Novel Oleanane-Type Triterpene Glycosides from the Saponaria officinalis L. Seeds and Apoptosis-Inducing Activity via Mitochondria
Abstract
:1. Introduction
2. Results and Discussion
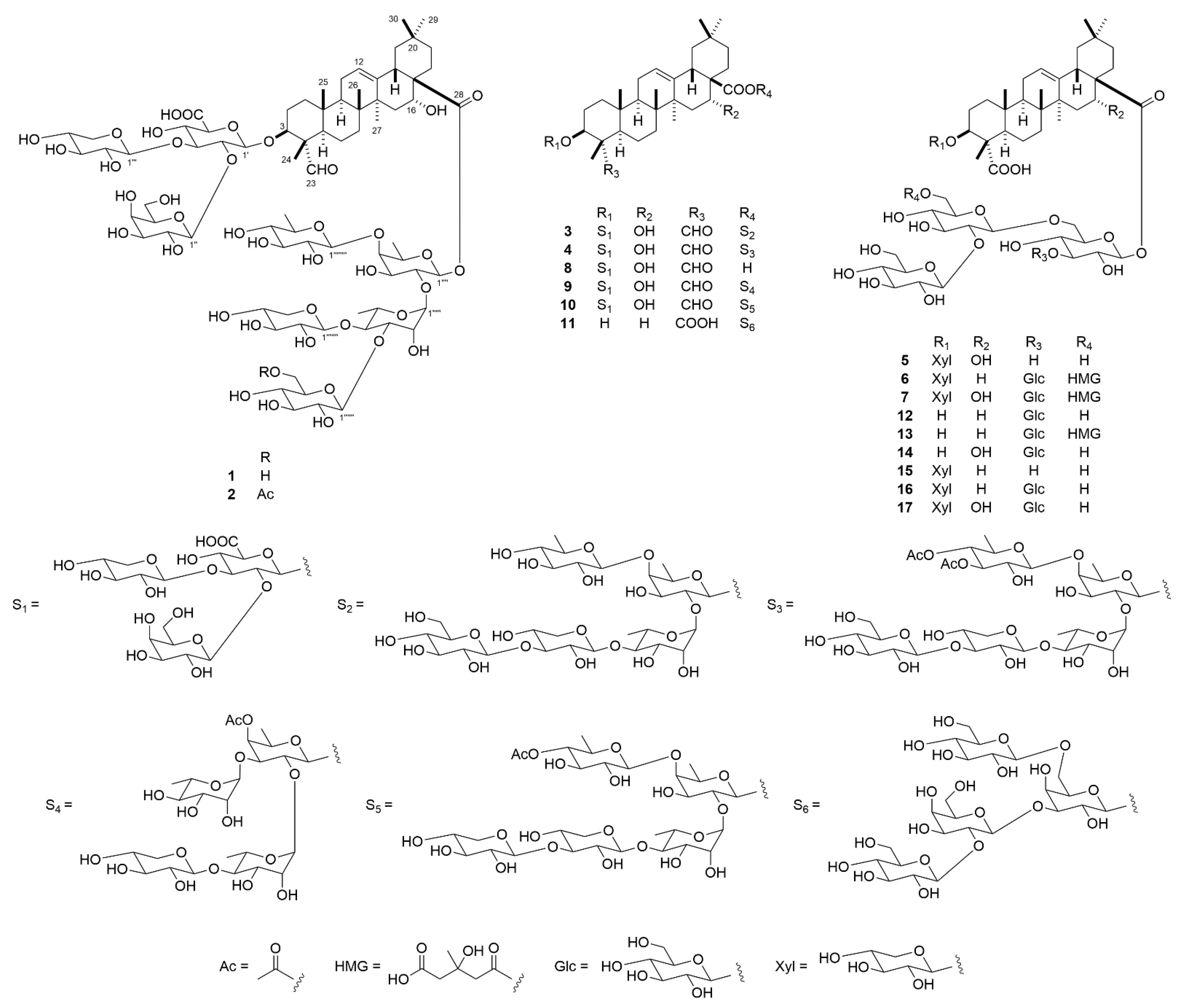
2.1. Structure Characterization of 1–17
2.2. Cytotoxic Activities of 1–17
2.3. Apotosis-Inducing Activity of 1
2.3.1. Apoptosis Induced by 1
2.3.2. Cell Cycle Arrest at the G2/M Phase by 1
2.3.3. Caspase Activation and PARP Cleavage by 1
2.3.4. Mitochondrial Dysfunction Induced by 1
2.3.5. ROS Generation by 1
2.4. Mitophagy Occurrence
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Structural Determination
3.5. Cell Culture and Cytotoxic Activity Assay
3.6. Apoptosis Induction Assay
3.7. Cell Cycle Distrubution Analysis
3.8. Western Blotting Analysis
3.9. Detection of Mitochondrial Membrane Potential
3.10. Measurment of ROS Level
3.11. Mitophagy Detection
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yokosuka, A.; Takayama, H.; Mimaki, Y. Triterpene glycosides from the seeds of Dolichos lablab. Chem. Pharm. Bull. 2019, 67, 604–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokosuka, A.; Okabe, G.; Tatsuno, S.; Mimaki, Y. Stryphnosides G–P, 10 new triterpene glycosides from the pericarps of Stryphnodendron fissuratum. Carbohydr. Res. 2016, 434, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Mimaki, Y.; Fukaya, H.; Matsuo, Y. Cycloartane and oleanane glycosides from the tubers of Eranthis cilicica. Molecules 2019, 24, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jitsuno, M.; Mimaki, Y. Triterpene glycosides from the aerial parts of Larrea tridentata. Phytochemistry 2010, 71, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, A.; Sano, T.; Hashimoto, K.; Sakagami, H.; Mimaki, Y. Triterpene glycosides from the whole plants of Anemone hupehensis var. japonica and their cytotoxic activity. Chem. Pharm. Bull. 2009, 57, 1425–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, Y.; Watanabe, K.; Mimaki, Y. Triterpene glycosides from the underground parts of Caulophyllum thalictroides. J. Nat. Prod. 2009, 72, 1155–1160. [Google Scholar] [CrossRef]
- Mimaki, Y.; Yokosuka, A.; Hamanaka, M.; Sakuma, C.; Yamori, T.; Sashida, Y. Triterpene saponins from the roots of Clematis chinensis. J. Nat. Prod. 2004, 67, 1511–1516. [Google Scholar] [CrossRef]
- Tsukamoto, Y. (Ed.) The Grand Dictionary of Horticulture; Shogakukan: Tokyo, Japan, 1989; Volume 2, pp. 475–476. [Google Scholar]
- Hotta, M.; Ogata, K.; Nitta, A.; Hoshikawa, K.; Yanagi, M.; Yamazaki, K. (Eds.) Useful Plants of the World; Heibonnsha: Tokyo, Japan, 1996; p. 952. [Google Scholar]
- Wichtl, M. (Ed.) Herbal Drugs and Phytopharmaceuticals; CRC Press: Boca Raton, FL, USA, 1994; pp. 453–454. [Google Scholar]
- Moniuszko-Szajwaj, B.; Masullo, M.; Kowalczyk, M.; Pecio, Ł.; Szumacher-Strabel, M.; Cieślak, A.; Piacente, S.; Oleszek, W.; Stochmal, A. Highly polar triterpenoid saponins from the roots of Saponaria officinalis L. Helv. Chim. Acta 2016, 99, 347–354. [Google Scholar] [CrossRef]
- Lu, Y.; Van, D.; Deibert, L.; Bishop, G.; Balsevich, J. Antiproliferative quillaic acid and gypsogenin saponins from Saponaria officinalis L. roots. Phytochemistry 2015, 113, 108–120. [Google Scholar] [CrossRef]
- Moniuszko-Szajwaj, B.; Pecio, Ł.; Kowalczyk, M.; Simonet, M.A.; Macias, A.F.; Szumacher-Strabel, M.; Cieślak, A.; Oleszek, W.; Stochmal, A. New triterpenoid saponins from the roots of Saponaria officinalis. Nat. Prod. Commun. 2013, 8, 1687–1690. [Google Scholar] [CrossRef]
- Koike, K.; Jia, Z.; Nikaido, T. New triterpenoid saponins and sapogenins from Saponaria officinalis. J. Nat. Prod. 1999, 62, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Koike, K.; Nikaido, T. Saponarioside C, the first α-D-galactose containing triterpenoid saponin, and five related compounds from Saponaria officinalis. J. Nat. Prod. 1999, 62, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Koike, K.; Nikaido, T. Major triterpenoid saponins from Saponaria officinalis. J. Nat. Prod. 1998, 61, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Miyashiro, I.; Ito, H.; Hosono, S.; Chihara, D.; Nakata-Yamada, K.; Nakayama, M.; Matsuzaka, M.; Hattori, M.; Sugiyama, H.; et al. Long-term survival and conditional survival of cancer patients in Japan using population-based cancer registry data. Cancer Sci. 2014, 105, 1480–1486. [Google Scholar] [CrossRef]
- Larhsini, M.; Marston, A.; Hostettmann, K. Triterpenoid saponins from the roots of Silene cucubalus. Fitoterapia 2003, 74, 237–241. [Google Scholar] [CrossRef]
- Guo, S.; Falk, E.; Kenne, L.; Rönnberg, B.; Sundquist, G.B. Triterpenoid saponins containing an acetylated branched D-fucosyl residue from Quillaja saponaria Molina. Phytochemistry 2000, 53, 861–868. [Google Scholar] [CrossRef]
- Elgamal, A.H.M.; Soliman, M.S.H.; Karawya, S.M.; Mikhova, B.; Duddeck, H. Isolation of triterpene saponins from Gypsophila capillaris. Phytochemistry 1995, 38, 1481–1485. [Google Scholar] [CrossRef]
- Morita, H.; Yun, S.Y.; Takeya, K.; Itokawa, H.; Yamada, K.; Shirota, O. Vaccaroid A, a new triterpenoid saponin with contractility of rat uterine from Vaccaria segetalis. Bioorg. Med. Chem. Lett. 1997, 7, 1095–1096. [Google Scholar] [CrossRef]
- Koike, K.; Jia, Z.; Nikaido, T. Triterpenoid saponins from Vaccaria segetalis. Phytochemistry 1998, 47, 1343–1349. [Google Scholar] [CrossRef]
- Sang, M.S.; Zou, L.M.; Lao, N.A.; Chen, L.Z.; Uzawa, J.; Fujimoto, Y. A new triterpenoid saponin from the seeds of Vaccaria segetalis. Chin. Chem. Lett. 2000, 11, 49–53. [Google Scholar]
- Postel, R.; Fuchs, H. Improved Cell-Targeting Binding Molecule. International Publication No. WO 2020/126627 A1, 25 June 2020.
- McIlwain, D.R.; Berger, T.; Mak, W.T. Caspase function in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.J.; Li, L.; Fattah, J.F.; Dong, Y.; Bey, A.E.; Patel, M.; Gao, J.; Boothman, A.D. Review of poly (ADP-ribose) polymerase (PARP) mechanism of action and rationale for targeting in cancer and other diseases. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eeva, J.; Nuutinen, U.; Ropponen, A.; Mättö, M.; Eray, M.; Pellinen, R.; Wahlfors, J.; Pelkonen, J. The involvement of mitochondria and the caspase-9 activation pathway in rituximab-induced apoptosis in FL cells. Apoptosis 2009, 14, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Pena-Blanco, A.; Garcia-Saez, A. Bax, Bak and beyond-mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef] [Green Version]
- Circu, L.M.; Aw, Y.T. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Bates-Averill, A.D.; Redza-Dutordoir, M. Activation of apoptosis signaling pathway by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar]
- Stergiou, E.I.; Kapsogeorgou, K.E. Autophagy and metabolism in normal and malignant hematopoiesis. Int. J. Mol. Sci. 2021, 22, 8540. [Google Scholar] [CrossRef]
- Wang, Y.; Nartiss, Y.; Steipe, B.; McQuibban, A.; Kim, K.P. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy 2012, 8, 1462–1476. [Google Scholar] [CrossRef] [Green Version]
- Xiao, B.; Goh, Y.J.; Xiao, L.; Xian, H.; Lim, L.K.; Liou, C.Y. Reactive oxygen species trigger Parkin/PINK1 pathway-dependent mitophagy by inducing mitochondrial recruitment of Parkin. J. Biol. Chem. 2017, 292, 16697–16708. [Google Scholar] [CrossRef] [Green Version]
- Castrejón-Jiménez, S.N.; Leyva-Paredes, K.; Baltierra-Uribe, L.S.; Castillo-Cruz, J.; Campillo-Navarro, M.; Hernández-Pérez, D.A.; Luna-Angulo, B.A.; Chacón-Salinas, R.; Coral-Vázquez, M.R.; Estrada-García, I.; et al. Ursolic and Oleanolic Acids Induce Mitophagy in A549 Human Lung Cancer Cells. Molecules 2019, 24, 3444. [Google Scholar] [CrossRef] [Green Version]












| Compounds | HL-60 Cells | A549 Cells | SBC-3 Cells | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | IC50 (μM) | IC50 (μM) | |||||||
| 1 | 1.8 | ± | 0.038 | 8.4 | ± | 0.055 | 0.84 | ± | 0.012 |
| 2 | 4.0 | ± | 0.039 | 21 | ± | 0.17 | 1.5 | ± | 0.052 |
| 3 | 5.4 | ± | 0.084 | 10 | ± | 0.10 | 1.7 | ± | 0.015 |
| 4 | 0.57 | ± | 0.0030 | 3.3 | ± | 0.21 | 0.59 | ± | 0.0015 |
| 5 | 21 | ± | 0.26 | >50 | 10 | ± | 0.15 | ||
| 6 | >50 | >50 | >50 | ||||||
| 7 | >50 | >50 | >50 | ||||||
| 8 | >50 | >50 | >50 | ||||||
| 9 | 11 | ± | 0.27 | 16 | ± | 0.020 | 1.7 | ± | 0.023 |
| 10 | 1.1 | ± | 0.010 | 7.7 | ± | 0.062 | 0.61 | ± | 0.0072 |
| 11 | >50 | >50 | >50 | ||||||
| 12 | >50 | >50 | >50 | ||||||
| 13 | >50 | >50 | >50 | ||||||
| 14 | >50 | >50 | >50 | ||||||
| 15 | >50 | >50 | >50 | ||||||
| 16 | >50 | >50 | >50 | ||||||
| 17 | >50 | >50 | >50 | ||||||
| Cisplatin | 2.0 | ± | 0.019 | 2.5 | ± | 0.079 | 0.23 | ± | 0.0049 |
| 1 | 2 | ||||||||
| Positions | δH | J (Hz) | Positions | δH | J (Hz) | ||||
| GlcUA | GlcUA | ||||||||
| 1’ | 4.89 | d | 7.8 | 1’ | 4.89 | d | 7.8 | ||
| 2’ | 4.34 | dd | 8.3, 7.8 | 2’ | 4.33 | dd | 8.4, 7.8 | ||
| 3’ | 4.24 | dd | 9.1, 8.3 | 3’ | 4.22 | dd | 9.0, 8.4 | ||
| 4’ | 4.45 | dd | 9.4, 9.1 | 4’ | 4.44 | dd | 9.6, 9.0 | ||
| 5’ | 4.50 | d | 9.4 | 5’ | 4.49 | d | 9.6 | ||
| Gal | Gal | ||||||||
| 1’’ | 5.54 | d | 7.7 | 1’’ | 5.53 | * | |||
| 2’’ | 4.46 | dd | 8.8, 7.7 | 2’’ | 4.44 | dd | 9.0, 7.8 | ||
| 3’’ | 4.16 | dd | 8.8, 3.2 | 3’’ | 4.15 | dd | 9.0, 3.6 | ||
| 4’’ | 4.58 | br d | 3.2 | 4’’ | 4.56 | br d | 3.6 | ||
| 5’’ | 4.02 | m | 5’’ | 4.00 | m | ||||
| 6’’ | a | 4.51 | m | 6’’ | a | 4.50 | m | ||
| b | 4.42 | m | b | 4.41 | dd | 10.8, 6.0 | |||
| Xyl (I) | Xyl (I) | ||||||||
| 1’’’ | 5.31 | d | 7.7 | 1’’’ | 5.30 | d | 7.8 | ||
| 2’’’ | 3.96 | dd | 9.1, 7.7 | 2’’’ | 3.96 | dd | 8.4, 7.8 | ||
| 3’’’ | 4.13 | m | 3’’’ | 4.11 | dd | 8.4, 7.8 | |||
| 4’’’ | 4.12 | m | 4’’’ | 4.12 | m | ||||
| 5’’’ | a | 4.23 | dd | 11.7, 5.3 | 5’’’ | a | 4.24 | m | |
| b | 3.65 | m | b | 3.65 | dd | 11.2, 9.3 | |||
| Fuc | Fuc | ||||||||
| 1’’’’ | 5.88 | d | 8.2 | 1’’’’ | 5.89 | d | 8.4 | ||
| 2’’’’ | 4.45 | dd | 9.1, 8.2 | 2’’’’ | 4.61 | dd | 9.0, 8.4 | ||
| 3’’’’ | 4.14 | dd | 9.1, 3.1 | 3’’’’ | 4.21 | m | |||
| 4’’’’ | 3.98 | br d | 3.1 | 4’’’’ | 4.01 | br d | 3.0 | ||
| 5’’’’ | 3.91 | m | 5’’’’ | 3.99 | m | ||||
| 6’’’’ | 1.53 | d | 5.8 | 6’’’’ | 1.59 | d | 6.0 | ||
| Rha | Rha | ||||||||
| 1’’’’’ | 6.00 | br s | 1’’’’’ | 6.32 | br s | ||||
| 2’’’’’ | 5.11 | br s | 2’’’’’ | 4.97 | br s | ||||
| 3’’’’’ | 4.80 | dd | 9.0, 2.7 | 3’’’’’ | 4.64 | br d | 9.0 | ||
| 4’’’’’ | 4.52 | m | 4’’’’’ | 4.53 | dd | 9.0, 8.7 | |||
| 5’’’’’ | 4.50 | m | 5’’’’’ | 4.55 | m | ||||
| 6’’’’’ | 1.66 | d | 5.8 | 6’’’’’ | 1.66 | d | 4.8 | ||
| Glc | Glc | ||||||||
| 1’’’’’’ | 5.39 | d | 7.7 | 1’’’’’’ | 5.24 | d | 7.8 | ||
| 2’’’’’’ | 4.02 | dd | 8.8, 7.7 | 2’’’’’’ | 3.97 | dd | 9.0, 7.8 | ||
| 3’’’’’’ | 4.13 | dd | 8.8, 8.8 | 3’’’’’’ | 4.04 | dd | 9.0, 9.0 | ||
| 4’’’’’’ | 3.91 | dd | 8.8, 8.8 | 4’’’’’’ | 3.91 | dd | 9.0, 9.0 | ||
| 5’’’’’’ | 4.19 | m | 5’’’’’’ | 4.09 | m | ||||
| 6’’’’’’ | a | 4.50 | m | 6’’’’’’ | a | 4.91 | br d | 11.4 | |
| b | 4.28 | dd | 11.8, 5.3 | b | 4.65 | m | |||
| Xyl (II) | Xyl (II) | ||||||||
| 1’’’’’’’ | 5.50 | d | 7.9 | 1’’’’’’’ | 5.48 | d | 7.8 | ||
| 2’’’’’’’ | 3.95 | dd | 9.1, 7.9 | 2’’’’’’’ | 3.91 | dd | 8.7, 7.8 | ||
| 3’’’’’’’ | 4.14 | m | 3’’’’’’’ | 4.09 | dd | 8.7, 7.8 | |||
| 4’’’’’’’ | 4.12 | m | 4’’’’’’’ | 4.12 | m | ||||
| 5’’’’’’’ | a | 4.17 | m | 5’’’’’’’ | a | 4.17 | m | ||
| b | 3.42 | dd | 9.1, 9.1 | b | 3.40 | dd | 10.2, 10.2 | ||
| Qui | Qui | ||||||||
| 1’’’’’’’’ | 4.98 | d | 7.9 | 1’’’’’’’’ | 4.97 | d | 6.6 | ||
| 2’’’’’’’’ | 3.98 | m | 2’’’’’’’’ | 4.01 | dd | 8.7, 6.6 | |||
| 3’’’’’’’’ | 3.98 | m | 3’’’’’’’’ | 4.04 | dd | 8.7, 8.7 | |||
| 4’’’’’’’’ | 4.08 | dd | 8.8, 8.8 | 4’’’’’’’’ | 3.62 | m | |||
| 5’’’’’’’’ | 3.63 | m | 5’’’’’’’’ | 3.65 | m | ||||
| 6’’’’’’’’ | 1.54 | d | 6.5 | 6’’’’’’’’ | 1.54 | d | 6.0 | ||
| Ac | 2.14 | s | |||||||
| 3 | 4 | ||||||||
| Positions | δH | J (Hz) | Positions | δH | J (Hz) | ||||
| GlcUA | GlcUA | ||||||||
| 1’ | 4.89 | d | 7.2 | 1’ | 4.89 | d | 7.2 | ||
| 2’ | 4.33 | dd | 9.0, 7.2 | 2’ | 4.33 | dd | 8.4, 7.2 | ||
| 3’ | 4.27 | dd | 9.0, 9.0 | 3’ | 4.25 | dd | 9.0, 8.4 | ||
| 4’ | 4.45 | dd | 9.6, 9.0 | 4’ | 4.45 | dd | 9.6, 9.0 | ||
| 5’ | 4.51 | d | 9.6 | 5’ | 4.50 | d | 9.6 | ||
| Gal | Gal | ||||||||
| 1’’ | 5.55 | d | 7.8 | 1’’ | 5.55 | * | |||
| 2’’ | 4.47 | dd | 9.0, 7.8 | 2’’ | 4.46 | dd | 9.0, 7.8 | ||
| 3’’ | 4.15 | dd | 9.0, 3.6 | 3’’ | 4.14 | dd | 9.0, 3.6 | ||
| 4’’ | 4.57 | br d | 3.6 | 4’’ | 4.57 | br d | 3.6 | ||
| 5’’ | 4.00 | m | 5’’ | 3.99 | m | ||||
| 6’’ | a | 4.53 | dd | 10.8, 6.6 | 6’’ | a | 4.52 | m | |
| b | 4.41 | dd | 10.8, 5.4 | b | 4.41 | dd | 10.2, 6.0 | ||
| Xyl (I) | Xyl (I) | ||||||||
| 1’’’ | 5.32 | d | 7.8 | 1’’’ | 5.32 | d | 7.8 | ||
| 2’’’ | 3.97 | dd | 9.0, 7.8 | 2’’’ | 3.96 | dd | 8.4, 7.8 | ||
| 3’’’ | 4.11 | m | 3’’’ | 4.11 | dd | 8.4, 7.8 | |||
| 4’’’ | 4.12 | m | 4’’’ | 4.12 | m | ||||
| 5’’’ | a | 4.25 | dd | 10.2, 5.4 | 5’’’ | a | 4.23 | m | |
| b | 3.65 | dd | 10.2, 9.0 | b | 3.65 | dd | 9.0, 8.1 | ||
| Fuc | Fuc | ||||||||
| 1’’’’ | 5.94 | d | 8.4 | 1’’’’ | 5.92 | d | 8.4 | ||
| 2’’’’ | 4.57 | dd | 9.0, 8.4 | 2’’’’ | 4.48 | dd | 9.0, 8.4 | ||
| 3’’’’ | 4.16 | dd | 9.0, 3.6 | 3’’’’ | 4.18 | dd | 9.0, 3.0 | ||
| 4’’’’ | 3.97 | br d | 3.6 | 4’’’’ | 3.99 | br d | 3.0 | ||
| 5’’’’ | 3.93 | dd | 6.6, 3.6 | 5’’’’ | 3.93 | m | |||
| 6’’’’ | 1.56 | d | 6.6 | 6’’’’ | 1.51 | d | 6.0 | ||
| Rha | Rha | ||||||||
| 1’’’’’ | 6.34 | br s | 1’’’’’ | 6.20 | br s | ||||
| 2’’’’’ | 4.74 | br s | 2’’’’’ | 4.70 | br s | ||||
| 3’’’’’ | 4.65 | dd | 9.0, 3.0 | 3’’’’’ | 4.61 | br d | 9.0 | ||
| 4’’’’’ | 4.37 | dd | 9.6, 9.0 | 4’’’’’ | 4.34 | dd | 9.0, 8.7 | ||
| 5’’’’’ | 4.44 | dd | 9.6, 6.0 | 5’’’’’ | 4.38 | m | |||
| 6’’’’’ | 1.62 | d | 6.0 | 6’’’’’ | 1.54 | d | 4.8 | ||
| Xyl (II) | Xyl (II) | ||||||||
| 1’’’’’’ | 5.16 | d | 7.2 | 1’’’’’’ | 5.15 | d | 7.2 | ||
| 2’’’’’’ | 4.00 | dd | 9.0, 7.2 | 2’’’’’’ | 3.98 | dd | 9.0, 7.2 | ||
| 3’’’’’’ | 4.01 | m | 3’’’’’’ | 4.01 | m | ||||
| 4’’’’’’ | 4.02 | m | 4’’’’’’ | 4.01 | m | ||||
| 5’’’’’’ | a | 4.14 | dd | 12.0, 4.8 | 5’’’’’’ | a | 4.12 | dd | 12.0, 5.4 |
| b | 3.40 | dd | 12.0, 10.2 | b | 3.39 | dd | 12.0, 9.6 | ||
| Glc | Glc | ||||||||
| 1’’’’’’’ | 5.18 | d | 7.8 | 1’’’’’’’ | 5.18 | d | 7.8 | ||
| 2’’’’’’’ | 4.08 | dd | 9.0, 7.8 | 2’’’’’’’ | 4.08 | dd | 9.0, 7.8 | ||
| 3’’’’’’’ | 4.22 | dd | 9.0, 9.0 | 3’’’’’’’ | 4.21 | dd | 9.0, 9.0 | ||
| 4’’’’’’’ | 4.16 | dd | 9.0, 9.0 | 4’’’’’’’ | 4.16 | dd | 9.0, 9.0 | ||
| 5’’’’’’’ | 3.96 | m | 5’’’’’’’ | 3.96 | m | ||||
| 6’’’’’’’ | a | 4.48 | dd | 10.8, 4.2 | 6’’’’’’’ | a | 4.48 | dd | 11.4, 4.2 |
| b | 4.26 | dd | 10.8, 6.0 | b | 4.26 | dd | 11.4, 5.4 | ||
| Qui | Qui | ||||||||
| 1’’’’’’’’ | 4.97 | d | 7.8 | 1’’’’’’’’ | 5.05 | d | 7.2 | ||
| 2’’’’’’’’ | 4.00 | dd | 9.0, 7.8 | 2’’’’’’’’ | 4.00 | dd | 9.6, 7.2 | ||
| 3’’’’’’’’ | 4.05 | dd | 9.0, 9.0 | 3’’’’’’’’ | 5.60 | dd | 9.6, 9.6 | ||
| 4’’’’’’’’ | 3.62 | dd | 9.0, 9.0 | 4’’’’’’’’ | 5.05 | dd | 9.6, 9.6 | ||
| 5’’’’’’’’ | 3.66 | dd | 9.0, 6.0 | 5’’’’’’’’ | 3.69 | dd | 9.6, 6.0 | ||
| 6’’’’’’’’ | 1.55 | d | 6.0 | 6’’’’’’’’ | 1.24 | d | 6.0 | ||
| 3’’’’’’’’-OAc | 1.96 | s | |||||||
| 4’’’’’’’’-OAc | 2.04 | s | |||||||
| 5 | 6 | ||||||||
| Positions | δH | J (Hz) | Positions | δH | J (Hz) | ||||
| Xyl | Xyl | ||||||||
| 1’ | 4.99 | d | 7.4 | 1’ | 4.97 | d | 7.4 | ||
| 2’ | 3.96 | dd | 8.6, 7.4 | 2’ | 3.94 | dd | 8.6, 7.4 | ||
| 3’ | 4.06 | dd | 8.6, 8.6 | 3’ | 4.05 | dd | 8.6, 8.6 | ||
| 4’ | 4.20 | m | 4’ | 4.18 | m | ||||
| 5’ | a | 4.35 | dd | 11.5, 5.2 | 5’ | a | 4.34 | dd | 11.6, 4.9 |
| b | 3.68 | dd | 11.5, 10.0 | b | 3.67 | dd | 11.6, 11.0 | ||
| Glc (I) | Glc (I) | ||||||||
| 1’’ | 6.22 | d | 7.7 | 1’’ | 6.17 | d | 7.1 | ||
| 2’’ | 4.18 | dd | 9.2, 7.7 | 2’’ | 4.24 | m | |||
| 3’’ | 4.21 | dd | 9.2, 9.2 | 3’’ | 4.25 | m | |||
| 4’’ | 4.55 | dd | 9.2, 9.2 | 4’’ | 4.33 | m | |||
| 5’’ | 4.05 | m | 5’’ | 4.11 | m | ||||
| 6’’ | a | 4.56 | br d | 10.8 | 6’’ | a | 4.53 | br d | 11.5 |
| b | 4.29 | dd | 10.8, 3.9 | b | 4.30 | dd | 11.5, 3.6 | ||
| Glc (II) | Glc (II) | ||||||||
| 1’’’ | 4.91 | d | 7.8 | 1’’’ | 5.28 | d | 7.8 | ||
| 2’’’ | 3.99 | dd | 9.1, 7.8 | 2’’’ | 4.05 | m | |||
| 3’’’ | 4.25 | dd | 9.1, 9.1 | 3’’’ | 4.14 | dd | 9.2, 9.2 | ||
| 4’’’ | 4.20 | dd | 9.1, 9.1 | 4’’’ | 4.13 | dd | 9.2, 9.2 | ||
| 5’’’ | 3.76 | m | 5’’’ | 3.91 | m | ||||
| 6’’’ | a | 4.38 | dd | 12.3, 2.2 | 6’’’ | a | 4.44 | br d | 11.1 |
| b | 4.31 | br d | 12.3 | b | 4.25 | dd | 11.1, 5.6 | ||
| Glc (III) | Glc (III) | ||||||||
| 1’’’’ | 5.26 | d | 7.8 | 1’’’’ | 4.99 | d | 7.7 | ||
| 2’’’’ | 4.03 | dd | 8.5, 7.8 | 2’’’’ | 4.06 | dd | 8.8, 7.7 | ||
| 3’’’’ | 4.17 | m | 3’’’’ | 4.22 | dd | 8.8, 8.8 | |||
| 4’’’’ | 4.18 | m | 4’’’’ | 3.99 | dd | 8.8, 8.8 | |||
| 5’’’’ | 3.90 | m | 5’’’’ | 3.89 | m | ||||
| 6’’’’ | a | 4.51 | dd | 12.1, 2.2 | 6’’’’ | a | 4.92 | br d | 11.2 |
| b | 4.34 | br d | 12.1 | b | 4.68 | dd | 11.2, 5.8 | ||
| Glc (IV) | |||||||||
| 1’’’’’ | 5.28 | d | 7.8 | ||||||
| 2’’’’’ | 4.05 | m | |||||||
| 3’’’’’ | 4.18 | dd | 8.5, 8.5 | ||||||
| 4’’’’’ | 4.15 | dd | 8.5, 8.5 | ||||||
| 5’’’’’ | 3.90 | m | |||||||
| 6’’’’’ | a | 4.54 | br d | 11.8 | |||||
| b | 4.34 | dd | 11.8, 5.2 | ||||||
| HMG | |||||||||
| 1’’’’’’ | – | ||||||||
| 2’’’’’’ | a | 3.16 | d | 14.3 | |||||
| b | 3.11 | d | 14.3 | ||||||
| 3’’’’’’ | – | ||||||||
| 4’’’’’’ | a | 3.18 | d | 15.0 | |||||
| b | 3.15 | d | 15.0 | ||||||
| 5’’’’’’ | – | ||||||||
| 6’’’’’’ | 1.75 | s | |||||||
| 7 | |||||||||
| Positions | δH | J (Hz) | |||||||
| Xyl | |||||||||
| 1’ | 4.97 | d | 7.7 | ||||||
| 2’ | 3.95 | dd | 8.2, 7.7 | ||||||
| 3’ | 4.05 | dd | 8.9, 8.2 | ||||||
| 4’ | 4.19 | m | |||||||
| 5’ | a | 4.35 | dd | 11.4, 5.0 | |||||
| b | 3.68 | dd | 11.4, 10.6 | ||||||
| Glc (I) | |||||||||
| 1’’ | 6.18 | d | 7.9 | ||||||
| 2’’ | 4.20 | m | |||||||
| 3’’ | 4.24 | dd | 9.1, 9.1 | ||||||
| 4’’ | 4.27 | dd | 9.1, 9.1 | ||||||
| 5’’ | 4.11 | m | |||||||
| 6’’ | a | 4.52 | br d | 11.6 | |||||
| b | 4.29 | m | |||||||
| Glc (II) | |||||||||
| 1’’’ | 5.28 | d | 7.7 | ||||||
| 2’’’ | 4.05 | m | |||||||
| 3’’’ | 4.14 | dd | 9.0, 9.0 | ||||||
| 4’’’ | 4.13 | dd | 9.0, 9.0 | ||||||
| 5’’’ | 3.90 | m | |||||||
| 6’’’ | a | 4.45 | dd | 11.8, 2.0 | |||||
| b | 4.25 | dd | 11.8, 5.1 | ||||||
| Glc (III) | |||||||||
| 1’’’’ | 4.98 | d | 7.3 | ||||||
| 2’’’’ | 4.05 | dd | 9.2, 7.3 | ||||||
| 3’’’’ | 4.23 | dd | 9.2, 9.2 | ||||||
| 4’’’’ | 3.99 | dd | 9.2, 9.2 | ||||||
| 5’’’’ | 3.90 | m | |||||||
| 6’’’’ | a | 4.93 | br d | 10.5 | |||||
| b | 4.69 | dd | 10.5, 5.8 | ||||||
| Glc (IV) | |||||||||
| 1’’’’’ | 5.29 | d | 7.6 | ||||||
| 2’’’’’ | 4.05 | m | |||||||
| 3’’’’’ | 4.17 | dd | 8.6, 8.6 | ||||||
| 4’’’’’ | 4.18 | dd | 8.6, 8.6 | ||||||
| 5’’’’’ | 3.90 | m | |||||||
| 6’’’’’ | a | 4.52 | br d | 11.7 | |||||
| b | 4.33 | dd | 11.7, 4.8 | ||||||
| HMG | |||||||||
| 1’’’’’’ | – | ||||||||
| 2’’’’’’ | a | 3.18 | d | 14.3 | |||||
| b | 3.12 | d | 14.3 | ||||||
| 3’’’’’’ | – | ||||||||
| 4’’’’’’ | a | 3.19 | d | 15.1 | |||||
| b | 3.15 | d | 15.1 | ||||||
| 5’’’’’’ | – | ||||||||
| 6’’’’’’ | 1.75 | s | |||||||
| Positions | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1 | 38.1 | 38.2 | 38.1 | 38.2 | 38.9 | 38.8 | 38.9 |
| 2 | 25.3 | 25.3 | 25.3 | 25.3 | 26.3 | 26.3 | 26.3 |
| 3 | 84.4 | 84.4 | 84.5 | 84.4 | 85.0 | 85.0 | 85.0 |
| 4 | 55.0 | 55.1 | 55.1 | 55.1 | 53.3 | 53.3 | 53.3 |
| 5 | 48.9 | 49.0 | 48.8 | 49.0 | 52.1 | 52.1 | 52.2 |
| 6 | 20.5 | 20.7 | 20.6 | 20.7 | 21.3 | 21.3 | 21.3 |
| 7 | 33.0 | 33.1 | 32.8 | 33.1 | 33.1 | 32.8 | 33.1 |
| 8 | 40.3 | 40.3 | 40.3 | 40.3 | 40.4 | 40.2 | 40.3 |
| 9 | 46.9 | 47.0 | 46.9 | 47.0 | 47.4 | 48.3 | 47.4 |
| 10 | 36.2 | 36.3 | 36.3 | 36.3 | 36.7 | 36.7 | 36.7 |
| 11 | 23.7 | 23.7 | 23.7 | 23.7 | 23.8 | 23.8 | 23.8 |
| 12 | 121.9 | 121.8 | 122.1 | 121.8 | 122.3 | 122.7 | 122.5 |
| 13 | 144.5 | 144.5 | 144.5 | 144.5 | 144.5 | 144.0 | 144.4 |
| 14 | 42.1 | 42.2 | 42.1 | 42.2 | 42.0 | 42.1 | 42.0 |
| 15 | 36.2 | 36.4 | 36.2 | 36.4 | 36.0 | 28.2 | 36.0 |
| 16 | 74.2 | 74.0 | 74.0 | 74.0 | 74.1 | 23.1 | 74.0 |
| 17 | 49.2 | 49.3 | 49.2 | 49.3 | 49.0 | 47.0 | 49.0 |
| 18 | 41.7 | 41.9 | 41.6 | 41.9 | 41.2 | 41.6 | 41.2 |
| 19 | 47.5 | 47.7 | 47.3 | 47.7 | 47.1 | 46.2 | 47.1 |
| 20 | 30.6 | 30.6 | 30.7 | 30.6 | 30.7 | 30.7 | 30.7 |
| 21 | 36.0 | 36.1 | 36.0 | 36.1 | 35.8 | 33.9 | 35.8 |
| 22 | 31.6 | 31.4 | 31.9 | 31.4 | 32.0 | 32.4 | 32.0 |
| 23 | 210.1 | 210.2 | 210.2 | 210.2 | 180.3 | 180.4 | 180.4 |
| 24 | 11.1 | 11.2 | 11.1 | 11.2 | 12.6 | 12.5 | 12.6 |
| 25 | 15.9 | 15.9 | 15.8 | 15.9 | 16.1 | 16.0 | 16.1 |
| 26 | 17.4 | 17.4 | 17.4 | 17.4 | 17.4 | 17.3 | 17.4 |
| 27 | 27.1 | 27.2 | 27.0 | 27.2 | 27.1 | 26.0 | 27.1 |
| 28 | 175.9 | 176.0 | 176.0 | 176.0 | 175.9 | 176.3 | 175.8 |
| 29 | 33.1 | 33.0 | 33.1 | 33.0 | 33.1 | 33.0 | 33.1 |
| 30 | 24.4 | 24.4 | 24.5 | 24.4 | 24.5 | 23.7 | 24.6 |
| GlcUA | GlcUA | GlcUA | GlcUA | Xyl | Xyl | Xyl | |
| 1’ | 103.9 | 103.9 | 103.9 | 103.9 | 106.2 | 106.2 | 106.2 |
| 2’ | 78.6 | 78.7 | 78.4 | 78.4 | 75.2 | 75.2 | 75.2 |
| 3’ | 86.0 | 86.0 | 86.1 | 86.1 | 78.0 | 78.0 | 78.0 |
| 4’ | 71.3 | 71.3 | 71.3 | 71.3 | 71.0 | 71.1 | 71.0 |
| 5’ | 77.2 | 77.2 | 77.3 | 77.3 | 67.0 | 67.0 | 67.0 |
| 6’ | 171.7 | 171.7 | 171.7 | 171.7 | |||
| Gal | Gal | Gal | Gal | Glc (I) | Glc (I) | Glc (I) | |
| 1’’ | 104.2 | 104.2 | 104.1 | 104.1 | 95.7 | 94.8 | 94.9 |
| 2’’ | 73.7 | 73.7 | 73.6 | 73.6 | 74.0 | 73.0 | 73.0 |
| 3’’ | 75.4 | 75.2 | 75.5 | 75.5 | 78.5 | 88.2 | 88.0 |
| 4’’ | 70.1 | 70.1 | 70.1 | 70.1 | 70.2 | 69.1 | 69.1 |
| 5’’ | 76.6 | 76.6 | 76.5 | 76.6 | 77.1 | 76.9 | 76.9 |
| 6’’ | 61.6 | 61.6 | 61.6 | 61.6 | 69.1 | 68.9 | 69.0 |
| Xyl (I) | Xyl (I) | Xyl (I) | Xyl (I) | Glc (II) | Glc (II) | Glc (II) | |
| 1’’’ | 105.0 | 105.0 | 105.0 | 105.0 | 102.7 | 105.7 | 105.7 |
| 2’’’ | 75.3 | 75.3 | 75.3 | 75.2 | 84.4 | 75.6 | 75.6 |
| 3’’’ | 78.5 | 78.6 | 78.6 | 78.6 | 78.1 | 77.9 | 78.1 |
| 4’’’ | 70.8 | 70.8 | 70.8 | 70.8 | 70.5 | 71.3 | 71.3 |
| 5’’’ | 67.3 | 67.3 | 67.3 | 67.3 | 78.3 | 78.5 | 78.5 |
| 6’’’ | 62.0 | 62.3 | 62.3 | ||||
| Fuc | Fuc | Fuc | Fuc | Glc (III) | Glc (III) | Glc (III) | |
| 1’’’’ | 94.8 | 94.7 | 94.6 | 94.5 | 106.1 | 102.5 | 102.5 |
| 2’’’’ | 75.2 | 73.8 | 74.4 | 74.3 | 76.3 | 83.3 | 83.2 |
| 3’’’’ | 75.9 | 76.8 | 76.8 | 76.2 | 78.1 | 77.7 | 77.7 |
| 4’’’’ | 83.4 | 84.0 | 84.0 | 83.5 | 70.9 | 71.0 | 70.9 |
| 5’’’’ | 71.5 | 71.8 | 71.6 | 71.4 | 78.6 | 75.0 | 75.0 |
| 6’’’’ | 17.1 | 17.1 | 17.1 | 17.0 | 62.0 | 64.4 | 64.3 |
| Rha | Rha | Rha | Rha | Glc (IV) | Glc (IV) | ||
| 1’’’’’ | 101.7 | 100.9 | 101.2 | 101.2 | 105.6 | 105.7 | |
| 2’’’’’ | 70.8 | 71.2 | 71.8 | 71.7 | 76.2 | 76.2 | |
| 3’’’’’ | 82.3 | 83.1 | 72.4 | 72.3 | 78.1 | 78.0 | |
| 4’’’’’ | 78.2 | 78.2 | 83.5 | 83.4 | 71.2 | 71.1 | |
| 5’’’’’ | 68.9 | 68.3 | 68.3 | 68.3 | 78.4 | 78.4 | |
| 6’’’’’ | 18.9 | 18.8 | 18.6 | 18.5 | 62.5 | 62.4 | |
| Glc | Glc | Xyl (II) | Xyl (II) | HMG | HMG | ||
| 1’’’’’’ | 105.2 | 105.3 | 106.2 | 106.1 | 171.7 | 171.7 | |
| 2’’’’’’ | 75.4 | 75.1 | 74.8 | 74.8 | 46.6 | 46.5 | |
| 3’’’’’’ | 78.3 | 78.1 | 88.2 | 88.3 | 70.0 | 70.0 | |
| 4’’’’’’ | 71.7 | 71.7 | 69.3 | 69.3 | 46.3 | 46.3 | |
| 5’’’’’’ | 78.4 | 75.2 | 66.7 | 66.6 | 174.6 | 174.7 | |
| 6’’’’’’ | 62.6 | 64.4 | 28.2 | 28.2 | |||
| Xyl (II) | Xyl (II) | Glc | Glc | ||||
| 1’’’’’’’ | 104.9 | 104.8 | 105.5 | 105.5 | |||
| 2’’’’’’’ | 75.6 | 75.7 | 75.4 | 75.3 | |||
| 3’’’’’’’ | 79.1 | 79.1 | 78.3 | 78.3 | |||
| 4’’’’’’’ | 71.2 | 71.2 | 71.5 | 71.5 | |||
| 5’’’’’’’ | 67.1 | 67.1 | 78.6 | 78.6 | |||
| 6’’’’’’’ | 62.5 | 62.4 | |||||
| Qui | Qui | Qui | Qui | ||||
| 1’’’’’’’’ | 106.3 | 106.7 | 106.7 | 105.7 | |||
| 2’’’’’’’’ | 75.7 | 75.8 | 75.8 | 73.1 | |||
| 3’’’’’’’’ | 78.1 | 78.2 | 78.4 | 76.3 | |||
| 4’’’’’’’’ | 76.7 | 76.7 | 76.6 | 74.4 | |||
| 5’’’’’’’’ | 73.1 | 73.2 | 73.2 | 70.2 | |||
| 6’’’’’’’’ | 18.5 | 18.5 | 18.5 | 17.7 | |||
| 6’’’’’’-OAc | 3’’’’’’’’-OAc | ||||||
| 21.0 | 20.6 | ||||||
| 170.9 | 170.4 | ||||||
| 4’’’’’’’’-OAc | |||||||
| 20.7 | |||||||
| 170.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, N.; Iguchi, T.; Kuroda, M.; Mishima, M.; Mimaki, Y. Novel Oleanane-Type Triterpene Glycosides from the Saponaria officinalis L. Seeds and Apoptosis-Inducing Activity via Mitochondria. Int. J. Mol. Sci. 2022, 23, 2047. https://doi.org/10.3390/ijms23042047
Takahashi N, Iguchi T, Kuroda M, Mishima M, Mimaki Y. Novel Oleanane-Type Triterpene Glycosides from the Saponaria officinalis L. Seeds and Apoptosis-Inducing Activity via Mitochondria. International Journal of Molecular Sciences. 2022; 23(4):2047. https://doi.org/10.3390/ijms23042047
Chicago/Turabian StyleTakahashi, Naoki, Tomoki Iguchi, Minpei Kuroda, Masaki Mishima, and Yoshihiro Mimaki. 2022. "Novel Oleanane-Type Triterpene Glycosides from the Saponaria officinalis L. Seeds and Apoptosis-Inducing Activity via Mitochondria" International Journal of Molecular Sciences 23, no. 4: 2047. https://doi.org/10.3390/ijms23042047
APA StyleTakahashi, N., Iguchi, T., Kuroda, M., Mishima, M., & Mimaki, Y. (2022). Novel Oleanane-Type Triterpene Glycosides from the Saponaria officinalis L. Seeds and Apoptosis-Inducing Activity via Mitochondria. International Journal of Molecular Sciences, 23(4), 2047. https://doi.org/10.3390/ijms23042047





