Histological and Immunohistochemical Studies to Determine the Mechanism of Cleft Palate Induction after Palatal Fusion in Mice Exposed to TCDD
Abstract
:1. Introduction
2. Results
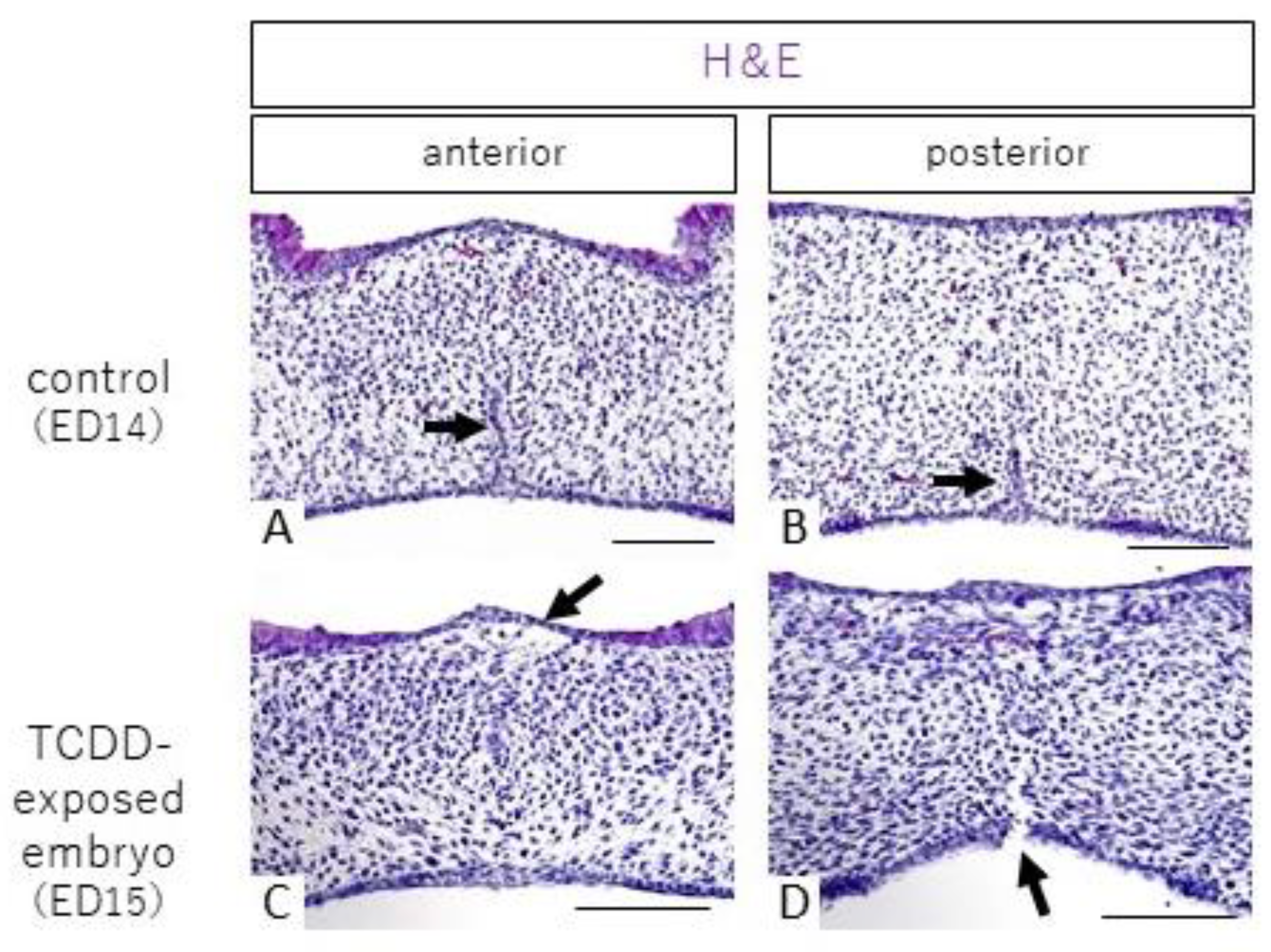
2.1. Histological Observations
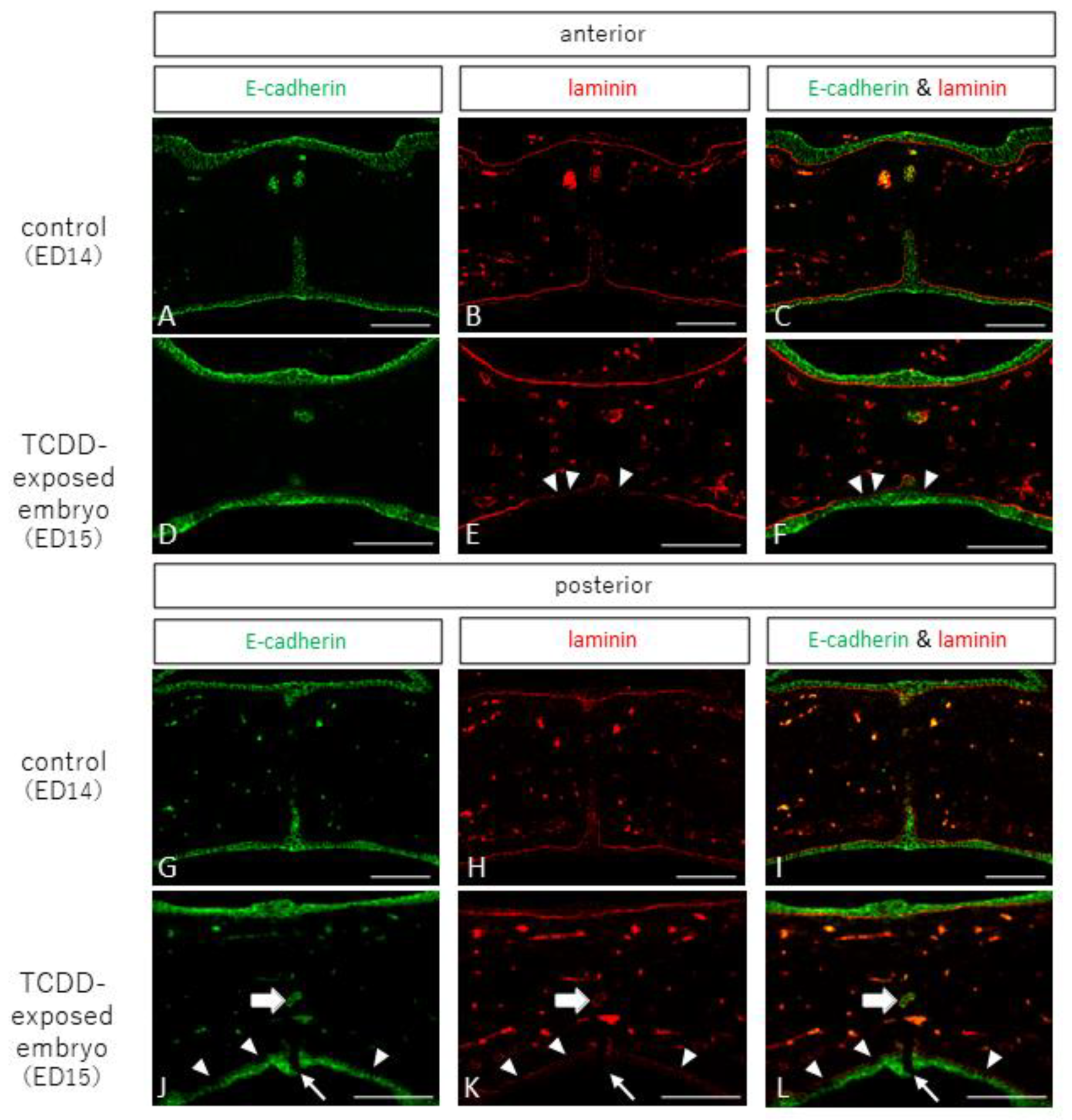
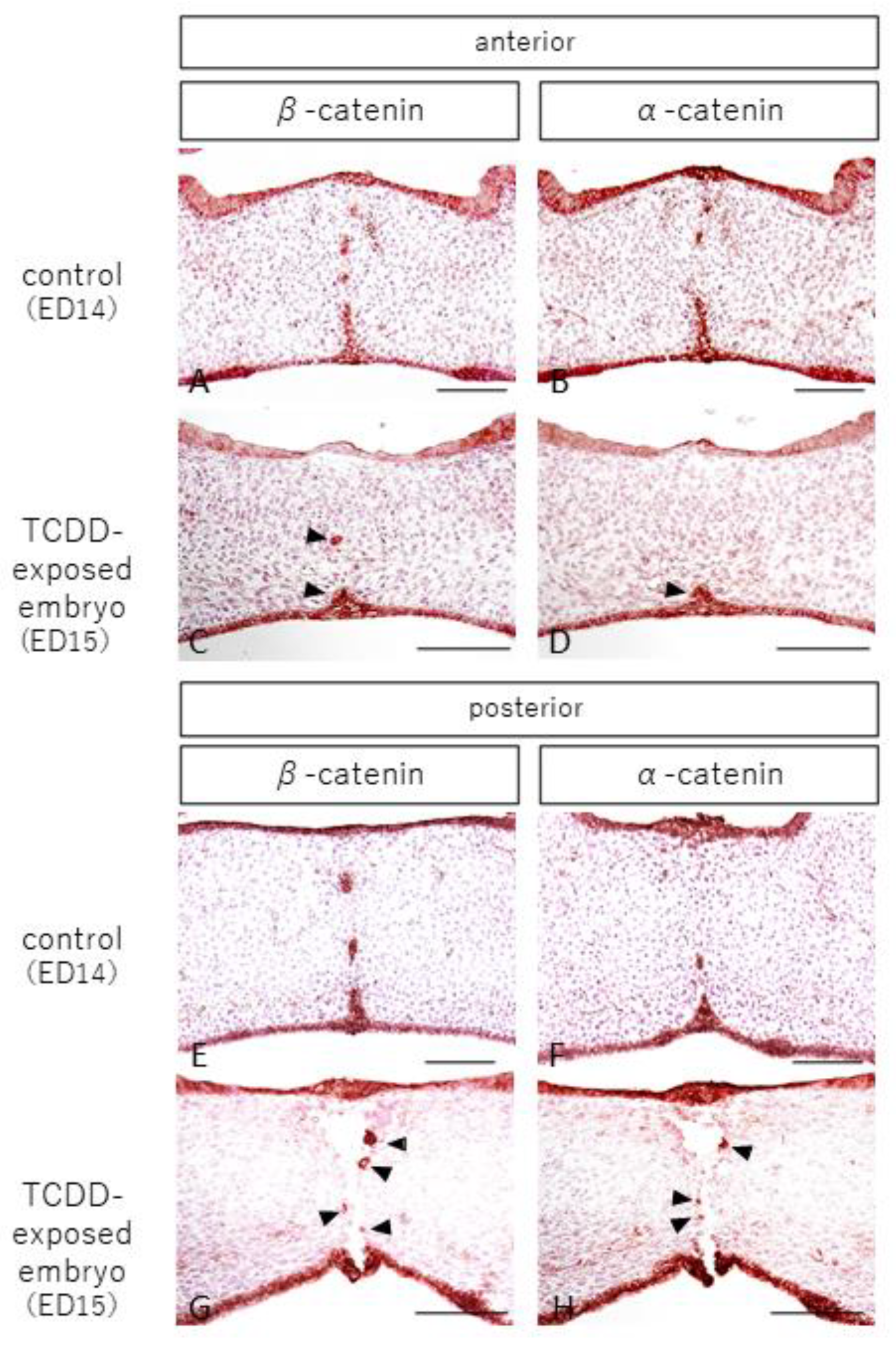
2.2. Observation of Epithelial and Interepithelial Cell Adhesion Factors and Basement Membrane
2.3. Observation of Apoptosis
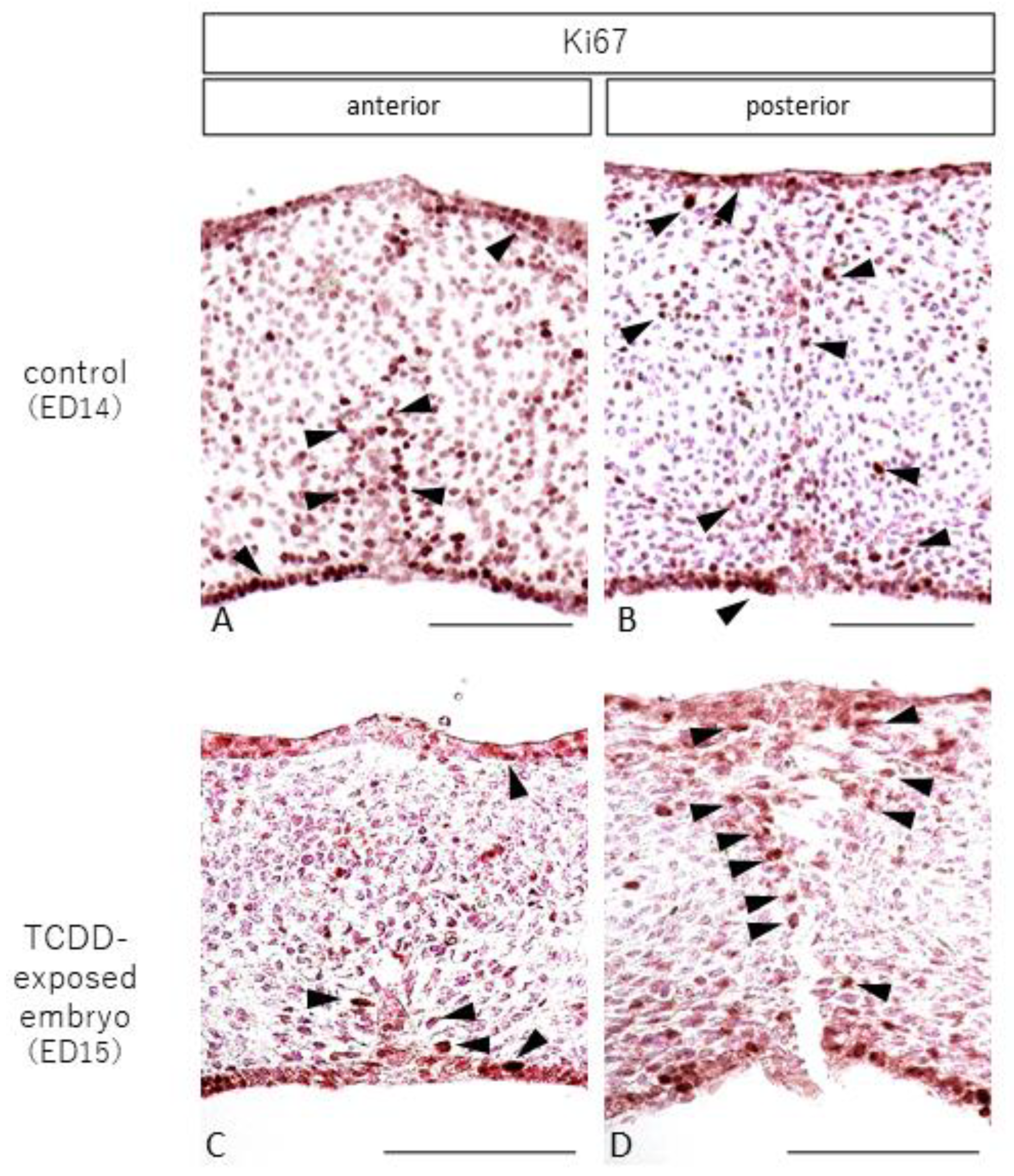
2.4. Observation of Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Embryo Extraction
4.2. Tissue Fixation and Section Preparation
4.3. Histological Observation
4.4. Immunohistological Observations
4.4.1. Observation of Epithelial Tissue, Interepithelial Cell Adhesion Factor, and Basement Membrane Tissue via Fluorescent Immunohistochemistry
4.4.2. Observation of Epithelial Cell Adhesion Factors
4.4.3. Observation of Apoptosis
4.4.4. Observation of Cell Proliferation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xue, A.S.; Buchanan, E.P.; Hollier, L.H. Update in Unilateral Cleft Lip Surgery. Plast. Reconstr. Surg. 2021, 148, 262e–274e. [Google Scholar] [CrossRef]
- Imura, H.; Suzuki, S.; Mizuno, S.; Sakuma, C.; Natsume, N. A case of Tetrasomy 15q with left cleft lip and alveolus. J. Oral Maxillofac. Surgery, Med. Pathol. 2017, 29, 427–429. [Google Scholar] [CrossRef]
- Natsume, N.; Furukawa, H.; Niimi, T.; Takeuchi, K.; Yoshida, W.; Sakuma, C.; Imura, H.; Fujiwara, K.; Akashi, J.; Hayami, K.; et al. Changes in the birth prevalence of orofacial clefts in Japan, Has the birth prevalence of orofacial clefts been affected by improved accuracy of prenatal diagnosis? Congenit. Anom. 2022, 62, 11–17. [Google Scholar] [CrossRef]
- Xu, J.; Liu, F.; Xiong, Z.; Huo, J.; Li, W.; Jiang, B.; Mao, W.; He, B.; Wang, X.; Li, G. The cleft palate candidate gene BAG6 supports FoxO1 acetylation to promote FasL-mediated apoptosis during palate fusion. Exp. Cell Res. 2020, 396, 112310. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.O.; Jiang, R. Palatogenesis: Morphogenetic and molecular mechanisms of secondary palate development. Development 2012, 139, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.A.; Oram, K.F.; Gridley, T. Multiple functions of Snail family genes during palate development in mice. Development 2007, 134, 1789–1797. [Google Scholar] [CrossRef] [Green Version]
- Fitch, N. Developmental of cleft palate in mice homozygous for the shorthead mutation. J. Morph. 1961, 109, 151–157. [Google Scholar] [CrossRef]
- Jin, J.-Z.; Warner, D.R.; Lu, Q.; Pisano, M.M.; Greene, R.M.; Ding, J. Deciphering TGF-β3 function in medial edge epithelium specification and fusion during mouse secondary palate development. Dev. Dyn. 2014, 243, 1536–1543. [Google Scholar] [CrossRef]
- Pratt, R.M. Mechanisms of fetal posture and casual mechanisms of congenital deformity of the palate, mandible and limbs. J. Dent. Res. 1966, 45, 584–596. [Google Scholar]
- Kitamura, H. Epithelial remnants and pearls in the secondary palate in the human abortus: A contribution to the study of the mechanism of cleft palate formation. Cleft Palate J. 1966, 3, 240–257. [Google Scholar] [PubMed]
- Kitamura, H. Evidence for cleft palate as a postfusion phenomenon. Cleft Palate J. 1990, 28, 195–211. [Google Scholar] [CrossRef]
- Mijiti, A.; Ling, W.; Guli; Moming, A. Association of single-nucleotide polymorphisms in the IRF6 gene with non-syndromic cleft lip with or without cleft palate in the Xinjiang Uyghur population. Br. J. Oral Maxillofac. Surg. 2015, 53, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Imura, H.; Suzuki, S.; Ono, M.; Hayakawa, T.; Minami, K.; Niimi, T.; Furukawa, H.; Komada, M.; Ikeda, Y.; et al. Genetic analysis of MEOX2 in Japanese non-syndromic cleft palate patients. Aichi Gakuin Dent. Sci. 2016, 29, 9–17. [Google Scholar]
- Tran, D.L.; Imura, H.; Mori, A.; Suzuki, S.; Niimi, T.; Ono, M.; Sakuma, C.; Nakahara, S.; Nguyen, T.T.; Pham, P.T.; et al. Association of MEOX2 polymorphism with nonsyndromic cleft palate only in a Vietnamese population. Congenit. Anomalies 2017, 58, 124–129. [Google Scholar] [CrossRef]
- Hirata, A.; Katayama, K.; Tsuji, T.; Imura, H.; Natsume, N.; Sugahara, T.; Kunieda, T.; Nakamura, H.; Otsuki, Y. Homeobox family Hoxc localization during murine palate formation. Congenit. Anomalies 2016, 56, 172–179. [Google Scholar] [CrossRef]
- Vaivads, M.; Akota, I.; Pilmane, M. Cleft Candidate Genes and Their Products in Human Unilateral Cleft Lip Tissue. Diseases 2021, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.M.; Suzuki, S.; Imura, H.; Niimi, T.; Furukawa, H.; Ta, T.V.; Tong, S.M.; Nguyen, T.T.; Pham, L.N.G.; Tran, D.L.; et al. Family based and case-control designs reveal an association of TFAP2A in non-syndromic cleft lip only among Vietnamese population. Mol. Genet. Genomic Med. 2021, 9, e1754. [Google Scholar] [CrossRef]
- Blanco, R.; Colombo, A.; Suazo, J. Maternal obesity is a risk factor for orofacial clefts: A meta-analysis. Br. J. Oral Maxillofac. Surg. 2015, 53, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Jaruga, A.; Ksiazkiewicz, J.; Kuzniarz, K.; Tylzanowski, P. Orofacial Cleft and Mandibular Prognathism—Human Genetics and Animal Models. Int. J. Mol. Sci. 2022, 23, 953. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, S.-N.; Yuan, X.-G.; Zhang, D.-W.; Jiang, H.; Qiu, L.; Fu, Y.-X. Common differentially expressed proteins were found in mouse cleft palate models induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin and retinoic acid. Environ. Toxicol. Pharmacol. 2019, 72, 103270. [Google Scholar] [CrossRef]
- Couture, L.A.; Harris, M.W.; Birnbaum, L.S. Characterization of the period of sensitivity for the induction of hydronephrosis in C57BL/6N mice following exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam. Appl. Toxicol. 1990, 15, 142–150. [Google Scholar] [CrossRef]
- Yamada, T.; Mishima, K.; Fujiwara, K.; Imura, H.; Sugahara, T. Cleft lip and palate in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin: A morphological in vivo study. Congenit. Anomalies 2006, 46, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Imura, H.; Yamada, T.; Mishima, K.; Fujiwara, K.; Kawaki, H.; Hirata, A.; Sogawa, N.; Ueno, T.; Sugahara, T. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin suggests abnormal palate development after palatal fusion. Congenit. Anomalies 2010, 50, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, C.; Imura, H.; Yamada, T.; Sugahara, T.; Hirata, A.; Ikeda, Y.; Natsume, N. Cleft palate formation after palatal fusion occurs due to the rupture of epithelial basement membranes. J. Cranio-Maxillofacial Surg. 2018, 46, 2027–2031. [Google Scholar] [CrossRef]
- Fujimaki, H.; Nohara, K.; Kobayashi, T.; Suzuki, K.; Eguchi-Kasai, K.; Tsukumo, S.; Kijima, M.; Tohyama, C. Effect of a single oral dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin on immune function in male NC/Nga mice. Toxicol. Sci. 2002, 66, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Ding, X.; Lei, J.; Qiu, L. Comparison of the biological behaviors of palatal mesenchymal and epithelial cells induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in vitro. Toxicol. Lett. 2020, 333, 90–96. [Google Scholar] [CrossRef]
- Puhvel, S.M.; Sakamoto, M.; Puhvel, M.S.S.M. Effect of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin on Murine Skin. J. Investig. Dermatol. 1988, 90, 354–358. [Google Scholar] [CrossRef] [Green Version]
- Paiva, K.B.S.; Maas, C.S.; Dos Santos, P.M.; Granjeiro, J.M.; Letra, A. Extracellular Matrix Composition and Remodeling: Current Perspectives on Secondary Palate Formation, Cleft Lip/Palate, and Palatal Reconstruction. Front. Cell Dev. Biol. 2019, 7, 340. [Google Scholar] [CrossRef] [Green Version]
- Al-Rugeebah, A.; Alanazi, M.; Parine, N.R. MEG3: An Oncogenic Long Non-coding RNA in Different Cancers. Pathol. Oncol. Res. 2019, 25, 859–874. [Google Scholar] [CrossRef]
- He, Z.; Liu, X.; Liu, X.; Cui, L.; Yuan, Y.; Zhang, H.; Chen, Y.; Tao, Y.; Yu, Z. The role of MEG3 in the proliferation of palatal mesenchymal cells is related to the TGFβ/Smad pathway in TCDD inducing cleft palate. Toxicol. Appl. Pharmacol. 2021, 419, 115517. [Google Scholar] [CrossRef]
- Mimura, J.; Yamashita, K.; Nakamura, K.; Morita, M.; Takagi, T.N.; Nakao, K.; Ema, M.; Sogawa, K.; Yasuda, M.; Katsuki, M.; et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 1997, 2, 645–654. [Google Scholar] [CrossRef]
- Yoshioka, W.; Tohyama, C. Mechanisms of Developmental Toxicity of Dioxins and Related Compounds. Int. J. Mol. Sci. 2019, 20, 617. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, X.; Tao, Y.; Li, N.; Ji, M.; Zhang, X.; Chen, Y.; He, Z.; Yu, K.; Yu, Z. TCDD inhibited the osteogenic differentiation of human fetal palatal mesenchymal cells through AhR and BMP-2/TGF-β/Smad signaling. Toxicology. 2020, 15, 152353. [Google Scholar] [CrossRef]
- Takagi, T.N.; Matsui, K.A.; Yamashita, K.; Ohmori, H.; Yasuda, M. Pathogenesis of cleft palate in mouse embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Teratog. Carcinog. Mutagen. 2000, 20, 73–86. [Google Scholar] [CrossRef]
- Hutson, M.S.; Leung, M.C.K.; Baker, N.C.; Spencer, R.M.; Knudsen, T.B. Computational Model of Secondary Palate Fusion and Disruption. Chem Res. Toxicol. 2017, 30, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Greenburg, G.; Hay, E.D. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J. Cell Biol. 1982, 95, 333–339. [Google Scholar] [CrossRef] [PubMed]
- García de Herreros, A. Epithelial to mesenchymal transition in tumor cells as consequence of phenotypic instability. Front. Cell. Dev. Biol. 2014, 2, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porto, L.P.A.; Dos Santos, J.N.; Ramalho, L.M.P.; Figueiredo, A.L.; Júnior, B.C.; Gurgel, C.A.; Paiva, K.B.S.; Xavier, F.C.A. E-cadherin regulators are differentially expressed in the epithelium and stroma of keratocystic odontogenic tumors. J. Oral Pathol. Med. 2015, 45, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Fitchett, J.E.; Hay, E.D. Medial edge epithelium transforms to mesenchyme after embryonic palatal shelves fuse. Dev. Biol. 1989, 131, 455–474. [Google Scholar] [CrossRef]
- Iseki, S. Disintegration of the medial epithelial seam: Is cell death important in palatogenesis? Dev. Growth. Differ. 2011, 53, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Bu, Y.; Zhang, G.; Liu, X.; Wang, X.; Ding, S.; Wang, E.; Shi, R.; Li, Q.; Fu, J.; et al. Effect of TCDD on the fate of epithelial cells isolated from human fetal palatal shelves (hFPECs). Toxicol. Appl. Pharmacol. 2016, 305, 186–193. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, X.; Cui, L.; Liu, X.; Chen, Y.; He, Z.; Ji, M.; Gao, Z.; Li, N.; Wan, Z.; et al. Oct4 plays a role in 2,3,7,8-tetrachlorobenzo-p-dioxin (TCDD) inducing cleft palate and inhibiting mesenchymal proliferation. Toxicology 2020, 30, 438. [Google Scholar]
- Barsky, S.H.; Siegal, G.P.; Jannotta, F.; A Liotta, L. Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab. Investig. 1983, 49, 140–147. [Google Scholar]
- Liotta, L.A. Adhere, Degrade, and Move: The Three-Step Model of Invasion. Cancer Res. 2016, 76, 3115–3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuyama, A.; Mochitate, K. Assembly of the exogenous extracellular matrix during basement membrane formation by alveolar epithelial cells in vitro. J. Cell Sci. 2000, 113, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Zhu, Z.; Yuan, L.; Chan, W.Y.; Sha, O. Extracellular Matrix Remodeling During Palate Development. Organogenesis 2020, 16, 43–60. [Google Scholar] [CrossRef] [Green Version]
- Halper, J.; Kjaer, M. Basic Components of Connective Tissues and Extracellular Matrix: Elastin, Fibrillin, Fibulins, Fibrinogen, Fibronectin, Laminin, Tenascins and Thrombospondins. Prog. Heritable Soft Connect. Tissue Dis. 2013, 802, 31–47. [Google Scholar] [CrossRef]
- Kjaer, M.; Magnusson, P.; Krogsgaard, M.; Moller, J.B.; Olesen, J.; Heinemeier, K.; Hansen, M.; Haraldsson, B.; Koskinen, S.; Esmarck, B.; et al. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J. Anat. 2006, 208, 445–450. [Google Scholar] [CrossRef]
- Abbott, B.D.; Birnbaum, L.S.; Pratt, R.M. TCDD-Induced hyperplasia of the ureteral epithelium produces hydronephrosis in murine fetuses. Teratology 1987, 35, 329–334. [Google Scholar] [CrossRef]
- Abbott, B.; Birnbaum, L. TCDD alters medial epithelial cell differentiation during palatogenesis. Toxicol. Appl. Pharmacol. 1989, 99, 276–286. [Google Scholar] [CrossRef]
- Bryant, P.L.; Reid, L.M.; E Schmid, J.; Buckalew, A.R.; Abbott, B.D. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on fetal mouse urinary tract epithelium in vitro. Toxicology 2001, 162, 23–34. [Google Scholar] [CrossRef]
- Deshpande, A.S.; Goudy, S.L. Cellular and molecular mechanisms of cleft palate development. Laryngoscope 2018, 4, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.; Nijhuis, A.; Kumagai, T.; Lindsay, J.; Silver, A. Defects in the adherens junction complex (E-cadherin/β-catenin) in inflammatory bowel disease. Cell Tissue Res. 2015, 360, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Cadherin Cell Adhesion Receptors as a Morphogenetic Regulator. Science 1991, 251, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Theiery, J.P. Epithelial-mesenchymal transitions in tumor progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Imura, H.; Sugahara, T.; Yamada, T.; Mishima, K. Morphological and immunohistochemical studies on cleft palates induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice. Congenit. Anom. 2008, 48, 68–73. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakuma, C.; Imura, H.; Yamada, T.; Hirata, A.; Ikeda, Y.; Ito, M.; Natsume, N. Histological and Immunohistochemical Studies to Determine the Mechanism of Cleft Palate Induction after Palatal Fusion in Mice Exposed to TCDD. Int. J. Mol. Sci. 2022, 23, 2069. https://doi.org/10.3390/ijms23042069
Sakuma C, Imura H, Yamada T, Hirata A, Ikeda Y, Ito M, Natsume N. Histological and Immunohistochemical Studies to Determine the Mechanism of Cleft Palate Induction after Palatal Fusion in Mice Exposed to TCDD. International Journal of Molecular Sciences. 2022; 23(4):2069. https://doi.org/10.3390/ijms23042069
Chicago/Turabian StyleSakuma, Chisato, Hideto Imura, Tomohiro Yamada, Azumi Hirata, Yayoi Ikeda, Masaaki Ito, and Nagato Natsume. 2022. "Histological and Immunohistochemical Studies to Determine the Mechanism of Cleft Palate Induction after Palatal Fusion in Mice Exposed to TCDD" International Journal of Molecular Sciences 23, no. 4: 2069. https://doi.org/10.3390/ijms23042069
APA StyleSakuma, C., Imura, H., Yamada, T., Hirata, A., Ikeda, Y., Ito, M., & Natsume, N. (2022). Histological and Immunohistochemical Studies to Determine the Mechanism of Cleft Palate Induction after Palatal Fusion in Mice Exposed to TCDD. International Journal of Molecular Sciences, 23(4), 2069. https://doi.org/10.3390/ijms23042069





