Central Nervous System Stimulants Limit Caffeine Transport at the Blood–Cerebrospinal Fluid Barrier
Abstract
:1. Introduction
2. Results
2.1. Number of Cases in Which Stimulants and Psychotropic Drugs Were Detected
2.2. Caffeine Concentration in Right Heart Blood and CSF
2.2.1. Caffeine Concentration in Groups A and B
2.2.2. Relationship between Caffein Concentration in Rs and CSF in Groups I, II, III, and IV
2.3. Permeability of Caffeine and 4-Aminopyridine in the Culture Cell Model of the BCSFB
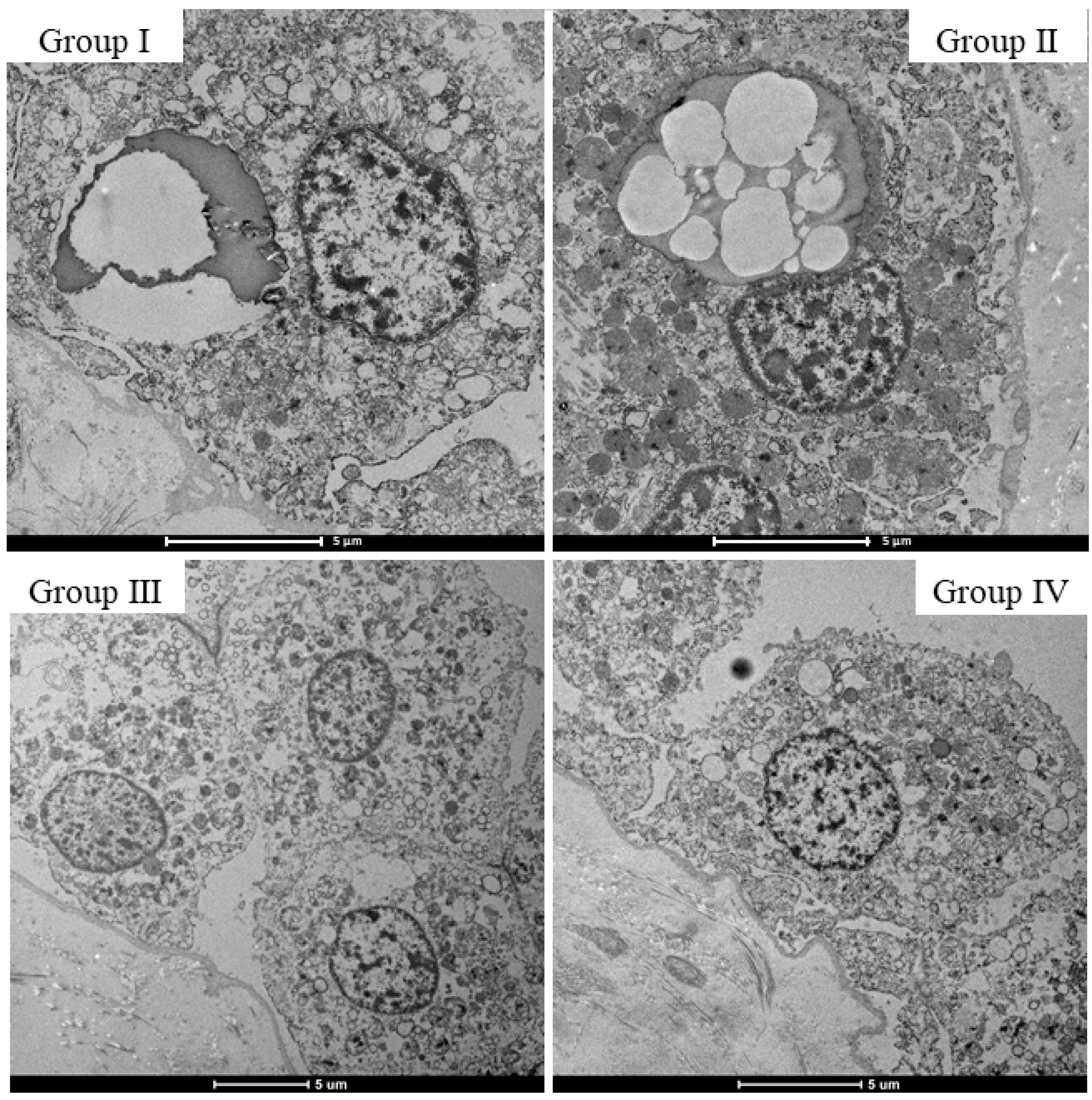
2.4. Morphological Changes in the Choroid Plexus with Drug Administration
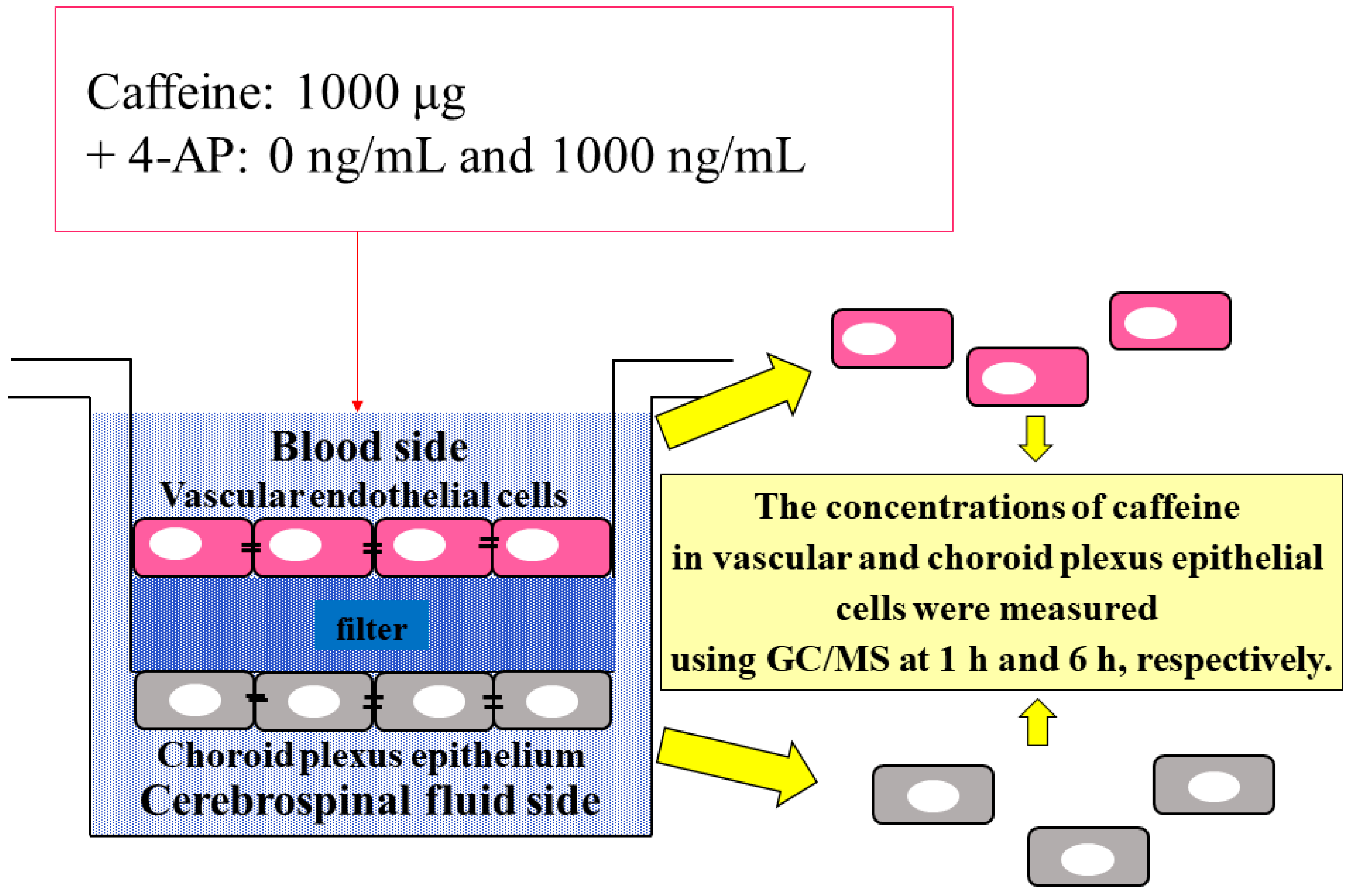
2.5. Comparison of Caffeine Concentrations in Choroid Plexus Epithelial Cells and Vascular Endothelial Cells in the BCSFB Model
3. Discussion
4. Materials and Methods
4.1. Autopsy Samples
4.2. Cell Culture Systems and Experiment
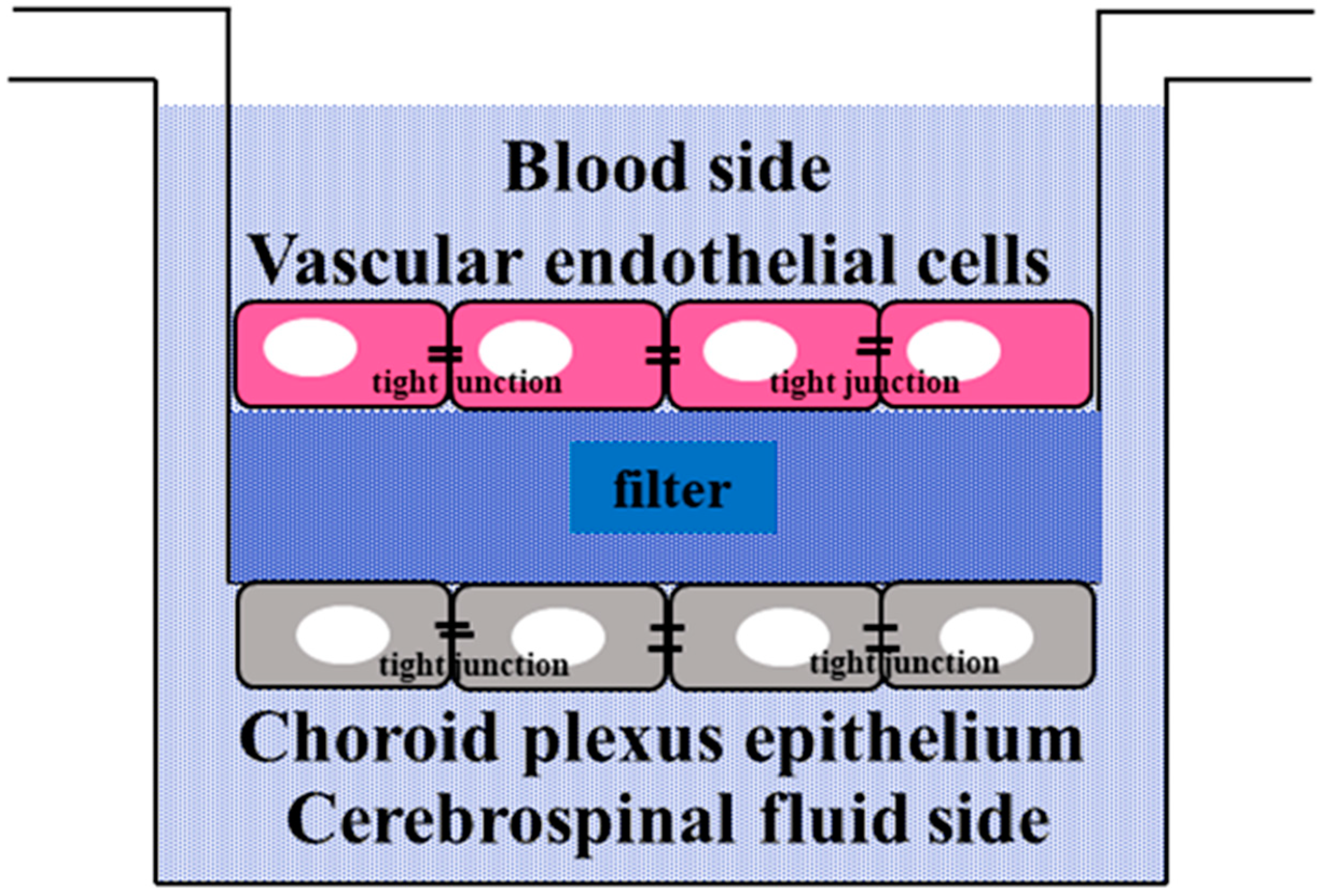
4.2.1. BCSFB Model
4.2.2. Culture Experiment
4.2.3. Caffeine Concentration in Cultured Cells
4.3. Measurement of Caffeine, Stimulants, and Psychotropic Drugs
4.3.1. Method for Preparing Reagents before Measuring Various Drugs in Autopsy Case Body Fluids and Culture Media
Caffeine and Stimulants
Psychotropic Drugs
4.3.2. Extraction Method of Various Drugs
Caffeine and Stimulants
Psychotropic Drugs (Psychiatric Drugs)
4.4. Preparation of Calibration Curves and ISs
4.5. GC/MS Equipment and Conditions
4.6. Histopathological and Micromorphological Changes
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curran, C.P.; Marczinski, C.A. Taurine, caffeine, and energy drinks: Reviewing the risks to the adolescent brain. Birth Defects Res. 2017, 109, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, J.; Yasuda, Y.; Kimura-Takaoka, K.; Hasegawa, M.; Kurata, S.; Takashita, H. Two fatal cases of caffeine poisoning and a review of the literature. Shimane J. Med. Sci. 2017, 34, 55–59. [Google Scholar]
- Yamamoto, T.; Yoshizawa, K.; Kubo, S.-I.; Emoto, Y.; Hara, K.; Waters, B.; Umehara, T.; Murase, T.; Ikematsu, K. Autopsy report for a caffeine intoxication case and review of the current literature. J. Toxicol. Pathol. 2015, 28, 33–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magdalan, J.; Zawadzki, M.; Skowronek, R.; Czuba, M.; Porębska, B.; Sozański, T.; Szpot, P. Nonfatal and fatal intoxications with pure caffeine—Report of three different cases. Forensic Sci. Med. Pathol. 2017, 13, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.E. Energy Drinks, Race, and Problem Behaviors Among College Students. J. Adolesc. Health 2008, 43, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Arria, A.M.; Caldeira, K.M.; Kasperski, S.J.; O’Grady, K.E.; Vincent, K.; Griffiths, R.R.; Wish, E.D. Increased Alcohol Consumption, Nonmedical Prescription Drug Use, and Illicit Drug Use Are Associated with Energy Drink Consumption Among College Students. J. Addict. Med. 2010, 4, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Terry-McElrath, Y.M.; O’Malley, P.; Johnston, L. Energy Drinks, Soft Drinks, and Substance Use Among United States Secondary School Students. J. Addict. Med. 2014, 8, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Peacock, A.; Bruno, R.; Ferris, J.; Winstock, A. Energy drink use frequency among an international sample of people who use drugs: Associations with other substance use and well-being. Drug Alcohol Depend. 2017, 174, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Sinchai, T.; Plasen, S.; Sanvarinda, Y.; Jaisin, Y.; Govitrapong, P.; Morales, N.P.; Ratanachamnong, P.; Plasen, D. Caffeine potentiates methamphetamine-induced toxicity both in vitro and in vivo. Neurosci. Lett. 2011, 502, 65–69. [Google Scholar] [CrossRef]
- Simola, N.; Tronci, E.; Pinna, A.; Morelli, M. Subchronic-intermittent caffeine amplifies the motor effects of amphetamine in rats. Amino Acids 2006, 31, 359–363. [Google Scholar] [CrossRef]
- Kuribara, H. Caffeine enhances the stimulant effect of methamphetamine, but may not affect induction of methamphetamine sensitization of ambulation in mice. Psychopharmacology 1994, 116, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Nieber, K. The Impact of Coffee on Health. Planta Med. 2017, 83, 1256–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Sugiyama, Y. Kinetic Analysis of the Disposition of Hydrophilic Drugs in the Central Nervous System (CNS): Prediction of the CNS Disposition from the Transport Properties in the Blood-Brain and Blood-Cerebrospinal Fluid Barriers. Yakugaku Zasshi 1994, 114, 950–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardridge, W.M. CNS Drug Design Based on Principles of Blood-Brain Barrier Transport. J. Neurochem. 1998, 70, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M. Elucidation of mechanism of blood-brain barrier damage for prevention and treatment of vascular dementia. Clin. Neurol. 2017, 57, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Strazielle, N.; Ghersi-Egea, J.-F. Physiology of Blood-Brain Interfaces in Relation to Brain Disposition of Small Compounds and Macromolecules. Mol. Pharm. 2013, 10, 1473–1491. [Google Scholar] [CrossRef]
- Alcolado, R.; Weller, R.O.; Parrish, E.P.; Garrod, D. The Cranial Arachnoid and Pia Mater in Man: Anatomical and Ultrastructural Observations. Neuropathol. Appl. Neurobiol. 1988, 14, 1–17. [Google Scholar] [CrossRef]
- Tominaga, M.; Michiue, T.; Ishikawa, T.; Inamori-Kawamoto, O.; Oritani, S.; Maeda, H. Evaluation of postmortem drug concentrations in cerebrospinal fluid compared with blood and pericardial fluid. Forensic Sci. Int. 2015, 254, 118–125. [Google Scholar] [CrossRef]
- Benowitz, N.L. Clinical Pharmacology of Caffeine. Annu. Rev. Med. 1990, 41, 277–288. [Google Scholar] [CrossRef]
- Ikeda, T.; Tani, N.; Watanabe, M.; Hirokawa, T.; Ikeda, K.; Morioka, F.; Ishikawa, T. Evaluation of cytokines and structural proteins to analyze the pathology of febrile central nervous system disease. Leg. Med. 2021, 51, 101864. [Google Scholar] [CrossRef]
- Liang, Z.; Yin, P.; Zhao, L. Effects of combined toxicity of methamphetamine and ketamine on apoptosis, oxidative stress and genotoxicity in HepG2 cells. Food Chem. Toxicol. 2019, 132, 110653. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Wiley, C.; Randell, K.; Darling-Reed, S.F.; Latinwo, L.M.; Agharahimi, M.; Soliman, K.F.A.; Goodman, C.B. Identification of cytotoxic markers in methamphetamine treated rat C6 astroglia-like cells. Sci. Rep. 2019, 9, 9412. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.; Koska, V.; Hecker, C.; Göttle, P.; Hilla, A.; Heskamp, A.; Lepka, K.; Issberner, A.; Hallenberger, A.; Baksmeier, C.; et al. Protective effects of 4-aminopyridine in experimental optic neuritis and multiple sclerosis. Brain 2020, 143, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Randall, C.; Baselt, R.C. Disposition of Toxic Drugs and Chemicals in Man, 9th ed.; Biomedical Publications: Seal Beach, CA, USA, 2011; pp. 63–64. [Google Scholar]
- Luca, C.C.; Singer, C. Can 4-aminopyridine modulate dysfunctional gait networks in Parkinson’s disease? Park. Relat. Disord. 2013, 19, 777–782. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.; Menke, N.B.; Katz, K.D.; Pizon, A. 4-Aminopyridine Toxicity: A Case Report and Review of the Literature. J. Med. Toxicol. 2012, 8, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, A.J.; Domínguez-Pinos, M.-D.; Guerra, M.M.; Fernández-Llebrez, P.; Pérez-Fígares, J.-M. Structure and function of the ependymal barrier and diseases associated with ependyma disruption. Tissue Barriers 2014, 2, e28426. [Google Scholar] [CrossRef]
- Johanson, C.; Stopa, E.; McMillan, P.; Roth, D.; Funk, J.; Krinke, G. The Distributional Nexus of Choroid Plexus to Cerebrospinal Fluid, Ependyma and Brain: Toxicologic/Pathologic Phenomena, Periventricular Destabilization, and Lesion Spread. Toxicol. Pathol. 2010, 39, 186–212. [Google Scholar] [CrossRef]
- Keep, R.F.; Jones, H.C.; Drewes, L.R. This was the year that was: Brain barriers and brain fluid research in 2019. Fluids Barriers CNS 2020, 17, 1–14. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005, 76, 22–76. [Google Scholar] [CrossRef]
- Girardin, F. Membrane transporter proteins: A challenge for CNS drug development. Dialog. Clin. Neurosci. 2006, 8, 311–321. [Google Scholar] [CrossRef]
- Zhou, K.; Khokhar, J.Y.; Zhao, B.; Tyndale, R.F. First demonstration that brain CYP2D-mediated opiate metabolic activation alters analgesia in vivo. Biochem. Pharmacol. 2013, 85, 1848–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanger, U.M.; Raimundo, S.; Eichelbaum, M. Cytochrome P450 2D6: Overview and update on pharmacology, genetics, biochemistry. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Fukuno, S.; Suzuki, H.; Konishi, H. Higher gene expression of CYP1A2, 2B1 and 2D2 in the brain of female compared with male rats. Die Pharm. Int. J. Pharm. Sci. 2016, 71, 334–336. [Google Scholar] [CrossRef]
- Soo, J.Y.; Wiese, M.; Dyson, R.M.; Gray, C.L.; Clarkson, A.N.; Morrison, J.L.; Berry, M.J. Methamphetamine administration increases hepatic CYP1A2 but not CYP3A activity in female guinea pigs. PLoS ONE 2020, 15, e0233010. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, A.; Blight, A. In vitro evaluation of the effects of 4-aminopyridine on cytochrome P450 enzymes. J. Drug Assess. 2012, 2, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Valois, A.A.; Webster, W.S. The choroid plexus as a target site for cadmium toxicity following chronic exposure in the adult mouse: An ultrastructural study. Toxicology 1989, 55, 193–205. [Google Scholar] [CrossRef]
- Koizumi, H.; Watanabe, M.; Numata, H.; Sakai, T.; Morishita, H. Species differences in vacuolation of the choroid plexus induced by the piperidine-ring drug disobutamide in the rat, dog, and monkey. Toxicol. Appl. Pharmacol. 1986, 84, 125–148. [Google Scholar] [CrossRef]
- Solinas, M.; Ferré, S.; You, Z.-B.; Karcz-Kubicha, M.; Popoli, P.; Goldberg, S.R. Caffeine Induces Dopamine and Glutamate Release in the Shell of the Nucleus Accumbens. J. Neurosci. 2002, 22, 6321–6324. [Google Scholar] [CrossRef]
- Okada, M.; Kiryu, K.; Kawata, Y.; Mizuno, K.; Wada, K.; Tasaki, H.; Kaneko, S. Determination of the effects of caffeine and carbamazepine on striatal dopamine release by in vivo microdialysis. Eur. J. Pharmacol. 1997, 321, 181–188. [Google Scholar] [CrossRef]
- Morgan, M.E.; Vestal, R.E. Methylxanthine effects on caudate dopamine release as measured by in vivo electrochemistry. Life Sci. 1989, 45, 2025–2039. [Google Scholar] [CrossRef]
- Nagai, T. Study on the mechanism of neuropsychological abnormalities induced by drugs of abuse. Folia Pharmacol. Jpn. 2007, 129, 354–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulzer, D.; Chen, T.K.; Lau, Y.Y.; Kristensen, H.; Rayport, S.; Ewing, A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J. Neurosci. 1995, 15, 4102–4108. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M.; Diaz-Corrales, F.J.; Fukuda, M.; Kitaichi, K.; Miyoshi, K.; Ogawa, N. Methamphetamine-induced dopaminergic neurotoxicity is regulated by quinone formation-related molecules. FASEB J. 2006, 20, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Pubill, D.; Chipana, C.; Camins, A.; Pallàs, M.; Camarasa, J.; Escubedo, E. Free radical production induced by methamphetamine in rat striatal synaptosomes. Toxicol. Appl. Pharmacol. 2005, 204, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Lunet, N.; Santos, C.; Santos, J.; Vaz-Carneiro, A. Caffeine Exposure and the Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis of Observational Studiess. J. Alzheimer’s Dis. 2010, 20, S221–S238. [Google Scholar] [CrossRef] [Green Version]
- Nakaso, K.; Ito, S.; Nakashima, K. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells. Neurosci. Lett. 2008, 432, 146–150. [Google Scholar] [CrossRef]
- Ishiwata, I.; Ishiwata, C.; Ishiwata, E.; Sato, Y.; Kiguchi, K.; Tachibana, T.; Hashimoto, H.; Ishikawa, H. Establishment and characterization of a human malignant choroids plexus papilloma cell line (HIBCPP). Hum. Cell 2008, 18, 67–72. [Google Scholar] [CrossRef]
- Dinner, S.; Borkowski, J.; Stump-Guthier, C.; Ishikawa, H.; Tenenbaum, T.; Schroten, H.; Schwerk, C. A Choroid Plexus Epithelial Cell-based Model of the Human Blood-Cerebrospinal Fluid Barrier to Study Bacterial Infection from the Basolateral Side. J. Vis. Exp. 2016, 111, e54061. [Google Scholar] [CrossRef] [Green Version]
- Tani, N.; Ikeda, T.; Watanabe, M.; Toyomura, J.; Ohyama, A.; Ishikawa, T. Prolactin selectively transported to cerebrospinal fluid from blood under hypoxic/ischemic conditions. PLoS ONE 2018, 13, e0198673. [Google Scholar] [CrossRef] [Green Version]
- Schwerk, C.; Papandreou, T.; Schuhmann, D.; Nickol, L.; Borkowski, J.; Steinmann, U.; Quednau, N.; Stump, C.; Weiss, C.; Berger, J.; et al. Polar Invasion and Translocation of Neisseria meningitidis and Streptococcus suis in a Novel Human Model of the Blood-Cerebrospinal Fluid Barrier. PLoS ONE 2012, 7, e30069. [Google Scholar] [CrossRef]
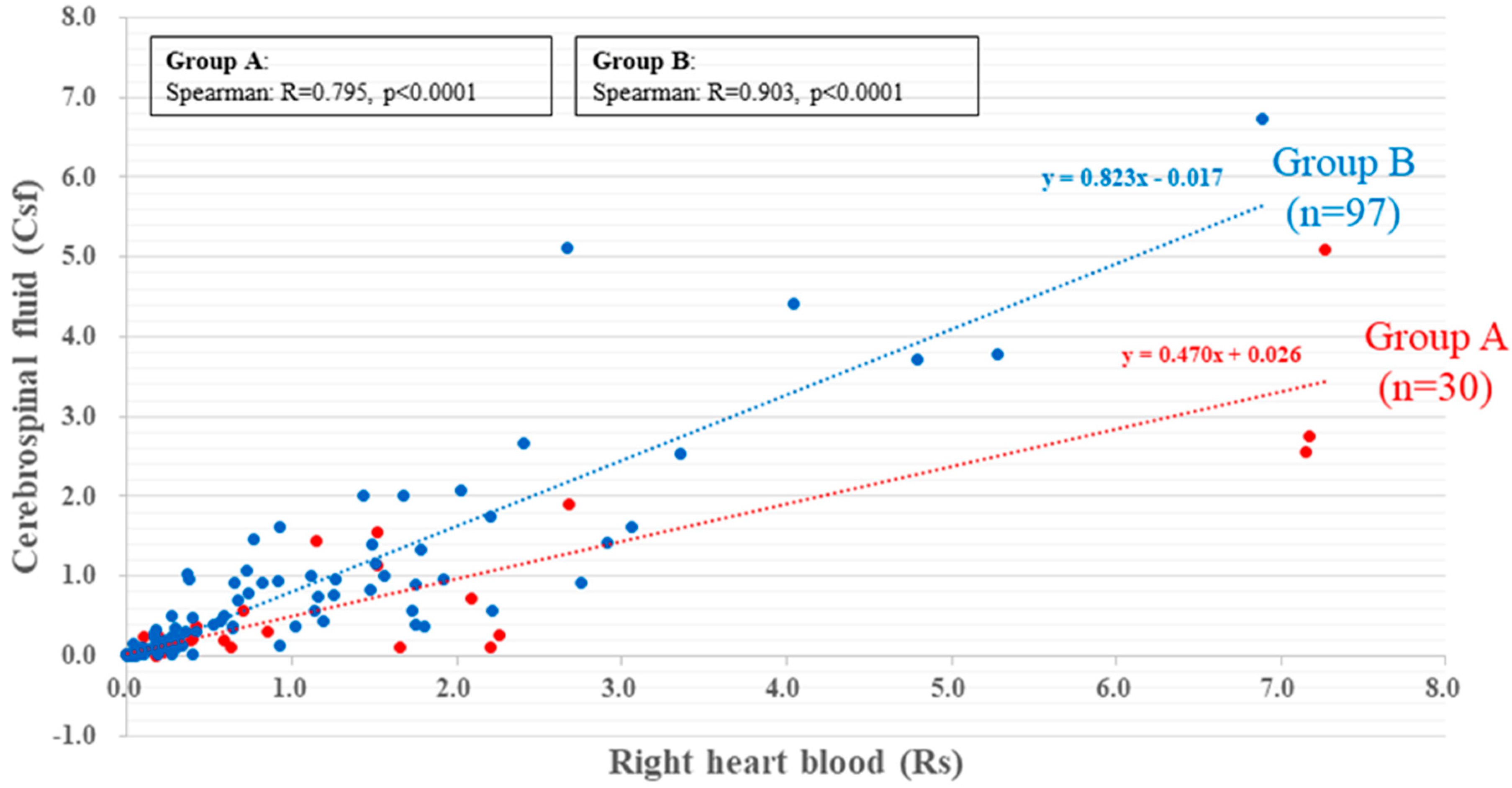





| Rs Level Range (µg/mL) (Mean/Median) | CSF Level Range (µg/mL) (Mean/Median) | Rs (x) CSF (y) | Rs Level Range (µg/mL) (Mean/Median) | CSF Level Range (µg/mL) (Mean/Median) | Rs (x) CSF (y) | ||
|---|---|---|---|---|---|---|---|
| Group A (n = 30) | Group Ⅰ: stimulants only (n = 17) | 0–7.27 (1.40/0.50) | 0–5.1 (0.68/0.21) | y = 0.470x + 0.026 (n = 30, r = 0.795, p < 0.0001) | 0–7.17 (1.41/0.85) | 0.01–2.75 (0.61/0.27) | y = 0.366x + 0.093 (n = 17, r = 0.651, p < 0.01) |
| Group Ⅱ: stimulants + psychotropic drugs(n = 13) | 0–7.27 (1.39/0.29) | 0–5.1 (0.78/0.18) | y = 0.532x + 0.044 (n = 13, r = 0.928, p < 0.0001) | ||||
| Group B (n = 97) | Group Ⅲ: psychotropic drugs (n = 26) | 0–6.89 (0.93/0.4) | 0–6.74 (0.75/0.34) | y = 0.823x − 0.017 (n = 97, r = 0.903, p < 0.0001) | 0–6.89 (1.39/0.77) | 0–5.12 (1.17/0.63) | y = 0.668x + 0.247 (n = 26, r = 0.917, p < 0.0001) |
| Group Ⅳ: Drug non-detection (n = 71) | 0–5.28 (0.76/0.31) | 0–3.78 (0.55/0.25) | y = 0.669x + 0.036 (n = 71. r = 0.884, p < 0.0001) |
| 4-AP Concentration (ng/mL) | 0 | 1 | 10 | 100 | 1000 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elapsed Time (h) | 1 | 3 | 6 | 1 | 3 | 6 | 1 | 3 | 6 | 1 | 3 | 6 | 1 | 3 | 6 | |
| Sample No. | No. 1 | 75.82 | 233.39 | 404.31 | 65.81 | 121.89 | 241.85 | 50.35 | 128.63 | 139.17 | 51.04 | 89.3 | 98.87 | 24.17 | 34.83 | 67.24 |
| 158.62 | 313.4 | 275.88 | 76.26 | 100.68 | 101.4 | 71.22 | 49.56 | 57.08 | 28.28 | 48.76 | 74.4 | 22.68 | 27.62 | 28.88 | ||
| No. 2 | 87.49 | 191.95 | 210.16 | 86.6 | 87.39 | 138.38 | 45.04 | 98.66 | 183.76 | 31.54 | 55.44 | 104 | 33.65 | 53.83 | 79.97 | |
| 127.16 | 148.46 | 157.52 | 106.1 | 139.12 | 188.56 | 62.02 | 60.44 | 75.86 | 35.58 | 65.5 | 46 | 19 | 37.08 | 29.46 | ||
| No. 3 | 113.69 | 348.85 | 359.48 | 71.72 | 56.69 | 128.75 | 42.05 | 64.81 | 62.42 | 25.65 | 74.21 | 145.77 | 26.51 | 48.98 | 50.96 | |
| 79.8 | 193.26 | 183.12 | 91.86 | 35.56 | 66.98 | 45.84 | 134.94 | 82.96 | 24.06 | 38.64 | 70.14 | 13.94 | 19.24 | 29.54 | ||
| Caffeine 1000 µg, 4-AP 0 ng/mL (Control) | Caffeine 1000 µg, 4-AP 1000 ng/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 6 h | 1 h | 6 h | ||||||
| Vascular Endothelial Cells | Choroid Plexus Epithelial Cells | Vascular Endothelial Cells | Choroid Plexus Epithelial Cells | Vascular Endothelial Cells | Choroid plexus epithelial cells | Vascular Endothelial Cells | Choroid Plexus Epithelial Cells | ||
| Sample No. | No. 1 | 0.77 | 1.86 | 1.29 | 2.48 | 0.87 | 0.56 | 0.36 | 3.15 |
| No. 2 | 0.12 | 0.25 | 0.41 | 1.75 | 0.19 | 0.24 | 0.19 | 1.69 | |
| No. 3 | 0.19 | 0.36 | 0.2 | 0.62 | 0.14 | 0.16 | 0.95 | 1.45 | |
| No. 4 | 0.18 | 1.16 | 0.13 | 0.57 | 0.14 | 0.14 | 0.64 | 1.03 | |
| No. 5 | 0.09 | 0.15 | 0.12 | 0.13 | 0.10 | 0.17 | 0.31 | 0.44 | |
| Group | Cause of Death | Sex (M/F) | Age (Mean) | Survival Period | Postmortem Period (Mean, h) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group A Stimulants detected | Group Ⅰ Stimulants only n = 17 | Blunt injury | n = 1 | Fire-related death | n = 1 | 16/1 | 34–85 (55) | acute | n = 10 | 20.2–61.3 (38) |
| Asphyxia | n = 3 | Hyperthermia | n = 1 | subacute | n = 7 | |||||
| Drowning | n = 1 | Other endogenous death | n = 4 | |||||||
| Intoxication | n = 6 | |||||||||
| Group Ⅱ Stimulants + Psychiatric drugs n = 13 | 8/5 | 26–75 (47.2) | acute | n = 4 | 17.7–61 (38.2) | |||||
| Intoxication | n = 12 | subacute | n = 6 | |||||||
| Blunt injury | n = 1 | prolonged | n = 2 | |||||||
| unknown | n = 1 | |||||||||
| Group BStimulants no-detected | Group Ⅲ Psychiatric drugs only n = 26 | Blunt injury | n = 3 | Fire-related death | n = 4 | 12/14 | 3–78 (52.7) | acute | n = 7 | 9.2–63 (36.8) |
| Sharp instrument injury | n = 3 | Other endogenous death | n = 4 | subacute | n = 9 | |||||
| Asphyxia | n = 3 | prolonged | n = 8 | |||||||
| Intoxication | n = 9 | unknown | n = 2 | |||||||
| Group Ⅳ Drug nondetection n = 71 | Blunt injury | n = 7 | Hyperthermia | n = 5 | 49/22 | 0–96 (62) | acute | n = 30 | 5–64 (33) | |
| Sharp instrument injury | n = 3 | Hypothermia | n = 4 | subacute | n = 25 | |||||
| Asphyxia | n = 13 | Acute cardiac death | n = 7 | prolonged | n = 7 | |||||
| Fire-related death | n = 8 | Other endogenous death | n = 16 | unknown | n = 9 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda-Murakami, K.; Tani, N.; Ikeda, T.; Aoki, Y.; Ishikawa, T. Central Nervous System Stimulants Limit Caffeine Transport at the Blood–Cerebrospinal Fluid Barrier. Int. J. Mol. Sci. 2022, 23, 1862. https://doi.org/10.3390/ijms23031862
Ikeda-Murakami K, Tani N, Ikeda T, Aoki Y, Ishikawa T. Central Nervous System Stimulants Limit Caffeine Transport at the Blood–Cerebrospinal Fluid Barrier. International Journal of Molecular Sciences. 2022; 23(3):1862. https://doi.org/10.3390/ijms23031862
Chicago/Turabian StyleIkeda-Murakami, Kei, Naoto Tani, Tomoya Ikeda, Yayoi Aoki, and Takaki Ishikawa. 2022. "Central Nervous System Stimulants Limit Caffeine Transport at the Blood–Cerebrospinal Fluid Barrier" International Journal of Molecular Sciences 23, no. 3: 1862. https://doi.org/10.3390/ijms23031862
APA StyleIkeda-Murakami, K., Tani, N., Ikeda, T., Aoki, Y., & Ishikawa, T. (2022). Central Nervous System Stimulants Limit Caffeine Transport at the Blood–Cerebrospinal Fluid Barrier. International Journal of Molecular Sciences, 23(3), 1862. https://doi.org/10.3390/ijms23031862







