Caffeine and MDMA (Ecstasy) Exacerbate ER Stress Triggered by Hyperthermia
Abstract
:1. Introduction
2. Results
2.1. Hyperthermia Triggers the Secretion of ER-Resident Proteins
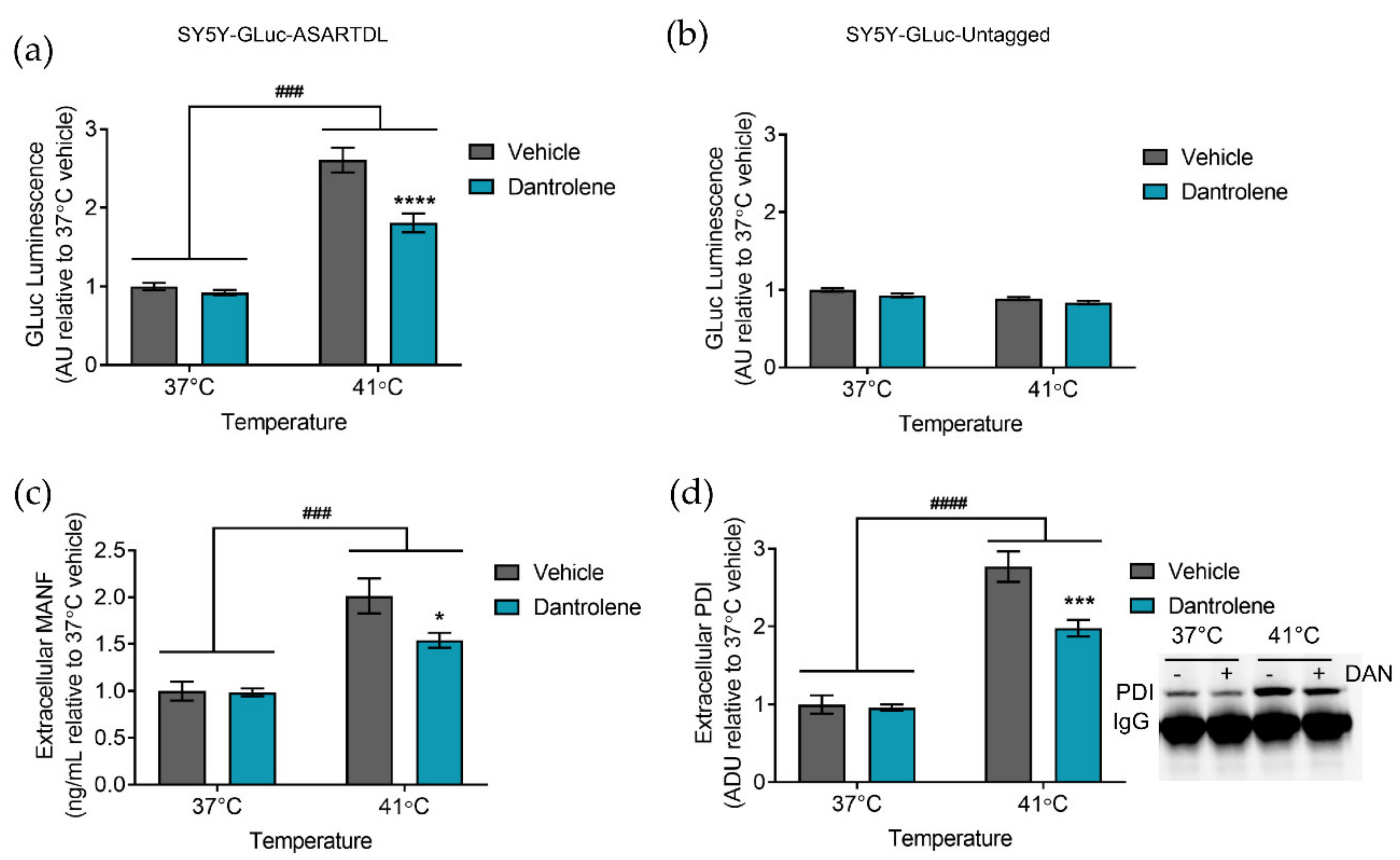
2.2. Manipulating ER Calcium Alters Hyperthermia-Induced ER Exodosis
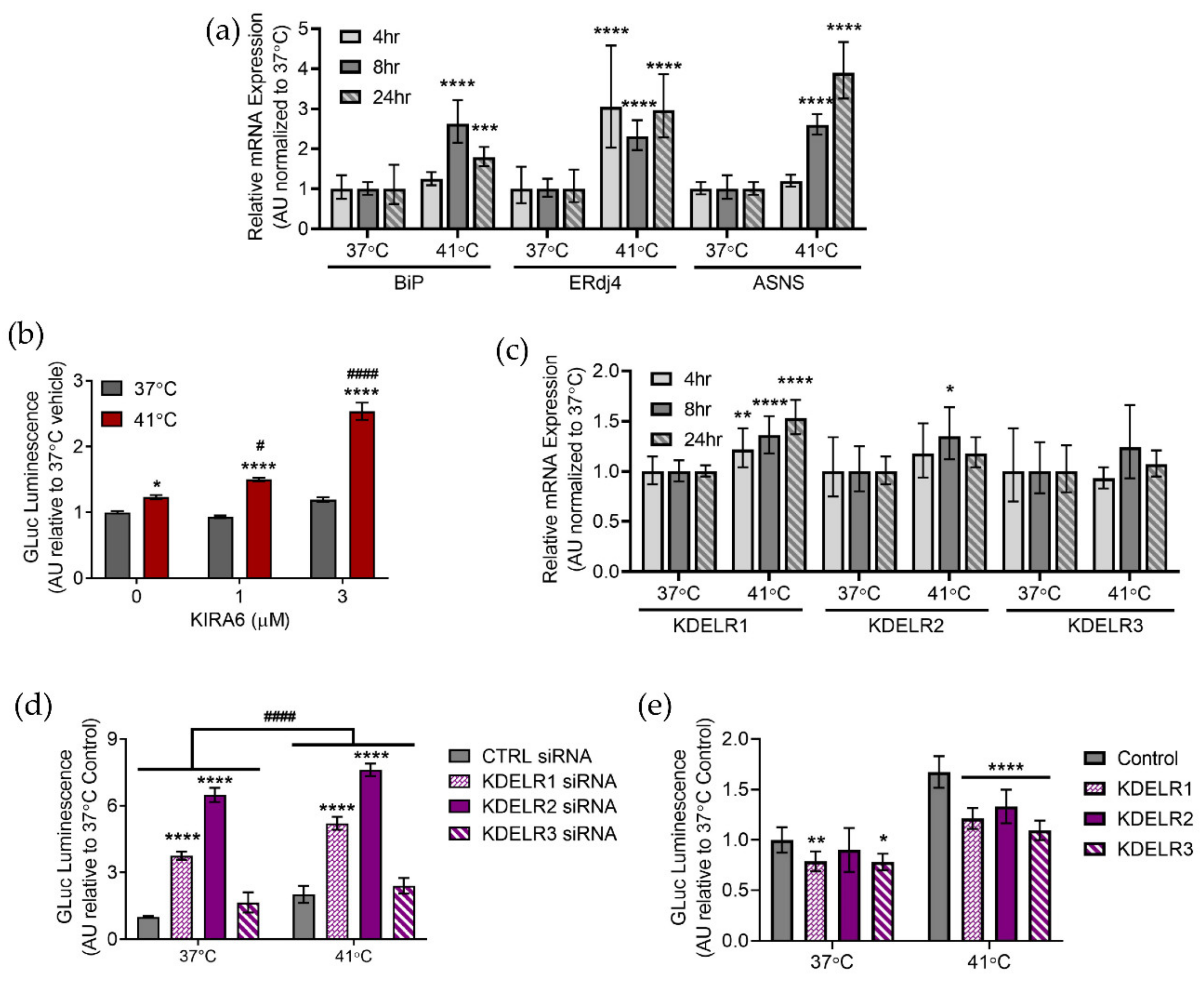
2.3. Hyperthermia Affects the Unfolded Protein Response
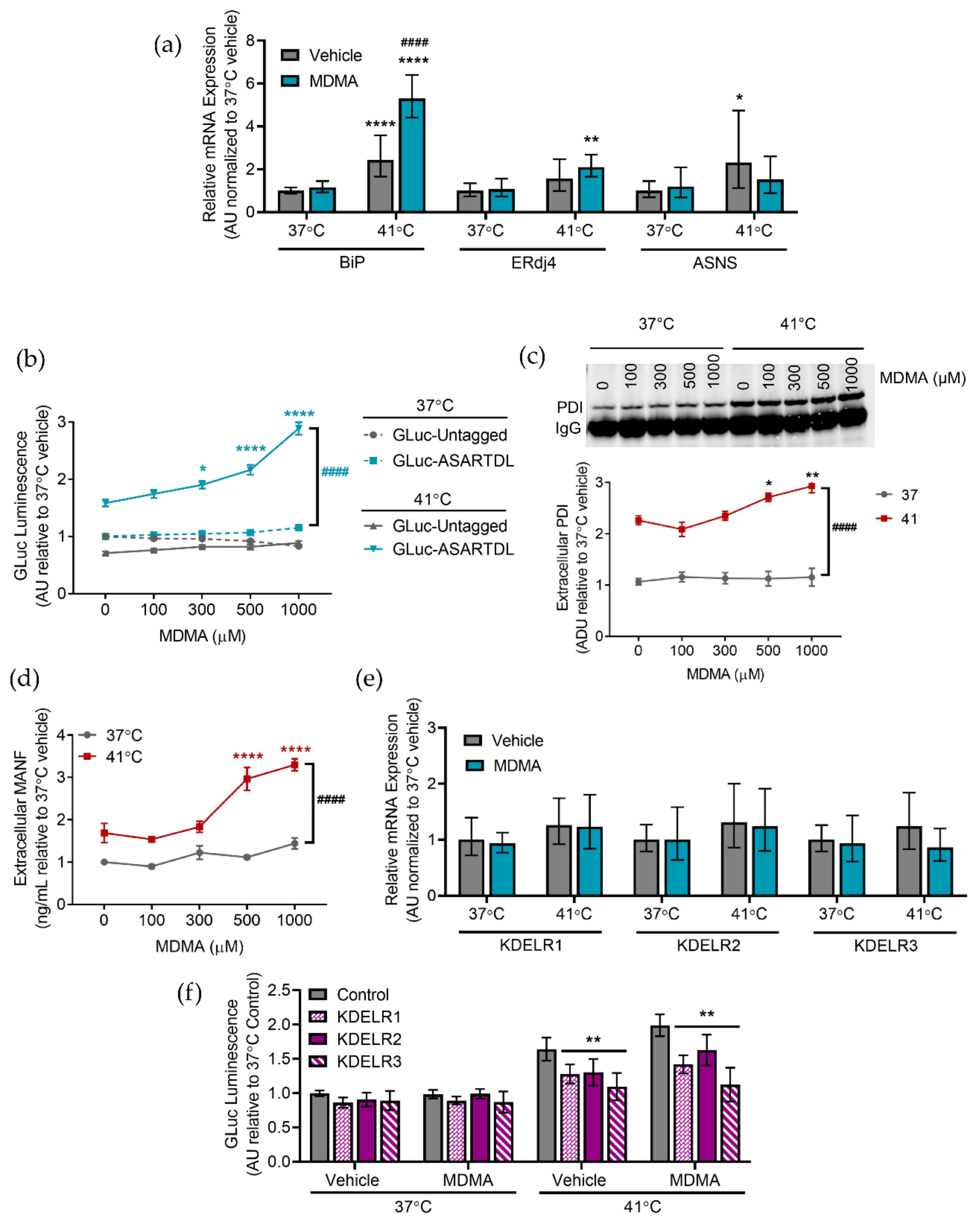
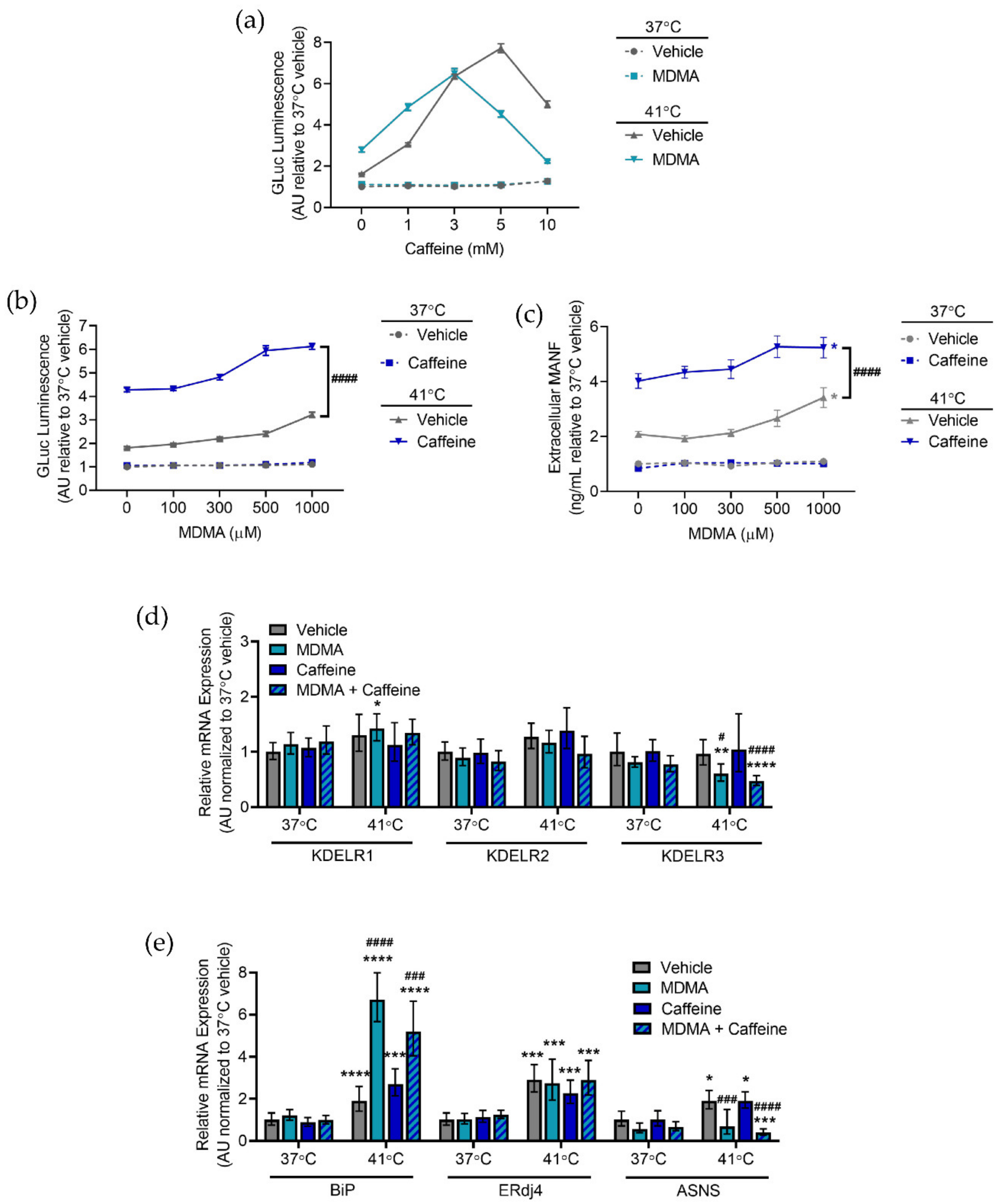
2.4. Caffeine and MDMA Affect ER Responses to Hyperthermia
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. SH-SY5Y Cell Culture
4.3. Primary Cortical Neuron Cell Culture
4.4. Gaussia Luciferase Assay
4.5. ATP Assay
4.6. PDI Immunoprecipitation
4.7. MANF Homogeneous Time-Resolved Fluorescence (HTRF) Assay
4.8. Esterase Assay
4.9. siRNA Transfection
4.10. Lentiviral Transduction
4.11. Real-Time qPCR
4.12. Quantification and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armenian, P.; Mamantov, T.M.; Tsutaoka, B.T.; Gerona, R.R.; Silman, E.F.; Wu, A.H.; Olson, K.R. Multiple MDMA (Ecstasy) overdoses at a rave event: A case series. J. Intensive Care Med. 2013, 28, 252–258. [Google Scholar] [CrossRef]
- Patel, M.M.; Belson, M.G.; Longwater, A.B.; Olson, K.R.; Miller, M.A. Methylenedioxymethamphetamine (ecstasy)-related hyperthermia. J. Emerg. Med. 2005, 29, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Musselman, M.E.; Saely, S. Diagnosis and treatment of drug-induced hyperthermia. Am. J. Health Syst. Pharm. 2013, 70, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Halpern, P.; Moskovich, J.; Avrahami, B.; Bentur, Y.; Soffer, D.; Peleg, K. Morbidity associated with MDMA (ecstasy) abuse: A survey of emergency department admissions. Hum. Exp. Toxicol. 2011, 30, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.A.; Jeffreys, K.J.; Dawling, S. Toxicity and deaths from 3,4-methylenedioxymethamphetamine (“ecstasy”). Lancet 1992, 340, 384–387. [Google Scholar] [CrossRef]
- Liechti, M.E.; Kunz, I.; Kupferschmidt, H. Acute medical problems due to Ecstasy use. Case-series of emergency department visits. Swiss Med. Wkly. 2005, 135, 652–657. [Google Scholar]
- Kiyatkin, E.A.; Kim, A.H.; Wakabayashi, K.T.; Baumann, M.H.; Shaham, Y. Effects of social interaction and warm ambient temperature on brain hyperthermia induced by the designer drugs methylone and MDPV. Neuropsychopharmacology 2015, 40, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Vanattou-Saïfoudine, N.; McNamara, R.; Harkin, A. Caffeine provokes adverse interactions with 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) and related psychostimulants: Mechanisms and mediators. Br. J. Pharmacol. 2012, 167, 946–959. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Yang, X.; Jiang, Q.; Liu, K.; Li, B.; Li, L.; Zhao, L.; Li, M. Hyperthermia impairs the executive function using the Attention Network Test. Int. J. Hyperth. 2012, 28, 621–626. [Google Scholar] [CrossRef]
- Yagoubi, N.; Jomni, Y.; Sakly, M. Hyperthermia-Induced Febrile Seizures Have Moderate and Transient Effects on Spatial Learning in Immature Rats. Behav. Neurol. 2015, 2015, 924303. [Google Scholar] [CrossRef] [Green Version]
- Greer David, M.; Funk Susan, E.; Reaven Nancy, L.; Ouzounelli, M.; Uman Gwen, C. Impact of Fever on Outcome in Patients With Stroke and Neurologic Injury. Stroke 2008, 39, 3029–3035. [Google Scholar] [CrossRef] [Green Version]
- Roti Roti, J.L. Cellular responses to hyperthermia (40–46 °C): Cell killing and molecular events. Int. J. Hyperth. 2008, 24, 3–15. [Google Scholar] [CrossRef]
- Lepock, J.R.; Frey, H.E.; Ritchie, K.P. Protein denaturation in intact hepatocytes and isolated cellular organelles during heat shock. J. Cell Biol. 1993, 122, 1267–1276. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science 2011, 334, 1081. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Gupta, S.; Hu, W.; McGrath, B.C.; Cavener, D.R. Hyperthermia induces the ER stress pathway. PLoS ONE 2011, 6, e23740. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.H.; Lin, F.L.; Hou, S.M.; Liu, J.F. Hyperthermia induces apoptosis through endoplasmic reticulum and reactive oxygen species in human osteosarcoma cells. Int. J. Mol. Sci. 2014, 15, 17380–17395. [Google Scholar] [CrossRef] [Green Version]
- Mantso, T.; Vasileiadis, S.; Anestopoulos, I.; Voulgaridou, G.P.; Lampri, E.; Botaitis, S.; Kontomanolis, E.N.; Simopoulos, C.; Goussetis, G.; Franco, R.; et al. Hyperthermia induces therapeutic effectiveness and potentiates adjuvant therapy with non-targeted and targeted drugs in an in vitro model of human malignant melanoma. Sci. Rep. 2018, 8, 10724. [Google Scholar] [CrossRef]
- Kwon, J.H.; Kim, H.K.; Ha, T.W.; Im, J.S.; Song, B.H.; Hong, K.S.; Oh, J.S.; Han, J.; Lee, M.R. Hyperthermia Disturbs and Delays Spontaneous Differentiation of Human Embryoid Bodies. Biomedicines 2020, 8, 176. [Google Scholar] [CrossRef]
- Spiess, C.; Beil, A.; Ehrmann, M. A Temperature-Dependent Switch from Chaperone to Protease in a Widely Conserved Heat Shock Protein. Cell 1999, 97, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Stephens, S.B.; Nicchitta, C.V. Divergent regulation of protein synthesis in the cytosol and endoplasmic reticulum compartments of mammalian cells. Mol. Biol. Cell 2008, 19, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Csala, M.; Bánhegyi, G.; Benedetti, A. Endoplasmic reticulum: A metabolic compartment. FEBS Lett. 2006, 580, 2160–2165. [Google Scholar] [CrossRef] [Green Version]
- Cribb, A.E.; Peyrou, M.; Muruganandan, S.; Schneider, L. The endoplasmic reticulum in xenobiotic toxicity. Drug Metab. Rev. 2005, 37, 405–442. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Munro, S.; Pelham, H.R. A C-terminal signal prevents secretion of luminal ER proteins. Cell 1987, 48, 899–907. [Google Scholar] [CrossRef]
- Raykhel, I.; Alanen, H.; Salo, K.; Jurvansuu, J.; Nguyen, V.D.; Latva-Ranta, M.; Ruddock, L. A molecular specificity code for the three mammalian KDEL receptors. J. Cell Biol. 2007, 179, 1193–1204. [Google Scholar] [CrossRef] [Green Version]
- Mazzarella, R.A.; Srinivasan, M.; Haugejorden, S.M.; Green, M. ERp72, an abundant luminal endoplasmic reticulum protein, contains three copies of the active site sequences of protein disulfide isomerase. J. Biol. Chem. 1990, 265, 1094–1101. [Google Scholar] [CrossRef]
- Mazzarella, R.A.; Marcus, N.; Haugejorden, S.M.; Balcarek, J.M.; Baldassare, J.J.; Roy, B.; Li, L.J.; Lee, A.S.; Green, M. ERp61 Is GRP58, a Stress-Inducible Luminal Endoplasmic Reticulum Protein, but Is Devoid of Phosphatidylinositide-Specific Phospholipase C Activity. Arch. Biochem. Biophys. 1994, 308, 454–460. [Google Scholar] [CrossRef]
- Fliegel, L.; Newton, E.; Burns, K.; Michalak, M. Molecular cloning of cDNA encoding a 55-kDa multifunctional thyroid hormone binding protein of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 15496–15502. [Google Scholar] [CrossRef]
- Orci, L.; Stamnes, M.; Ravazzola, M.; Amherdt, M.; Perrelet, A.; Sollner, T.H.; Rothman, J.E. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell 1997, 90, 335–349. [Google Scholar] [CrossRef] [Green Version]
- Booth, C.; Koch, G.L. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell 1989, 59, 729–737. [Google Scholar] [CrossRef]
- Glembotski, C.C.; Thuerauf, D.J.; Huang, C.; Vekich, J.A.; Gottlieb, R.A.; Doroudgar, S. Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion. J. Biol. Chem. 2012, 287, 25893–25904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, M.J.; Richie, C.T.; Airavaara, M.; Wang, Y.; Harvey, B.K. Mesencephalic astrocyte-derived neurotrophic factor (MANF) secretion and cell surface binding are modulated by KDEL receptors. J. Biol. Chem. 2013, 288, 4209–4225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trychta, K.A.; Heathward, E.J.; Sulima, A.; Back, S.; Farokhnia, M.; Richie, C.T.; Leggio, L.; Rice, K.C.; Harvey, B.K. Extracellular esterase activity as an indicator of endoplasmic reticulum calcium depletion. Biomarkers 2018, 23, 756–765. [Google Scholar] [CrossRef]
- Henderson, M.J.; Wires, E.S.; Trychta, K.A.; Richie, C.T.; Harvey, B.K. SERCaMP: A carboxy-terminal protein modification that enables monitoring of ER calcium homeostasis. Mol. Biol. Cell 2014, 25, 2828–2839. [Google Scholar] [CrossRef]
- Trychta, K.A.; Back, S.; Henderson, M.J.; Harvey, B.K. KDEL Receptors Are Differentially Regulated to Maintain the ER Proteome under Calcium Deficiency. Cell Rep. 2018, 25, 1829. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Stevenson, M.A.; Hahn, G.M. Effects of Heat on Cell Calcium and Inositol Lipid Metabolism. Radiat. Res. 1988, 113, 414–425. [Google Scholar] [CrossRef]
- Barbosa, D.J.; Capela, J.P.; Silva, R.; Ferreira, L.M.; Branco, P.S.; Fernandes, E.; Bastos, M.L.; Carvalho, F. “Ecstasy”-induced toxicity in SH-SY5Y differentiated cells: Role of hyperthermia and metabolites. Arch. Toxicol. 2014, 88, 515–531. [Google Scholar] [CrossRef]
- Zhou, X.; Bouitbir, J.; Liechti, M.E.; Krähenbühl, S.; Mancuso, R.V. Hyperthermia Increases Neurotoxicity Associated with Novel Methcathinones. Cells 2020, 9, 965. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Smith, D.J.; Anderson, R.L.; Nagley, P. Human neuroblastoma SH-SY5Y cells show increased resistance to hyperthermic stress after differentiation, associated with elevated levels of Hsp72. Int. J. Hyperth. 2011, 27, 415–426. [Google Scholar] [CrossRef]
- Li, L.; Tan, H.; Gu, Z.; Liu, Z.; Geng, Y.; Liu, Y.; Tong, H.; Tang, Y.; Qiu, J.; Su, L. Heat Stress Induces Apoptosis through a Ca2+-Mediated Mitochondrial Apoptotic Pathway in Human Umbilical Vein Endothelial Cells. PLoS ONE 2015, 9, e111083. [Google Scholar] [CrossRef]
- Taylor, C.W.; Tovey, S.C. IP(3) Receptors: Toward Understanding Their Activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a004010. [Google Scholar] [CrossRef]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef] [Green Version]
- Lytton, J.; Westlin, M.; Burk, S.E.; Shull, G.E.; MacLennan, D.H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J. Biol. Chem. 1992, 267, 14483–14489. [Google Scholar] [CrossRef]
- Lytton, J.; Westlin, M.; Hanley, M.R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J. Biol. Chem. 1991, 266, 17067–17071. [Google Scholar] [CrossRef]
- Duksin, D.; Mahoney, W.C. Relationship of the structure and biological activity of the natural homologues of tunicamycin. J. Biol. Chem. 1982, 257, 3105–3109. [Google Scholar] [CrossRef]
- Wei, H.; Perry, D.C. Dantrolene is cytoprotective in two models of neuronal cell death. J. Neurochem. 1996, 67, 2390–2398. [Google Scholar] [CrossRef]
- Zhao, F.; Li, P.; Chen, S.R.; Louis, C.F.; Fruen, B.R. Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. J. Biol. Chem. 2001, 276, 13810–13816. [Google Scholar] [CrossRef] [Green Version]
- Abu-Omar, N.; Das, J.; Szeto, V.; Feng, Z.P. Neuronal Ryanodine Receptors in Development and Aging. Mol. Neurobiol. 2018, 55, 1183–1192. [Google Scholar] [CrossRef]
- Saleem, H.; Tovey, S.C.; Molinski, T.F.; Taylor, C.W. Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br. J. Pharm. 2014, 171, 3298–3312. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Ryno, L.M.; Genereux, J.C.; Moresco, J.J.; Tu, P.G.; Wu, C.; Yates, J.R.; Su, A.I.; Kelly, J.W.; Wiseman, R.L. Stress-Independent Activation of XBP1s and/or ATF6 Reveals Three Functionally Diverse ER Proteostasis Environments. Cell Rep. 2013, 3, 1279–1292. [Google Scholar] [CrossRef] [Green Version]
- Gjymishka, A.; Su, N.; Kilberg, M.S. Transcriptional induction of the human asparagine synthetase gene during the unfolded protein response does not require the ATF6 and IRE1/XBP1 arms of the pathway. Biochem. J. 2009, 417, 695–703. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, R.; Wang, L.; Wang, E.S.; B Perera, G.K.; Igbaria, A.; Morita, S.; Prado, K.; Thamsen, M.; Caswell, D.; Macias, H.; et al. Allosteric Inhibition of the IRE1α RNase Preserves Cell Viability and Function during Endoplasmic Reticulum Stress. Cell 2014, 158, 534–548. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.L.; Kiyatkin, E.A. Brain hyperthermia induced by MDMA (ecstasy): Modulation by environmental conditions. Eur. J. Neurosci. 2004, 20, 51–58. [Google Scholar] [CrossRef]
- Valente, M.J.; Bastos, M.d.L.; Fernandes, E.; Carvalho, F.; Guedes de Pinho, P.; Carvalho, M. Neurotoxicity of β-Keto Amphetamines: Deathly Mechanisms Elicited by Methylone and MDPV in Human Dopaminergic SH-SY5Y Cells. ACS Chem. Neurosci. 2017, 8, 850–859. [Google Scholar] [CrossRef]
- Li, I.H.; Shih, J.-H.; Yeh, T.-Y.; Lin, H.-C.; Chen, M.-H.; Huang, Y.-S. Lysosomal Dysfunction and Autophagy Blockade Contribute to MDMA-Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Chem. Res. Toxicol. 2020, 33, 903–914. [Google Scholar] [CrossRef]
- Burdakov, D.; Petersen, O.H.; Verkhratsky, A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium 2005, 38, 303–310. [Google Scholar] [CrossRef]
- Hofer, A.M.; Schulz, I. Quantification of intraluminal free [Ca] in the agonist-sensitive internal calcium store using compartmentalized fluorescent indicators: Some considerations. Cell Calcium 1996, 20, 235–242. [Google Scholar] [CrossRef]
- Mogami, H.; Tepikin, A.V.; Petersen, O.H. Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. EMBO J. 1998, 17, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Robert, V.; De Giorgi, F.; Massimino, M.L.; Cantini, M.; Pozzan, T. Direct monitoring of the calcium concentration in the sarcoplasmic and endoplasmic reticulum of skeletal muscle myotubes. J. Biol. Chem. 1998, 273, 30372–30378. [Google Scholar] [CrossRef] [Green Version]
- Miyawaki, A.; Llopis, J.; Heim, R.; McCaffery, J.M.; Adams, J.A.; Ikura, M.; Tsien, R.Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997, 388, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ratés, S.; Camarasa, J.; Sánchez-García, A.I.; Gandía, L.; Escubedo, E.; Pubill, D. The effects of 3,4-methylenedioxymethamphetamine (MDMA) on nicotinic receptors: Intracellular calcium increase, calpain/caspase 3 activation, and functional upregulation. Toxicol. Appl. Pharmacol. 2010, 244, 344–353. [Google Scholar] [CrossRef]
- Beitia, G.; Cobreros, A.; Sainz, L.; Cenarruzabeitia, E. 3,4-Methylenedioxymethamphetamine (ecstasy)-induced hepatotoxicity: Effect on cytosolic calcium signals in isolated hepatocytes. Liver 1999, 19, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Usachev, Y.; Shmigol, A.; Pronchuk, N.; Kostyuk, P.; Verkhratsky, A. Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience 1993, 57, 845–859. [Google Scholar] [CrossRef]
- Konieczny, V.; Tovey, S.C.; Mataragka, S.; Prole, D.L.; Taylor, C.W. Cyclic AMP Recruits a Discrete Intracellular Ca2+ Store by Unmasking Hypersensitive IP3 Receptors. Cell Rep. 2017, 18, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Masson, J.; Emerit, M.B.; Hamon, M.; Darmon, M. Serotonergic signaling: Multiple effectors and pleiotropic effects. Wiley Interdiscip. Rev. Membr. Transport. Signal. 2012, 1, 685–713. [Google Scholar] [CrossRef]
- Bonati, M.; Latini, R.; Tognoni, G.; Young, J.F.; Garattini, S. Interspecies Comparison of In Vivo Caffeine Pharmacokinetics in Man, Monkey, Rabbit, Rat and Mouse. Drug Metab. Rev. 1984, 15, 1355–1383. [Google Scholar] [CrossRef]
- Dickson, J.A.; Shah, D.M. The effects of hyperthermia (42 °C) on the biochemistry and growth of a malignant cell line. Eur. J. Cancer 1972, 8, 561–571. [Google Scholar] [CrossRef]
- Bergmann, J.E.; Tokuyasu, K.T.; Singer, S.J. Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc. Natl. Acad. Sci. USA 1981, 78, 1746–1750. [Google Scholar] [CrossRef] [Green Version]
- Bienz, M.; Pelham, H.R.B. Heat shock regulatory elements function as an inducible enhancer in the xenopus hsp70 gene and when linked to a heterologous promoter. Cell 1986, 45, 753–760. [Google Scholar] [CrossRef]
- Ortner, V.; Ludwig, A.; Riegel, E.; Dunzinger, S.; Czerny, T. An artificial HSE promoter for efficient and selective detection of heat shock pathway activity. Cell Stress Chaperones 2015, 20, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Zawadzka, M.; Szmuda, M.; Mazurkiewicz-Bełdzińska, M. Thermoregulation disorders of central origin—How to diagnose and treat. Anaesthesiol. Intensive Ther. 2017, 49, 227–234. [Google Scholar] [CrossRef]
- Henderson, M.J.; Trychta, K.A.; Yang, S.-M.; Bäck, S.; Yasgar, A.; Wires, E.S.; Danchik, C.; Yan, X.; Yano, H.; Shi, L.; et al. A target-agnostic screen identifies approved drugs to stabilize the endoplasmic reticulum-resident proteome. Cell Rep. 2021, 35, 109040. [Google Scholar] [CrossRef]
- Singarajah, C.; Lavies, N.G. An overdose of ecstasy. A role for dantrolene. Anaesthesia 1992, 47, 686–687. [Google Scholar] [CrossRef]
- Grunau, B.; Wiens, M.; Greidanus, M. Dantrolene for the treatment of MDMA toxicity. CJEM 2010, 12, 457–459. [Google Scholar] [CrossRef] [Green Version]
- Grandjean, J.M.D.; Madhavan, A.; Cech, L.; Seguinot, B.O.; Paxman, R.J.; Smith, E.; Scampavia, L.; Powers, E.T.; Cooley, C.B.; Plate, L.; et al. Pharmacologic IRE1/XBP1s activation confers targeted ER proteostasis reprogramming. Nat. Chem. Biol. 2020, 16, 1052–1061. [Google Scholar] [CrossRef]
- Howard, D.B.; Powers, K.; Wang, Y.; Harvey, B.K. Tropism and toxicity of adeno-associated viral vector serotypes 1,2,5,6,7,8,9 in rat neurons and glia in vitro. Virology 2008, 372, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Wires, E.S.; Trychta, K.A.; Back, S.; Sulima, A.; Rice, K.C.; Harvey, B.K. High fat diet disrupts endoplasmic reticulum calcium homeostasis in the rat liver. J. Hepatol. 2017, 67, 1009–1017. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trychta, K.A.; Harvey, B.K. Caffeine and MDMA (Ecstasy) Exacerbate ER Stress Triggered by Hyperthermia. Int. J. Mol. Sci. 2022, 23, 1974. https://doi.org/10.3390/ijms23041974
Trychta KA, Harvey BK. Caffeine and MDMA (Ecstasy) Exacerbate ER Stress Triggered by Hyperthermia. International Journal of Molecular Sciences. 2022; 23(4):1974. https://doi.org/10.3390/ijms23041974
Chicago/Turabian StyleTrychta, Kathleen A., and Brandon K. Harvey. 2022. "Caffeine and MDMA (Ecstasy) Exacerbate ER Stress Triggered by Hyperthermia" International Journal of Molecular Sciences 23, no. 4: 1974. https://doi.org/10.3390/ijms23041974





