Genome-Wide Expression and Anti-Proliferative Effects of Electric Field Therapy on Pediatric and Adult Brain Tumors
Abstract
:1. Introduction
2. Results
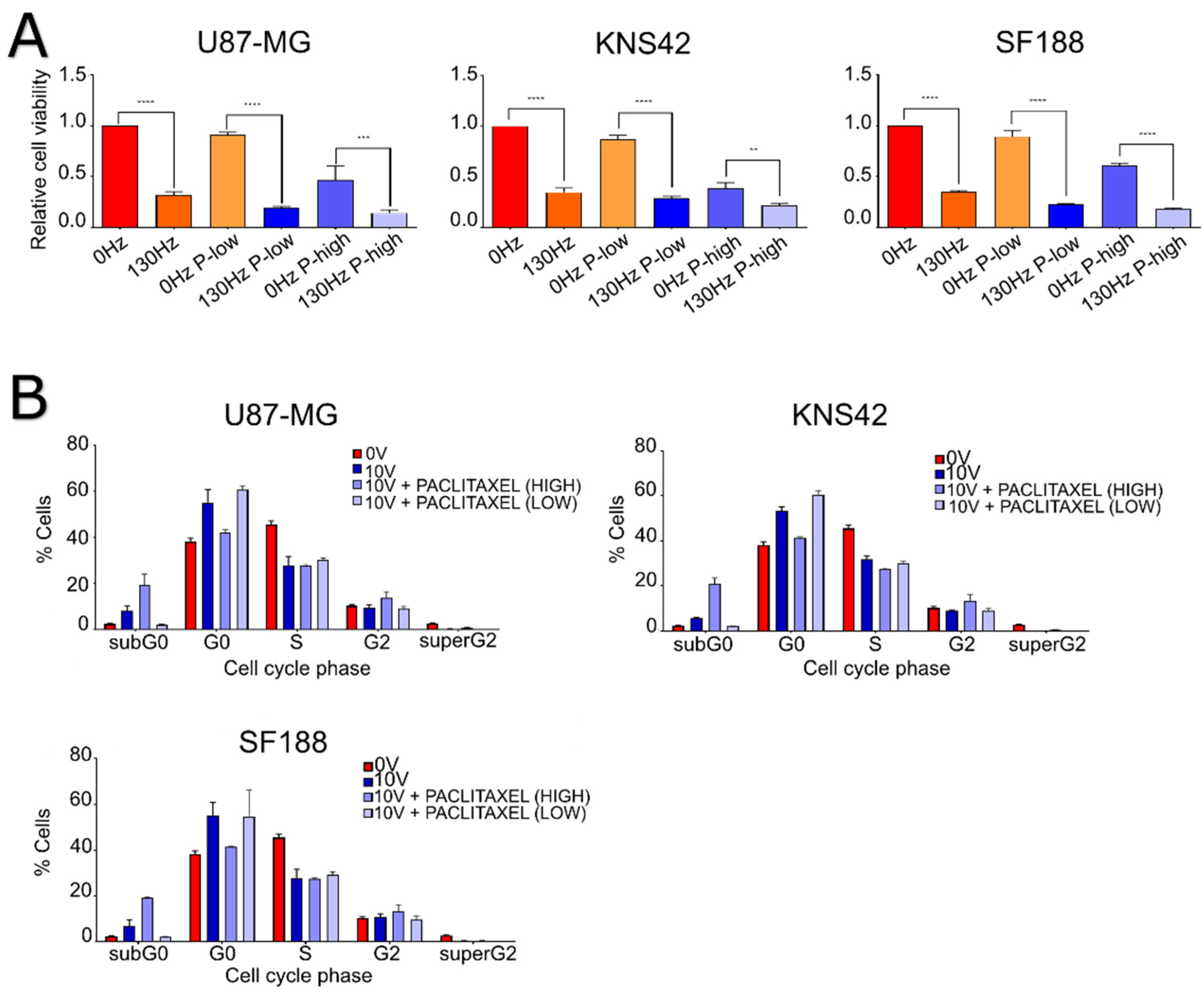
2.1. Tumor Treating Fields Demonstrate Efficacy That Varies in a Frequency-Dependent Manner
2.2. Electric Fields Delivered from DBS Reduce Metabolic Viability in a Voltage- and Frequency-Dependent Manner in GBM Cell Lines
2.3. Electric Fields Perturb Cell Cycling of Brain Tumor Cell Lines in a Frequency-Dependent Manner
2.4. The Efficacy of Electric Fields Appears to Be Increased with the Addition of Mitotic Inhibitors in GBM Cell Lines
2.5. The Efficacy of TTFields and DBS Is Increased with the Addition of Chemotherapies in Brain Tumor Cell Lines
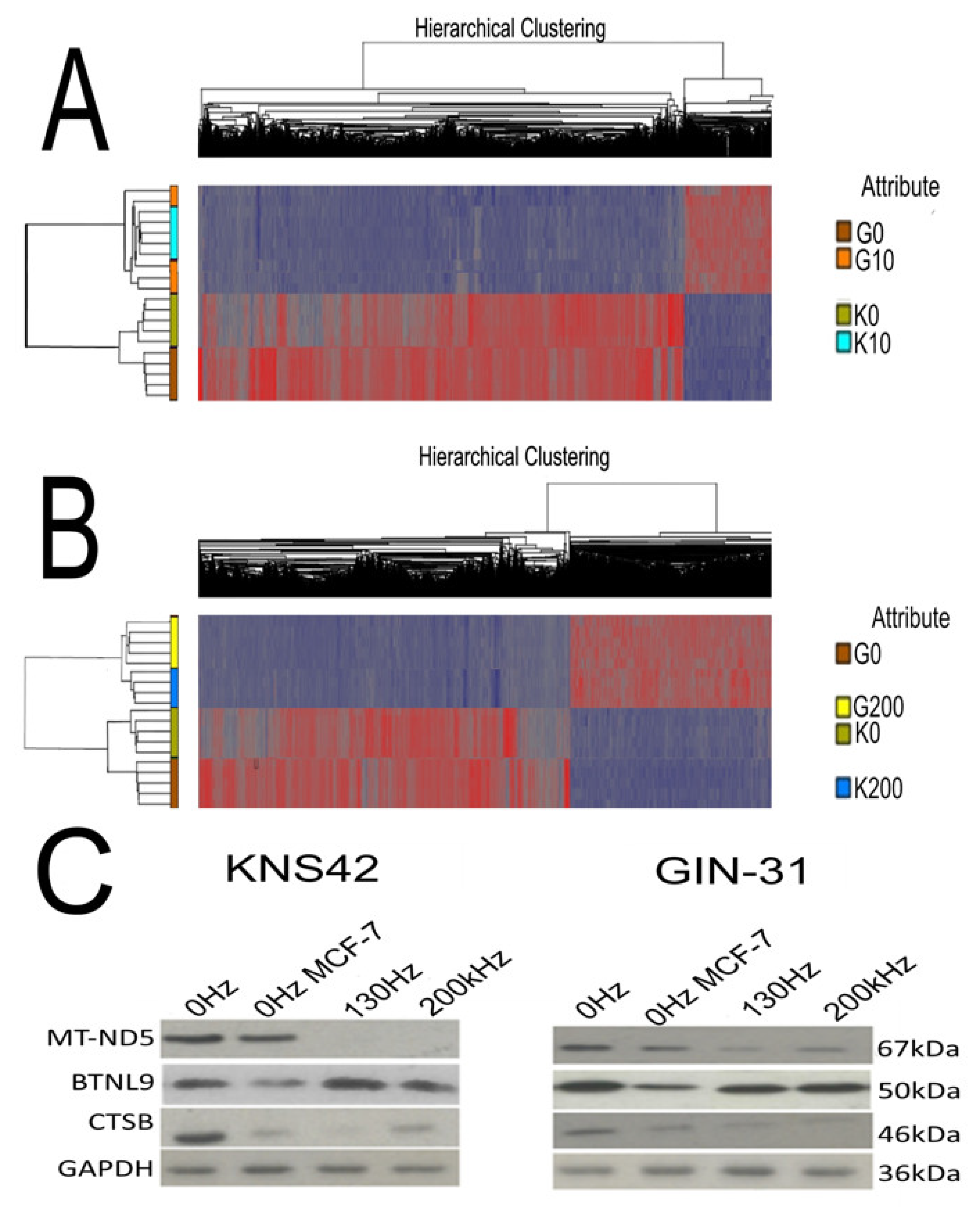
2.6. Electric Field Treatments Cause Gene Expression Changes in GBM Cell Lines
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Cell Lines
4.2. TTFields Application
4.3. Deep Brain Stimulation Electric Fields Application
4.4. Intensity and Frequency Titration
4.5. Cell Viability Assay
4.6. Cell Cycle Analysis
4.7. Chemotherapeutic Combinations
4.8. Gene Expression Analysis
4.9. Gene Expression Data Processing
4.10. Western Blot
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartzbaum, J.A.; Fisher, J.L.; Aldape, K.D.; Wrensch, M. Epidemiology and molecular pathology of glioma. Nat. Clin. Pract. Neurol. 2006, 2, 494–503. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbalý, V.; et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma. JAMA 2017, 318, 2306–2311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gera, N.; Yang, A.; Holtzman, T.S.; Lee, S.X.; Wong, E.T.; Swanson, K.D. Tumor Treating Fields Perturb the Localization of Septins and Cause Aberrant Mitotic Exit. PLoS ONE 2015, 10, e0125269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirson, E.D.; Dbalý, V.; Tovarys, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 10152–10157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, E.; Patel, C.B.; Pohling, C.; Young, C.; Song, J.; Flores, T.A.; Zeng, Y.; Joubert, L.-M.; Arami, H.; Natarajan, A.; et al. Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov. 2018, 4, 113. [Google Scholar] [CrossRef] [PubMed]
- Shteingauz, A.; Porat, Y.; Voloshin, T.; Schneiderman, R.S.; Munster, M.; Zeevi, E.; Kaynan, N.; Gotlib, K.; Giladi, M.; Kirson, E.D.; et al. AMPK-dependent autophagy upregulation serves as a survival mechanism in response to Tumor Treating Fields (TTFields). Cell Death Dis. 2018, 9, 1074–1114. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, H.S.; Johung, T.B.; Caretti, V.; Noll, A.; Tang, Y.; Nagaraja, S.; Gibson, E.M.; Mount, C.W.; Polepalli, J.; Mitra, S.S.; et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 2015, 161, 803–816. [Google Scholar] [CrossRef] [Green Version]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 1–29. [Google Scholar] [CrossRef]
- Wick, W. TTFields: Where does all the skepticism come from? Neuro-Oncology 2016, 18, 303–305. [Google Scholar] [CrossRef] [Green Version]
- Korshoej, A.R.; Hansen, F.L.; Mikic, N.; Oettingen, G.; Sørensen, J.C.H.; Thielscher, A. Importance of electrode position for the distribution of tumor treating fields (TTFields) in a human brain. Identification of effective layouts through systematic analysis of array positions for multiple tumor locations. PLoS ONE 2018, 13, e0201957. [Google Scholar] [CrossRef]
- Bernard-Arnoux, F.; Lamure, M.; Ducray, F.; Aulagner, G.; Honnorat, J.; Armoiry, X. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro-Oncology 2016, 18, 1129–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.U.; Bihari, F.; Whitehead, S.; Wong, E.; Schmid, S.; Hebb, M.O. In Vitro Validation of Intratumoral Modulation Therapy for Glioblastoma. Anticancer Res. 2016, 36, 71–80. [Google Scholar] [PubMed]
- Sebastiano, A.R.; Deweyert, A.; Benoit, S.; Iredale, E.; Xu, H.; Oliveira, C.; Wong, E.; Schmid, S.; Hebb, M.O. Preclinical outcomes of Intratumoral Modulation Therapy for glioblastoma. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chiken, S.; Nambu, A. Mechanism of Deep Brain Stimulation: Inhibition, Excitation, or Disruption? Neuroscientist 2016, 22, 313–322. [Google Scholar] [CrossRef]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef] [Green Version]
- Giladi, M.; Schneiderman, R.S.; Voloshin, T.; Porat, Y.; Munster, M.; Blat, R.; Sherbo, S.; Bomzon, Z.; Urman, N.; Itzhaki, A.; et al. Mitotic Spindle Disruption by Alternating Electric Fields Leads to Improper Chromosome Segregation and Mitotic Catastrophe in Cancer Cells. Nat. Publ. Group 2015, 5, 1–16. [Google Scholar] [CrossRef]
- Voloshin, T.; Munster, M.; Blatt, R.; Shteingauz, A.; Roberts, P.C.; Schmelz, E.M.; Giladi, M.; Schneiderman, R.S.; Zeevi, E.; Porat, Y.; et al. Alternating electric fields (TTFields) in combination with paclitaxel are therapeutically effective against ovarian cancer cells in vitro and in vivo. Int. J. Cancer 2016, 139, 2850–2858. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Shakeley, R.M.; Attardi, G. Tight control of respiration by NADH dehydrogenase ND5 subunit gene expression in mouse mitochondria. Mol. Cell. Biol. 2000, 20, 805–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnesi, F.; Johnson, M.D.; Vitek, J.L. Deep brain stimulation: How does it work? Handb. Clin. Neurol. 2013, 116, 39–54. [Google Scholar] [PubMed]
- Porat, Y.; Giladi, M.; Schneiderman, R.S.; Blat, R.; Shteingauz, A.; Zeevi, E.; Munster, M.; Voloshin, T.; Kaynan, N.; Tal, O.; et al. Determining the Optimal Inhibitory Frequency for Cancerous Cells Using Tumor Treating Fields (TTFields). JoVE 2017, 123, e55820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirson, E.D.; Schneiderman, R.S.; Dbalý, V.; Tovarys, F.; Vymazal, J.; Itzhaki, A.; Mordechovich, D.; Gurvich, Z.; Shmueli, E.; Goldsher, D.; et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med. Phys. 2009, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wenger, C.; Miranda, P.C.; Salvador, R.; Thielscher, A.; Bomzon, Z.; Giladi, M.; Mrugala, M.M.; Korshoej, A.R. A Review on Tumor-Treating Fields (TTFields): Clinical Implications Inferred From Computational Modeling. IEEE Rev. Biomed. Eng. 2018, 11, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.; Hwang, S.-G.; Jin, Y.B.; Sung, J.; Jeong, Y.K.; Baek, J.-H.; Cho, J.-M.; Kim, E.H.; Yoon, M. Selective toxicity of tumor treating fields to melanoma: An in vitro and in vivo study. Cell Death Discov. 2018, 4, 46. [Google Scholar] [CrossRef]
- Silginer, M.; Weller, M.; Stupp, R.; Roth, P. Biological activity of tumor-treating fields in preclinical glioma models. Cell Death Dis. 2017, 8, e2753. [Google Scholar] [CrossRef] [Green Version]
- Kessler, A.F.; Frömbling, G.E.; Gross, F.; Hahn, M.; Dzokou, W.; Ernestus, R.-I.; Löhr, M.; Hagemann, C. Effects of tumor treating fields (TTFields) on glioblastoma cells are augmented by mitotic checkpoint inhibition. Cell Death Discov. 2018, 4, 77. [Google Scholar] [CrossRef] [Green Version]
- Cucullo, L.; Dini, G.; Hallene, K.L.; Fazio, V.; Ilkanich, E.V.; Igboechi, C.; Kight, K.M.; Agarwal, M.K.; Garrity-Moses, M.; Janigro, D. Very low intensity alternating current decreases cell proliferation. Glia 2005, 51, 65–72. [Google Scholar] [CrossRef]
- Yang, M.; Brackenbury, W.J. Membrane potential and cancer progression. Front. Physiol. 2013, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, P.A.; Gaal, J.T.; Strebe, J.K.; Pasch, C.A.; Deming, D.A.; Kuo, J.S.; Robins, H.I. The effects of tumor treating fields and temozolomide in MGMT expressing and non-expressing patient-derived glioblastoma cells. J. Clin. Neurosci. 2016, 36, 120–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.; Kim, S.S.; Lee, J. Cancer cell metabolism: Implications for therapeutic targets. Exp. Mol. Med. 2013, 45, e45. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branter, J.; Estevez-Cebrero, M.; Diksin, M.; Griffin, M.; Castellanos-Uribe, M.; May, S.; Rahman, R.; Grundy, R.; Basu, S.; Smith, S. Genome-Wide Expression and Anti-Proliferative Effects of Electric Field Therapy on Pediatric and Adult Brain Tumors. Int. J. Mol. Sci. 2022, 23, 1982. https://doi.org/10.3390/ijms23041982
Branter J, Estevez-Cebrero M, Diksin M, Griffin M, Castellanos-Uribe M, May S, Rahman R, Grundy R, Basu S, Smith S. Genome-Wide Expression and Anti-Proliferative Effects of Electric Field Therapy on Pediatric and Adult Brain Tumors. International Journal of Molecular Sciences. 2022; 23(4):1982. https://doi.org/10.3390/ijms23041982
Chicago/Turabian StyleBranter, Joshua, Maria Estevez-Cebrero, Mohammed Diksin, Michaela Griffin, Marcos Castellanos-Uribe, Sean May, Ruman Rahman, Richard Grundy, Surajit Basu, and Stuart Smith. 2022. "Genome-Wide Expression and Anti-Proliferative Effects of Electric Field Therapy on Pediatric and Adult Brain Tumors" International Journal of Molecular Sciences 23, no. 4: 1982. https://doi.org/10.3390/ijms23041982






