Transferrin Saturation/Hepcidin Ratio Discriminates TMPRSS6-Related Iron Refractory Iron Deficiency Anemia from Patients with Multi-Causal Iron Deficiency Anemia
Abstract
1. Introduction
2. Results
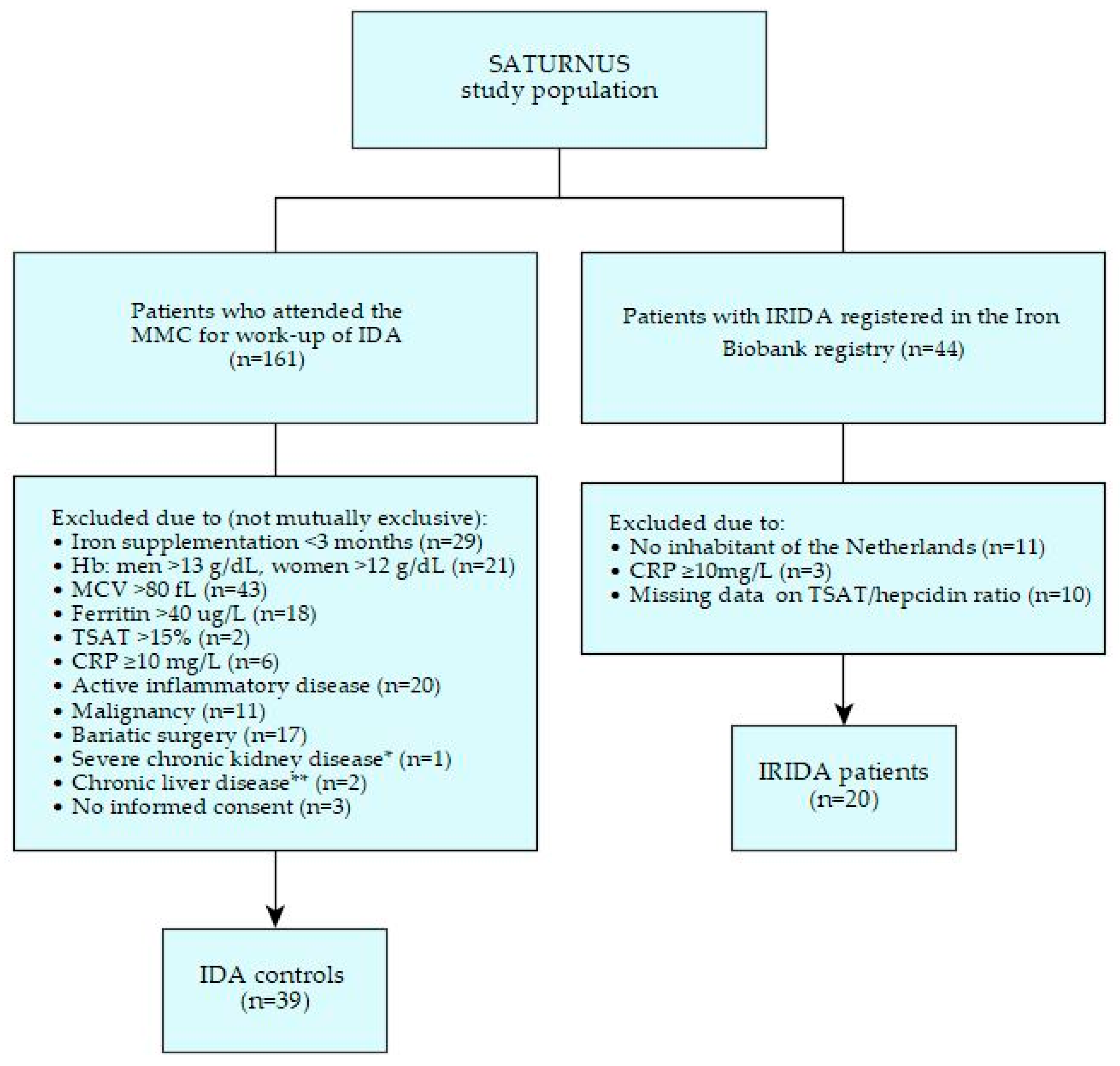
2.1. Patient Characteristics
2.1.1. IRIDA Patients
2.1.2. IDA Controls
2.1.3. Comparison of Baseline Characteristics
2.2. TSAT, Hepcidin Levels, and TSAT/Hepcidin Ratios
2.3. Diagnostic Properties of the TSAT/Hepcidin Ratio
2.4. Sensitivity Analyses
3. Discussion
4. Materials and Methods
4.1. IRIDA Patients
4.2. IDA Controls
4.3. Laboratory Measurements
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, L.; Pagani, A.; Nai, A.; De Domenico, I.; Kaplan, J.; Camaschella, C. The Serine Protease Matriptase-2 (TMPRSS6) Inhibits Hepcidin Activation by Cleaving Membrane Hemojuvelin. Cell Metab. 2008, 8, 502–511. [Google Scholar] [CrossRef]
- Silvestri, L.; Nai, A.; Dulja, A.; Pagani, A. Hepcidin and the BMP-SMAD pathway: An unexpected liaison. Vitam. Horm. 2019, 110, 71–99. [Google Scholar] [CrossRef] [PubMed]
- E Finberg, K.; Heeney, M.; Campagna, D.R.; Aydınok, Y.; A Pearson, H.; Hartman, K.R.; Mayo, M.M.; Samuel, S.M.; Strouse, J.J.; Markianos, K.; et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat. Genet. 2008, 40, 569–571. [Google Scholar] [CrossRef]
- Silvestri, L.; Guillem, F.; Pagani, A.; Nai, A.; Oudin, C.; Silva, M.; Toutain, F.; Kannengiesser, C.; Beaumont, C.; Camaschella, C.; et al. Molecular mechanisms of the defective hepcidin inhibition in TMPRSS6 mutations associated with iron-refractory iron deficiency anemia. Blood 2009, 113, 5605–5608. [Google Scholar] [CrossRef]
- De Falco, L.; Sanchez, M.; Silvestri, L.; Kannengiesser, C.; Muckenthaler, M.; Iolascon, A.; Gouya, L.; Camaschella, C.; Beaumont, C. Iron refractory iron deficiency anemia. Haematol. 2013, 98, 845–853. [Google Scholar] [CrossRef]
- Girelli, D.; Nemeth, E.; Swinkels, D.W. Hepcidin in the diagnosis of iron disorders. Blood 2016, 127, 2809–2813. [Google Scholar] [CrossRef]
- Heeney, M.; Finberg, K.E. Iron-Refractory Iron Deficiency Anemia (IRIDA). Hematol. Clin. N. Am. 2014, 28, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.A.; Cau, M.; Congiu, R.; Sole, G.; Barella, S.; Cao, A.; Westerman, M.; Cazzola, M.; Galanello, R. A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematol. 2008, 93, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Donker, A.E.; Schaap, C.C.; Novotny, V.M.J.; Smeets, R.; Peters, T.; Heuvel, B.L.P.V.D.; Raphael, M.F.; Rijneveld, A.W.; Appel, I.M.; Vlot, A.J.; et al. Iron refractory iron deficiency anemia: A heterogeneous disease that is not always iron refractory. Am. J. Hematol. 2016, 91, E482–E490. [Google Scholar] [CrossRef]
- Donker, A.E.; Raymakers, R.A.P.; Vlasveld, L.T.; Van Barneveld, T.; Terink, R.; Dors, N.; Brons, P.P.T.; Knoers, N.V.A.M.; Swinkels, D.W. Practice guidelines for the diagnosis and management of microcytic anemias due to genetic disorders of iron metabolism or heme synthesis. Blood 2014, 123, 3873–3886. [Google Scholar] [CrossRef] [PubMed]
- Heeney, M.M.; Guo, D.; De Falco, L.; Campagna, D.R.; Olbina, G.; Kao, P.P.-C.; Schmitz-Abe, K.; Rahimov, F.; Gutschow, P.; Westerman, K.; et al. Normalizing hepcidin predicts TMPRSS6 mutation status in patients with chronic iron deficiency. Blood 2018, 132, 448–452. [Google Scholar] [CrossRef]
- Heeney, M.M.; Campagna, D.R.; Westerman, M.; Fleming, M.D. The Clinical and Genetic Spectrum of TMPRSS6 Mutations Leading to Inappropriate Hepcidin Expression and Iron Refractory Iron Deficiency Anemia (IRIDA). Blood 2009, 114, 629. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Troesch, B.; Biebinger, R.; Egli, I.; Zeder, C.; Hurrell, R.F. Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am. J. Clin. Nutr. 2009, 90, 1280–1287. [Google Scholar] [CrossRef]
- Bregman, D.B.; Morris, D.; Koch, T.A.; He, A.; Goodnough, L.T. Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. Am. J. Hematol. 2013, 88, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef]
- Stoffel, N.U.; Lazrak, M.; Bellitir, S.; El Mir, N.; El Hamdouchi, A.; Barkat, A.; Zeder, C.; Moretti, D.; Aguenaou, H.; Zimmermann, M.B. The opposing effects of acute inflammation and iron deficiency anemia on serum hepcidin and iron absorption in young women. Haematol. 2019, 104, 1143–1149. [Google Scholar] [CrossRef]
- Camaschella, C. Iron deficiency: New insights into diagnosis and treatment. Hematol. 2015, 2015, 8–13. [Google Scholar] [CrossRef]
- Stoffel, N.U.; Zeder, C.; Brittenham, G.M.; Moretti, D.; Zimmermann, M.B. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematol. 2020, 105, 1232–1239. [Google Scholar] [CrossRef]
- Diepeveen, L.E.; Laarakkers, C.M.; Martos, G.; Pawlak, M.E.; Uğuz, F.F.; Verberne, K.E.; van Swelm, R.P.; Klaver, S.; de Haan, A.F.; Pitts, K.R.; et al. Provisional standardization of hepcidin assays: Creating a traceability chain with a primary reference material, candidate reference method and a commutable secondary reference material. Clin. Chem. Lab. Med. (CCLM) 2019, 57, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Aune, E.T.; Diepeveen, L.E.; Laarakkers, C.M.; Klaver, S.; Armitage, A.E.; Bansal, S.; Chen, M.; Fillet, M.; Han, H.; Herkert, M.; et al. Optimizing hepcidin measurement with a proficiency test framework and standardization improvement. Clin. Chem. Lab. Med. (CCLM) 2021, 59, 315–323. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Body Mass Index (BMI). Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 23 May 2021).
- Stoffel, N.U.; El-Mallah, C.; Herter-Aeberli, I.; Bissani, N.; Wehbe, N.; Obeid, O.; Zimmermann, M.B. The effect of central obesity on inflammation, hepcidin, and iron metabolism in young women. Int. J. Obes. 2020, 44, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Schrier, S.L. So you know how to treat iron deficiency anemia. Blood 2015, 126, 1971. [Google Scholar] [CrossRef]
- Khuong-Quang, D.-A.; Schwartzentruber, J.; Westerman, M.; Lepage, P.; Finberg, K.E.; Majewski, J.; Jabado, N. Iron Refractory Iron Deficiency Anemia: Presentation With Hyperferritinemia and Response to Oral Iron Therapy. Pediatr. 2013, 131, e620–e625. [Google Scholar] [CrossRef]
- Arsenault, V.; Mailloux, C.; Bonnefoy, A.; Lemyre, E.; Pastore, Y. Iron-Refractory Iron Deficiency Anemia May Not Lead to Neurocognitive Dysfunction: A Case Report. Pediatr. 2016, 138, 20153608. [Google Scholar] [CrossRef]
- McDonald, C.; Ostini, L.; Bennett, N.; Subramaniam, N.; Hooper, J.; Velasco, G.; Wallace, D.; Subramaniam, V.N. Functional analysis of matriptase-2 mutations and domains: Insights into the molecular basis of iron-refractory iron deficiency anemia. Am. J. Physiol. Physiol. 2015, 308, C539–C547. [Google Scholar] [CrossRef]
- Hepcidinanalysis. Reference Values WCX-TOF MS for Serum Hepcidin-25. Available online: http://www.hepcidinanalysis.com/provided-service/reference-values/ (accessed on 20 August 2021).
- Donker, A.E.; Galesloot, T.E.; Laarakkers, C.M.; Klaver, S.M.; Bakkeren, D.L.; Swinkels, D.W. Standardized serum hepcidin values in Dutch children: Set point relative to body iron changes during childhood. Pediatr. Blood Cancer 2019, 67, e28038. [Google Scholar] [CrossRef]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef]
- Theurl, I.; Aigner, E.; Theurl, M.; Nairz, M.; Seifert, M.; Schroll, A.; Sonnweber, T.; Eberwein, L.; Witcher, D.R.; Murphy, A.T.; et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: Diagnostic and therapeutic implications. Blood 2009, 113, 5277–5286. [Google Scholar] [CrossRef]
- Manders, P.; Lutomski, J.E.; Smit, C.; Swinkels, D.W.; Zielhuis, G.A. The Radboud Biobank: A Central Facility for Disease-Based Biobanks to Optimise Use and Distribution of Biomaterial for Scientific Research in the Radboud University Medical Center, Nijmegen. Open J. Bioresour. 2018, 5, 2. [Google Scholar] [CrossRef]
- Wallis, Y.; Payne, S.; McAnulty, C.; Bodmer, D.; Sister-mans, E.; Robertson, K.; Moore, D.; Abbs, S.; Deans, Z.; Devereau, A. Practice Guidelines for the Evaluation of Pathogenicity and the Reporting of Sequence Variants in Clinical Molecular Genetics. Association for Clinical Genetic Science and the Dutch Society of Clinical Genetic Laboratory Specialists. 2013. Available online: https://www.acgs.uk.com/media/10791/evaluation_and_reporting_of_sequence_variants_bpgs_june_2013_-_finalpdf.pdf (accessed on 13 June 2021).
- White, S.J.; Breuning, M.H.; Dunnen, J.T.D. Detecting Copy Number Changes in Genomic DNA: MAPH and MLPA. Methods Cell Biol. 2004, 75, 751–768. [Google Scholar] [CrossRef][Green Version]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Alamut Visual Software. Available online: http://www.interactive-biosoftware.com/alamut-visual/ (accessed on 20 August 2021).
- World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention, and Control; a Guide for Programme Managers. Geneva: World Health Organization. 2001. Available online: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf (accessed on 3 July 2021).
- World Health Organization. Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations. Geneva: World Health Organization. 2020. Available online: https://www.who.int/publications/i/item/9789240000124 (accessed on 12 September 2021).
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.C.H.; Crawford, D.H.G.; Franklin, M.E.; Jaskowski, L.A.; Macdonald, G.A.; Jonsson, J.R.; Watson, M.J.; Taylor, P.J.; Fletcher, L.M. The serum hepcidin:ferritin ratio is a potential biomarker for cirrhosis. Liver Int. 2012, 32, 1391–1399. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Kemna, E.; Pickkers, P.; Nemeth, E.; Van Der Hoeven, H.; Swinkels, D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood 2005, 106, 1864–1866. [Google Scholar] [CrossRef]
- Hiatt, J.B.; Pritchard, C.C.; Salipante, S.J.; O’Roak, B.J.; Shendure, J. Single molecule molecular inversion probes for targeted, high-accuracy detection of low-frequency variation. Genome Res. 2013, 23, 843–854. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; E Bruns, D.; A Gatsonis, C.; Glasziou, P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; De Vet, H.C.W.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef]
- Donker, A.E. SATURNUS Study: The Transferrin Saturation/Hepcidin Ratio: A Study on the Diagnostic Utility in the Differentiation of TMPRSS6-Related Iron Refractory Iron Deficiency Anemia from TMPRSS6-Unrelated Iron Deficiency Anemia. Available online: https://www.trialregister.nl/trial/6845 (accessed on 1 October 2021).
- Laarakkers, C.M.M.; Wiegerinck, E.T.; Klaver, S.; Kolodziejczyk, M.; Gille, H.; Hohlbaum, A.M.; Tjalsma, H.; Swinkels, D.W. Improved Mass Spectrometry Assay For Plasma Hepcidin: Detection and Characterization of a Novel Hepcidin Isoform. PLoS ONE 2013, 8, e75518. [Google Scholar] [CrossRef]
- Westgard QC. Westgard Rules and Multirules. 2019. Available online: https://www.westgard.com/mltirule.htm (accessed on 20 August 2021).
- Galesloot, T.E.; Vermeulen, S.H.; Geurts-Moespot, A.J.; Klaver, S.M.; Kroot, J.J.; Van Tienoven, D.; Wetzels, J.F.M.; Kiemeney, L.A.L.M.; Sweep, F.C.; Heijer, M.D.; et al. Serum hepcidin: Reference ranges and biochemical correlates in the general population. Blood 2011, 117, e218–e225. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Tian, L. Joint confidence region estimation for area underROCcurve and Youden index. Stat. Med. 2014, 33, 985–1000. [Google Scholar] [CrossRef] [PubMed]

| IRIDA Patients (n = 20) | IDA Controls (n = 39) | |||||
|---|---|---|---|---|---|---|
| Characteristics | N | Median or Count (%) | IQR | N | Median or Count (%) | IQR |
| Age at presentation with anemia, years | 20 | N/A | N/A | N/A | ||
| 10 | 2–31 | ||||
| 4 | 2–10 | ||||
| 31 | 10–43 | ||||
| Age at TSAT/hepcidin assessment, years | 20 | 39 | ||||
| 25 | 7–40 | 62 | 52–71 | ||
| 9 | 6–21 | N/A | N/A | ||
| 40 | 31–48 | N/A | N/A | ||
| Women | 20 | 15 (75) | N/A | 39 | 29 (74) | N/A |
| Systemic disease | 20 | N/A | 39 | N/A | ||
| 1 (5) | 12 (31) | ||||
| NP | 4 (10) | ||||
| NP | 1 (3) | ||||
| NP | 1 (3) | ||||
| 19 (95) | 21 (54) | ||||
| Hb, g/dL a | 19 | 11.4 | 9.8–12.0 | 39 | 9.3 | 4.7–6.5 |
| MCV, fL a | 19 | 70.0 | 63.0–80.0 | 39 | 73.0 | 66.0–77.0 |
| Ferritin, µg/L a | 19 | 130.0 | 33.0–293.0 | 39 | 9.0 | 6.0–14.0 |
| TSAT, % a | 20 | 6.0 | 4.0–9.8 | 39 | 5.0 | 3.0–7.0 |
| CRP, mg/L a | 20 | <5.0 | <5.0–<5.0 | 39 | 1.8 | 0.7–2.5 |
| Pathogenic TMPRSS6 variant (s) | 20 | N/A | 36 | N/A | ||
| 11 (55) | NP | ||||
| 9 (45) | 1 (3) | ||||
| NP | 35 (97) | ||||
| IDA Controls (n = 39) | |||
|---|---|---|---|
| Characteristics | N | Median or Count (%) | IQR |
| ALT, U/L | 21 | 18.0 | 16.0–25.0 |
| eGFR (CKD-EPI), mL/min/1.73 m2 a | 33 | 89 | 65–≥90 |
| BMI, kg/m2 | 39 | 28 | 24–34 |
| 11 (28) | ||
| 9 (23) | ||
| 17 (44) | ||
| 2 (5) | ||
| Assessment of underlying disorder of IDA | 39 | N/A | |
| 12 (31) | ||
| 5 (13) | ||
| 4 (10) | ||
| 18 (46) | ||
| Assessment of medication use | 39 | N/A | |
| 6 (15) | ||
| 12 (31) | ||
| 1 (3) | ||
| 14 (36) | ||
| 1 (3) | ||
| Assessment of response to iron therapy in unexplained IDA b | 18 | N/A | |
| 1 (6) | ||
| 1 (6) | ||
| 4 (22) | ||
| 9 (50) | ||
| 3 (17) | ||
| IRIDA Patients | IDA Controls | Reference Range (General Population) a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median | IQR | N | Median | IQR | Mann–Whitney U Test p-Value | Median | P2.5–P97.5 | |
| Hepcidin level (nM) b | |||||||||
| Total group | 20 | 7.6 | 6.7–15.3 | 39 | 0.3 | 0.3–0.3 | <0.001 | N/A | N/A |
| Men | 5 | 7.6 | 7.1–19.2 | 10 | 0.3 | 0.3–0.3 | <0.001 | 4.7 | <0.5–15.5 |
| Premenopausal women (age < 55 years) | 14 | 7.6 | 6.9–14.2 | 10 | 0.3 | 0.3–0.3 | <0.001 | 2.1 | <0.5–13.0 |
| Postmenopausal women (age ≥ 55 years) | 1 | 2.4 | N/A | 19 | 0.3 | 0.3–0.3 | N/A | 5.2 | <0.5–16.5 |
| Adults | 12 | 7.5 | 6.0–12.5 | 39 | 0.3 | 0.3–0.3 | <0.001 | N/A | N/A |
| Children (<18 years) | 8 | 7.7 | 7.2–20.9 | N/A | N/A | N/A | N/A | 1.5 | 0.1–9.8 |
| Biallelic pathogenic TMPRSS6 variants c | 11 | 10.0 | 7.1–19.4 | N/A | N/A | N/A | N/A | N/A | N/A |
| Monoallelic pathogenic TMPRSS6 variants c | 9 | 7.5 | 6.1–7.6 | N/A | N/A | N/A | N/A | N/A | N/A |
| TSAT/hepcidin ratio (%/nM) | |||||||||
| Total group | 20 | 0.6 | 0.4–1.1 | 39 | 16.7 | 12.0–24.0 | <0.001 | N/A | N/A |
| Men | 5 | 0.5 | 0.2–1.1 | 10 | 20.0 | 15.0–34.0 | 0.002 | 6.9 | 1.6–243.0 |
| Premenopausal women (age < 55 years) | 14 | 0.7 | 0.5–1.1 | 10 | 16.0 | 8.0–24.0 | <0.001 | 13.2 | 1.9–312.9 |
| Postmenopausal women (age ≥ 55 years) | 1 | 3.8 | N/A | 19 | 20.0 | 12.0–24.0 | N/A | 5.4 | 1.4–69.6 |
| Adults (≥18 years) | 12 | 0.9 | 0.5–2.2 | 39 | 16.7 | 12.0–24.0 | <0.001 | N/A | N/A |
| Children (<18 years) | 8 | 0.5 | 0.3–0.7 | N/A | N/A | N/A | N/A | 15.4 | 1.4–665.5 |
| Biallelic pathogenic TMPRSS6 variants d | 11 | 0.5 | 0.3–0.6 | N/A | N/A | N/A | N/A | N/A | N/A |
| Monoallelic pathogenic TMPRSS6 variants d | 9 | 1.1 | 0.7–2.6 | N/A | N/A | N/A | N/A | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Staaij, H.; Donker, A.E.; Bakkeren, D.L.; Salemans, J.M.J.I.; Mignot-Evers, L.A.A.; Bongers, M.Y.; Dieleman, J.P.; Galesloot, T.E.; Laarakkers, C.M.; Klaver, S.M.; et al. Transferrin Saturation/Hepcidin Ratio Discriminates TMPRSS6-Related Iron Refractory Iron Deficiency Anemia from Patients with Multi-Causal Iron Deficiency Anemia. Int. J. Mol. Sci. 2022, 23, 1917. https://doi.org/10.3390/ijms23031917
van der Staaij H, Donker AE, Bakkeren DL, Salemans JMJI, Mignot-Evers LAA, Bongers MY, Dieleman JP, Galesloot TE, Laarakkers CM, Klaver SM, et al. Transferrin Saturation/Hepcidin Ratio Discriminates TMPRSS6-Related Iron Refractory Iron Deficiency Anemia from Patients with Multi-Causal Iron Deficiency Anemia. International Journal of Molecular Sciences. 2022; 23(3):1917. https://doi.org/10.3390/ijms23031917
Chicago/Turabian Stylevan der Staaij, Hilde, Albertine E. Donker, Dirk L. Bakkeren, Jan M. J. I. Salemans, Lisette A. A. Mignot-Evers, Marlies Y. Bongers, Jeanne P. Dieleman, Tessel E. Galesloot, Coby M. Laarakkers, Siem M. Klaver, and et al. 2022. "Transferrin Saturation/Hepcidin Ratio Discriminates TMPRSS6-Related Iron Refractory Iron Deficiency Anemia from Patients with Multi-Causal Iron Deficiency Anemia" International Journal of Molecular Sciences 23, no. 3: 1917. https://doi.org/10.3390/ijms23031917
APA Stylevan der Staaij, H., Donker, A. E., Bakkeren, D. L., Salemans, J. M. J. I., Mignot-Evers, L. A. A., Bongers, M. Y., Dieleman, J. P., Galesloot, T. E., Laarakkers, C. M., Klaver, S. M., & Swinkels, D. W. (2022). Transferrin Saturation/Hepcidin Ratio Discriminates TMPRSS6-Related Iron Refractory Iron Deficiency Anemia from Patients with Multi-Causal Iron Deficiency Anemia. International Journal of Molecular Sciences, 23(3), 1917. https://doi.org/10.3390/ijms23031917






