. https://doi.org/10.3390/ijms23031777
APA StyleElyamny, S., Hamdy, A., Ali, R., & Hamad, H. (2022). Role of Combined Na2HPO4 and ZnCl2 in the Unprecedented Catalysis of the Sequential Pretreatment of Sustainable Agricultural and Agro-Industrial Wastes in Boosting Bioethanol Production. International Journal of Molecular Sciences, 23(3), 1777. https://doi.org/10.3390/ijms23031777






