Collagen Peptide in a Combinatorial Treatment with Lactobacillus rhamnosus Inhibits the Cariogenic Properties of Streptococcus mutans: An In Vitro Study
Abstract
:1. Introduction
2. Results
2.1. Effects of Prebiotic Collagen Peptide and Probiotic L. rhamnosus on Bacterial Cell Growth
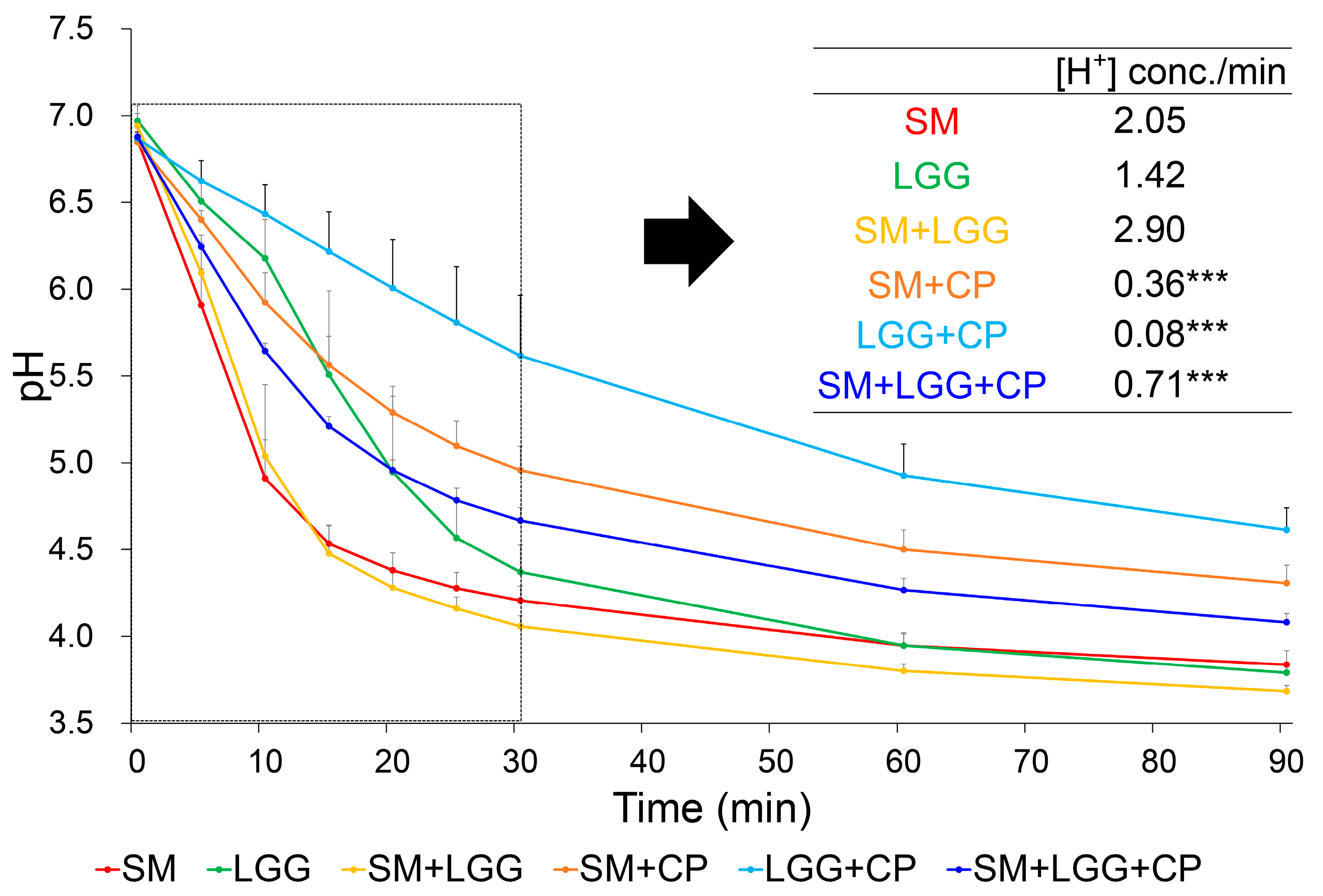
2.2. Effect of Collagen Peptide on Glycolytic Acid Production by S. mutans and L. rhamnosus
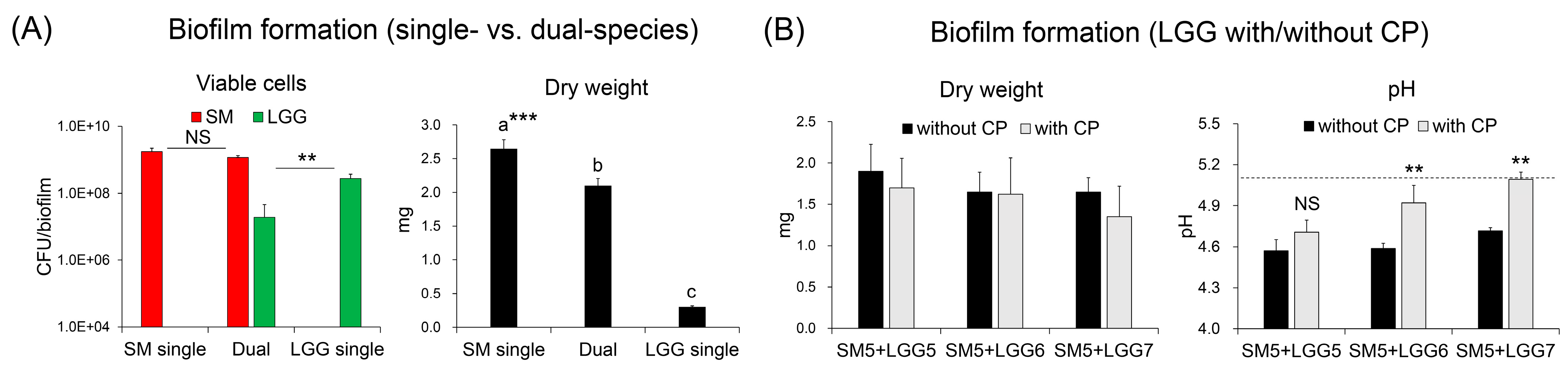
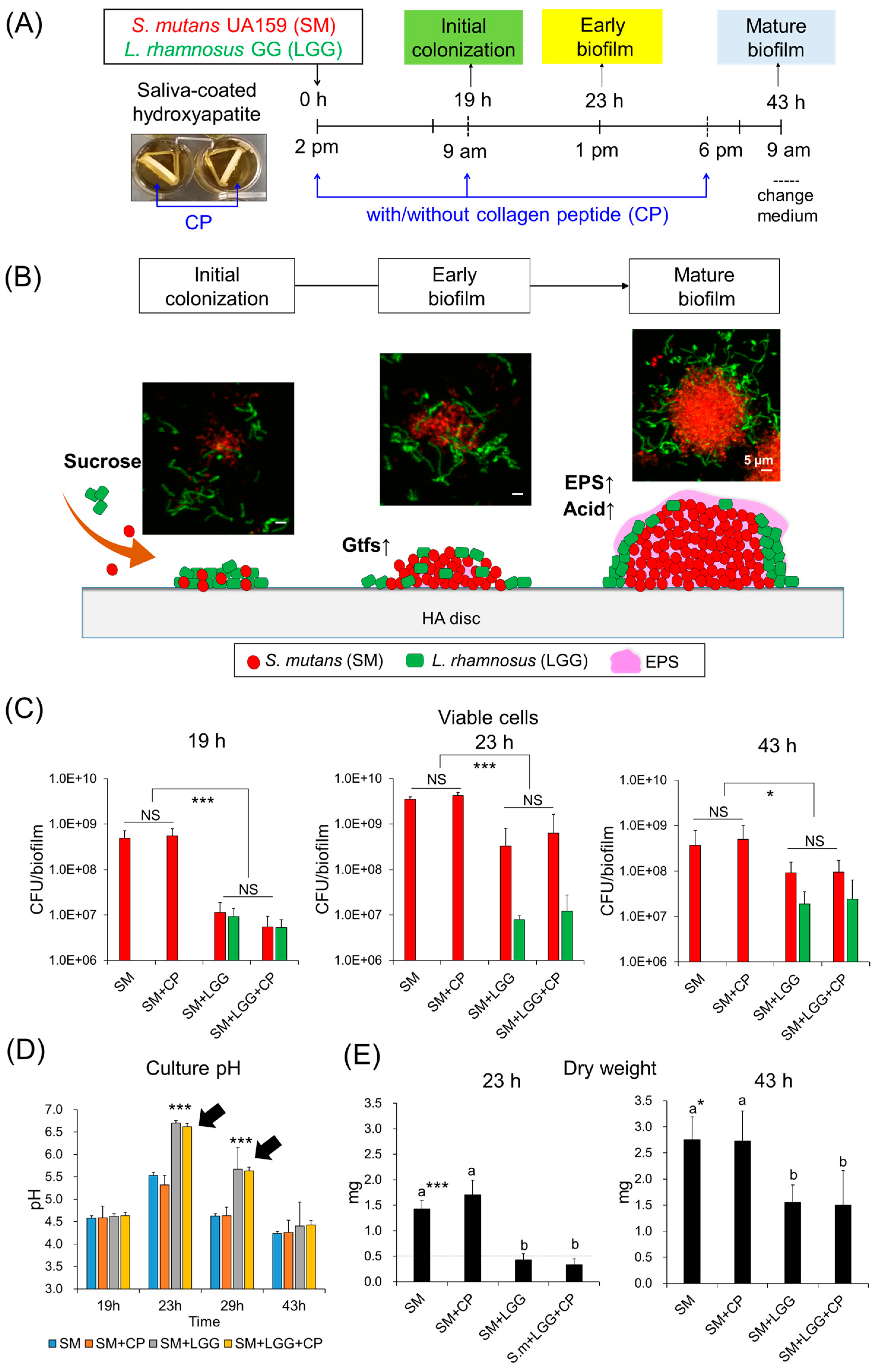
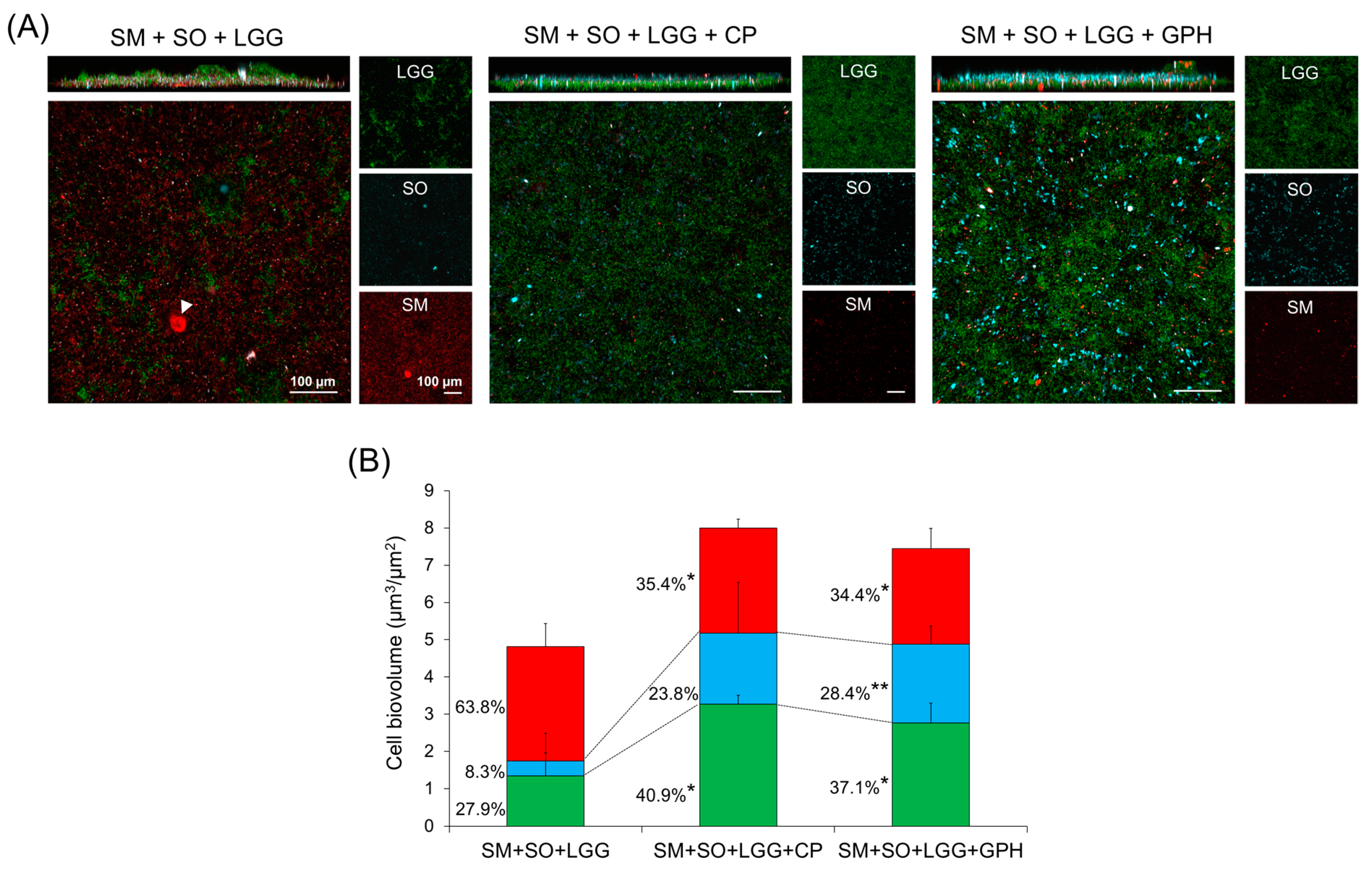
2.3. Effect of Collagen Peptide in a Combination of L. rhamnosus on the Biofilm Formation of S. mutans
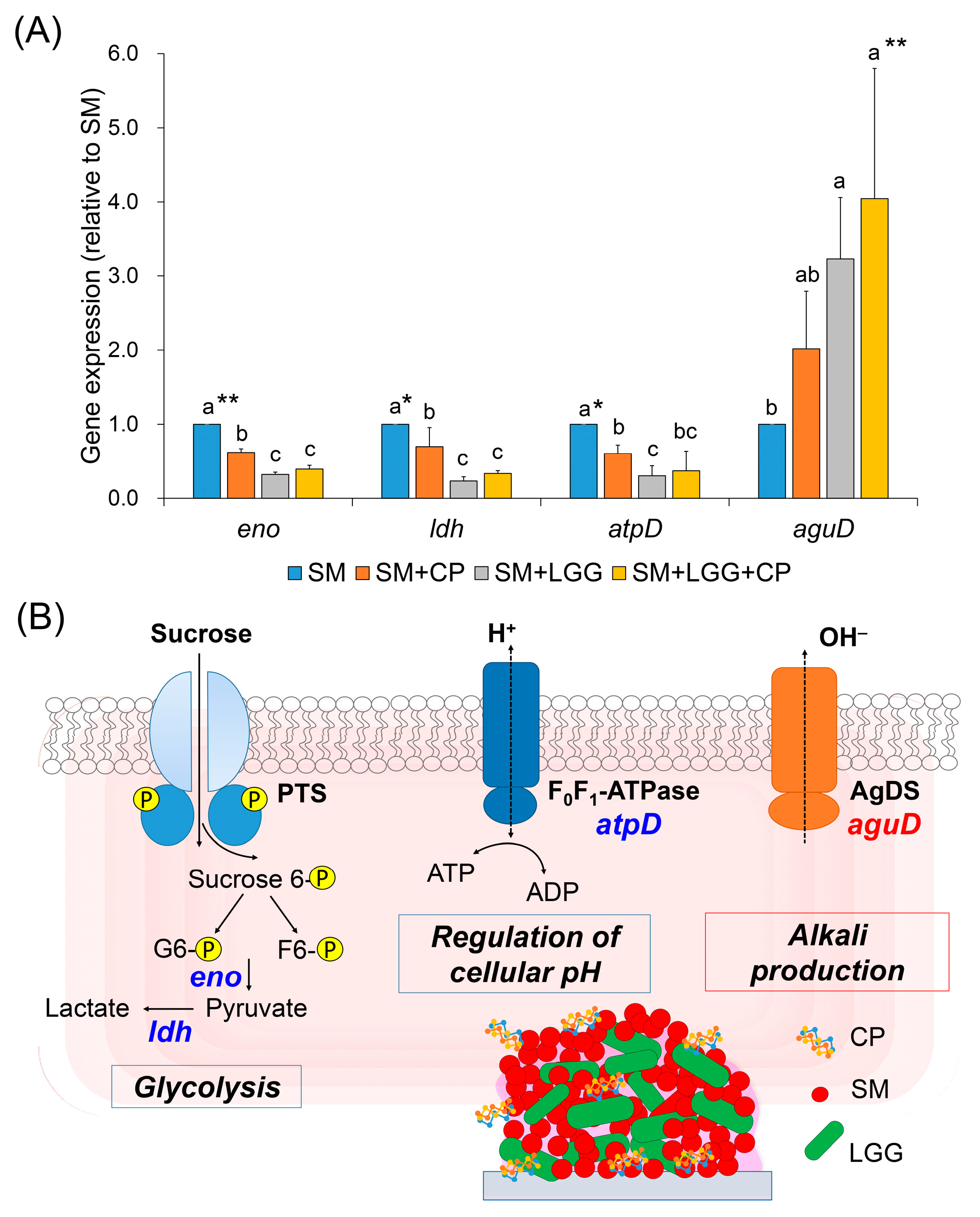
2.4. Effect of Collagen Peptide in a Combination of L. rhamnosus on the Transcriptomic Changes of S. mutans Biofilm
2.5. Effects of Glycine-Proline-Hydroxyproline (Gly-Pro-Hyp) and the Presence of L. rhamnosus on the Acid Production Ability of S. mutans
2.6. Gly-Pro-Hyp Treatment Prevented S. mutans Outgrowth and Promoted S. oralis Dominance
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Condition
4.2. Bacterial Cell Response to Collagen Peptide (CP)
4.3. Glycolytic pH Drop Assay
4.4. In Vitro Biofilm Model
4.5. Assessment of Gene Expression via qRT-PCR
4.6. Fluorescence In Situ Hybridization for Bacterial Cell Labeling in Biofilms
4.7. Confocal Laser Scanning Microscopy (CLSM)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- Peres, M.A.; Macpherson, L.M.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Hajishengallis, E.; Parsaei, Y.; Klein, M.; Koo, H. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol. Oral Microbiol. 2017, 32, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Huang, X.; Alkhers, N.; AlZamil, H.; Alzoubi, S.; Wu, T.T.; Castillo, D.A.; Campbell, F.; Davis, J.; Herzog, K.; et al. Candida albicans and Early Childhood Caries: A Systematic Review and Meta-Analysis. Caries Res. 2018, 52, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Koo, H. Spatial Design of Polymicrobial Oral Biofilm in Its Native Disease State. J. Dent. Res. 2020, 99, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Kim, D.; Liu, Y.; Benhamou, R.; Sanchez, H.; Simon-Soro, A.; Li, Y.; Hwang, G.; Fridman, M.; Andes, D.; Koo, H. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018, 12, 1427–1442. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Kim, D.; Paula, A.; Hwang, G.; Liu, Y.; Li, J.; Daniell, H.; Koo, H. Dual-Targeting Approach Degrades Biofilm Matrix and Enhances Bacterial Killing. J. Dent. Res. 2019, 98, 322–330. [Google Scholar] [CrossRef]
- Culp, D.J.; Hull, W.; Bremgartner, M.J.; Atherly, T.A.; Christian, K.N.; Killeen, M.; Dupuis, M.R.; Schultz, A.C.; Chakraborty, B.; Lee, K.; et al. In Vivo Colonization with Candidate Oral Probiotics Attenuates Streptococcus mutans Colonization and Virulence. Appl. Environ. Microbiol. 2021, 87, e02490-20. [Google Scholar] [CrossRef]
- Jiang, Q.; Kainulainen, V.; Stamatova, I.; Korpela, R.; Meurman, J.H. Lactobacillus rhamnosus GG in Experimental Oral Biofilms Exposed to Different Carbohydrate Sources. Caries Res. 2018, 52, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Fernández, C.E.; Giacaman, R.A.; Tenuta, L.M.; Cury, J.A. Effect of the Probiotic Lactobacillus rhamnosus LB21 on the Cariogenicity of Streptococcus mutans UA159 in a Dual-Species Biofilm Model. Caries Res. 2015, 49, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Toiviainen, A.; Jalasvuori, H.; Lahti, E.; Gursoy, U.; Salminen, S.; Fontana, M.; Flannagan, S.; Eckert, G.; Kokaras, A.; Paster, B.; et al. Impact of orally administered lozenges with Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 on the number of salivary mutans streptococci, amount of plaque, gingival inflammation and the oral microbiome in healthy adults. Clin. Oral Investig. 2014, 19, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, L.C.; Hoogenkamp, M.A.; Exterkate, R.A.; Terefework, Z.; De Soet, J.J.; Cate, J.M.T.; Crielaard, W.; Zaura, E. Effects of Lactobacillus rhamnosus GG on saliva-derived microcosms. Arch. Oral Biol. 2011, 56, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Aminabadi, N.A.; Erfanparast, L.; Ebrahimi, A.; Oskouei, S.G. Effect of Chlorhexidine Pretreatment on the Stability of Salivary Lactobacilli Probiotic in Six- to Twelve-Year-Old Children: A Randomized Controlled Trial. Caries Res. 2011, 45, 148–154. [Google Scholar] [CrossRef]
- Zaura, E.; Twetman, S. Critical Appraisal of Oral Pre- and Probiotics for Caries Prevention and Care. Caries Res. 2019, 53, 514–526. [Google Scholar] [CrossRef]
- Bijle, M.N.; Ekambaram, M.; Lo, E.C.M.; Yiu, C.K.Y. Synbiotics in caries prevention: A scoping review. PLoS ONE 2020, 15, e0237547. [Google Scholar] [CrossRef]
- Bijle, M.N.; Neelakantan, P.; Ekambaram, M.; Lo, E.C.M.; Yiu, C.K.Y. Effect of a novel synbiotic on Streptococcus mutans. Sci. Rep. 2020, 10, 7951. [Google Scholar] [CrossRef]
- Steinert, R.E.; Sadabad, M.S.; Harmsen, H.J.M.; Weber, P. The prebiotic concept and human health: A changing landscape with riboflavin as a novel prebiotic candidate? Eur. J. Clin. Nutr. 2016, 70, 1348–1353. [Google Scholar] [CrossRef]
- Griswold, A.R.; Jameson-Lee, M.; Burne, R.A. Regulation and Physiologic Significance of the Agmatine Deiminase System of Streptococcus mutans UA159. J. Bacteriol. 2006, 188, 834–841. [Google Scholar] [CrossRef] [Green Version]
- Kanasi, E.; Dewhirst, F.; Chalmers, N.; Kent, R., Jr.; Moore, A.; Hughes, C.; Pradhan, N.; Loo, C.; Tanner, A.C.R. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010, 44, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Eren, A.M.; Borisy, G.G.; Huse, S.M.; Welch, J.L.M. PNAS Plus: From the Cover: Oligotyping analysis of the human oral microbiome. Proc. Natl. Acad. Sci. USA 2014, 111, E2875–E2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, I.; Witkowska, E.; Kaveh, B.; Lif Holgerson, P.; Tanner, A.C.R. The Microbiome in Populations with a Low and High Prevalence of Caries. J. Dent. Res. 2016, 95, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell–cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I. Organization of supragingival plaque at the micron scale. J. Oral Microbiol. 2018, 10, 1438722. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Barraza, J.P.; Arthur, R.; Hara, A.; Lewis, K.; Liu, Y.; Scisci, E.L.; Hajishengallis, E.; Whiteley, M.; Koo, H. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc. Natl. Acad. Sci. USA 2020, 117, 12375–12386. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Koopman, J.E.; Röling, W.F.M.; Buijs, M.J.; Sissons, C.H.; Cate, J.M.T.; Keijser, B.J.F.; Crielaard, W.; Zaura, E. Stability and Resilience of Oral Microcosms Toward Acidification and Candida Outgrowth by Arginine Supplementation. Microb. Ecol. 2014, 69, 422–433. [Google Scholar] [CrossRef]
- He, J.; Hwang, G.; Liu, Y.; Gao, L.; Kilpatrick-Liverman, L.; Santarpia, P.; Zhou, X.; Koo, H. L-Arginine Modifies the Exopolysaccharide Matrix and Thwarts Streptococcus mutans Outgrowth within Mixed-Species Oral Biofilms. J. Bacteriol. 2016, 198, 2651–2661. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, M.; Browngardt, C.; Xiaohui, X.; Klepac-Ceraj, V.; Paster, B.; Burne, R. The effect of arginine on oral biofilm communities. Mol. Oral Microbiol. 2014, 29, 45–54. [Google Scholar] [CrossRef]
- Stage, M.; Wichmann, A.; Jørgensen, M.; Vera-Jimenéz, N.I.; Wielje, M.; Nielsen, D.S.; Sandelin, A.; Chen, Y.; Baker, A. Lactobacillus rhamnosus GG Genomic and Phenotypic Stability in an Industrial Production Process. Appl. Environ. Microbiol. 2020, 86, e02780-19. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Sengupta, A.; Niepa, T.H.R.; Lee, B.-H.; Weljie, A.; Freitas-Blanco, V.; Murata, R.M.; Stebe, K.J.; Lee, D.; Koo, H. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci. Rep. 2017, 7, srep41332. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.G.; Pandit, S.; Xiao, J.; Gregoire, S.; Falsetta, M.L.; Klein, M.I.; Koo, H. Influences of transtrans farnesol, a membranetargeting sesquiterpenoid, on Streptococcus mutans physiology and survival within mixedspecies oral biofilms. Int. J. Oral. Sci. 2011, 3, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Klein, M.I.; Falsetta, M.L.; Lu, B.; Delahunty, C.M.; Yates, J.R.; Heydorn, A.; Koo, H. The Exopolysaccharide Matrix Modulates the Interaction between 3D Architecture and Virulence of a Mixed-Species Oral Biofilm. PLOS Pathog. 2012, 8, e1002623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philip, N.; Suneja, B.; Walsh, L.J. Ecological Approaches to Dental Caries Prevention: Paradigm Shift or Shibboleth? Caries Res. 2018, 52, 153–165. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Kim, D.; Zhou, X.; Ahn, S.-J.; Burne, R.A.; Richards, V.; Koo, H. RNA-Seq Reveals Enhanced Sugar Metabolism in Streptococcus mutans Co-cultured with Candida albicans within Mixed-Species Biofilms. Front. Microbiol. 2017, 8, 1036. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.-G.; Klein, M.I.; Xiao, J.; Gregoire, S.; Rosalen, P.L.; Koo, H. Influences of naturally occurring agents in combination with fluoride on gene expression and structural organization of Streptococcus mutans in biofilms. BMC Microbiol. 2009, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.-N.; Jung, J.-E.; Lee, M.-H.; Choi, H.-M.; Jeon, J.-G. Sucrose challenges to Streptococcus mutans biofilms and the curve fitting for the biofilm changes. FEMS Microbiol. Ecol. 2018, 94, fiy091. [Google Scholar] [CrossRef] [Green Version]
- Cury, J.A.; Koo, H. Extraction and purification of total RNA from Sreptococcus mutans biofilms. Anal. Biochem. 2007, 365, 208–214. [Google Scholar] [CrossRef]
- Quevedo, B.; Giertsen, E.; Zijnge, V.; Lüthi-Schaller, H.; Guggenheim, B.; Thurnheer, T.; Gmür, R. Phylogenetic group- and species-specific oligonucleotide probes for single-cell detection of lactic acid bacteria in oral biofilms. BMC Microbiol. 2011, 11, 14. [Google Scholar] [CrossRef] [Green Version]


| Group | pH at 30 min | Delta pH 1 | [H+] Conc. (µM) 2 | Rate ([H+]/min) | Relative Ratio 3 |
|---|---|---|---|---|---|
| Control | 4.73 ± 0.13 c,4 | 2.26 ± 0.08 a | 19.27 ± 5.52 a | 0.64 ± 0.18 a | 1.00 ± 0.00 a |
| CP | 5.53 ± 0.28 ab | 1.18 ± 0.15 c | 3.24 ± 2.05 bc | 0.11 ± 0.07 bc | 0.15 ± 0.06 bc |
| Gly-Pro-Hyp | 5.97 ± 0.37 a | 0.75 ± 0.22 d | 1.20 ± 1.06 c | 0.04 ± 0.04 c | 0.05 ± 0.04 c |
| Gly | 5.31 ± 0.20 b | 1.44 ± 0.10 b | 5.10 ± 2.35 b | 0.17 ± 0.08 b | 0.25 ± 0.05 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.-Y.; Cai, J.-N.; Yoo, S.C.; Kim, S.-H.; Jeon, J.-G.; Kim, D. Collagen Peptide in a Combinatorial Treatment with Lactobacillus rhamnosus Inhibits the Cariogenic Properties of Streptococcus mutans: An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 1860. https://doi.org/10.3390/ijms23031860
Jung H-Y, Cai J-N, Yoo SC, Kim S-H, Jeon J-G, Kim D. Collagen Peptide in a Combinatorial Treatment with Lactobacillus rhamnosus Inhibits the Cariogenic Properties of Streptococcus mutans: An In Vitro Study. International Journal of Molecular Sciences. 2022; 23(3):1860. https://doi.org/10.3390/ijms23031860
Chicago/Turabian StyleJung, Hee-Young, Jian-Na Cai, Sung Chul Yoo, Seon-Hwa Kim, Jae-Gyu Jeon, and Dongyeop Kim. 2022. "Collagen Peptide in a Combinatorial Treatment with Lactobacillus rhamnosus Inhibits the Cariogenic Properties of Streptococcus mutans: An In Vitro Study" International Journal of Molecular Sciences 23, no. 3: 1860. https://doi.org/10.3390/ijms23031860
APA StyleJung, H.-Y., Cai, J.-N., Yoo, S. C., Kim, S.-H., Jeon, J.-G., & Kim, D. (2022). Collagen Peptide in a Combinatorial Treatment with Lactobacillus rhamnosus Inhibits the Cariogenic Properties of Streptococcus mutans: An In Vitro Study. International Journal of Molecular Sciences, 23(3), 1860. https://doi.org/10.3390/ijms23031860







