Kaposi’s Sarcoma Lesion Progression in BKV-Tat Transgenic Mice Is Increased by Inflammatory Cytokines and Blocked by Treatment with Anti-Tat Antibodies
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
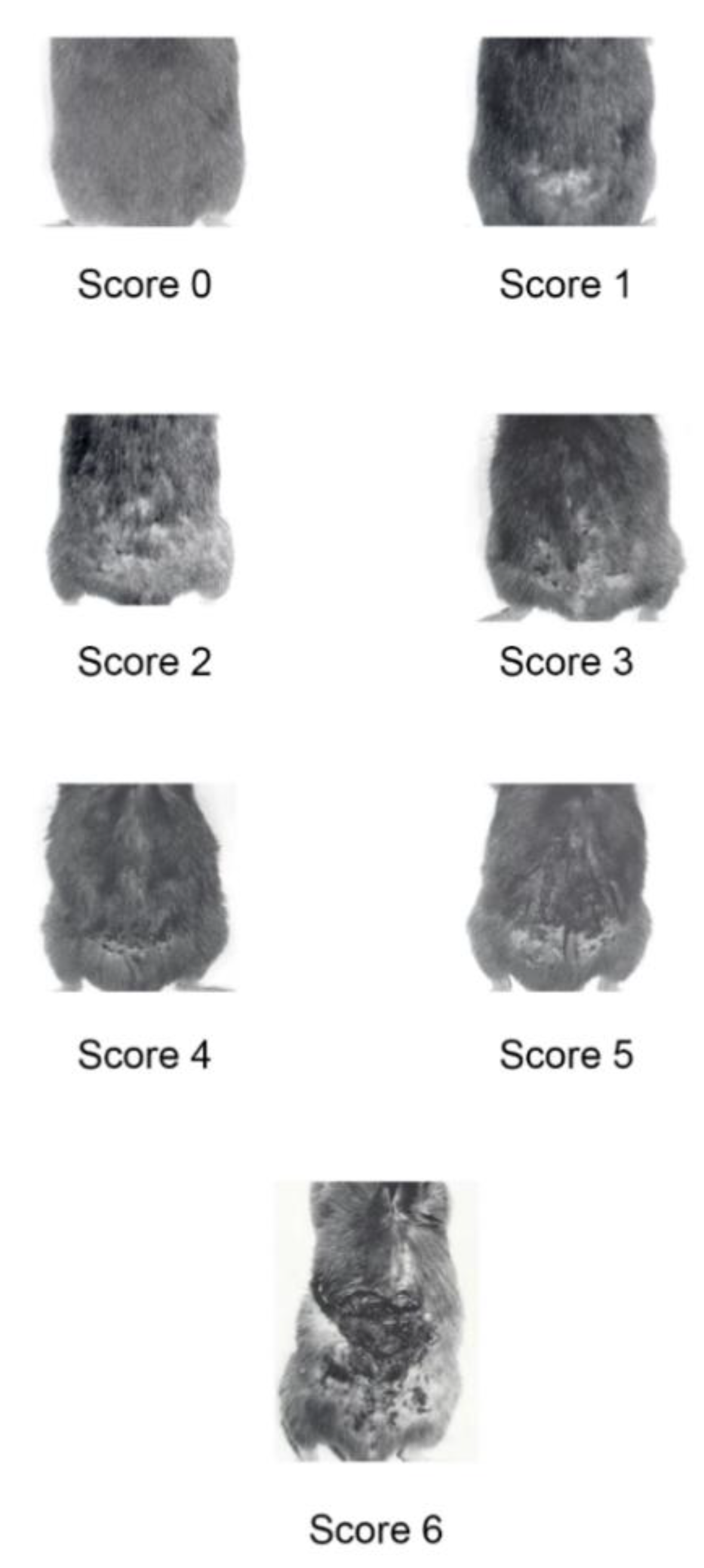
4.1. BKV/Tat Transgenic Mice Monitoring and Treatment
4.2. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ensoli, B.; Sgadari, C.; Barillari, G.; Sirianni, M.C.; Stürzl, M.; Monini, P. Biology of Kaposi’s sarcoma. Eur. J. Cancer 2001, 37, 1251–1269. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, Q.; Zuo, J.; Minhas, V.; Wood, C.; Zhang, T. The world-wide incidence of Kaposi’s sarcoma in the HIV/AIDS era. HIV Med. 2018, 19, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Sabranski, M.; Esser, S. HIV-Associated Kaposi’s Sarcoma. Oncol. Res. Treat. 2017, 40, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Regezi, J.; McPhail, L.A.; Daniels, T.E.; De Souza, Y.G.; Greenspan, J.S.; Greenspan, D. Human immunodeficiency virus-associated oral Kaposi’s sarcoma. A heterogeneous population dominated by spindle shaped endothelial cells. Am. J. Pathol. 1993, 143, 240–249. [Google Scholar] [PubMed]
- Safai, B.; Johnson, K.G.; Myskowski, S.; Koziner, B.; Yang, S.Y.; Cunningham-Rundles, S.; Godbold, J.H.; Dupont, B. The natural history of Kaposi’s sarcoma in the acquired immunodeficiency syndrome. Ann. Intern. Med. 1985, 103, 744–750. [Google Scholar] [CrossRef]
- Hober, D.; Haque, A.; Wattre, P.; Beaucaire, G.; Moutonj, Y.; Capron, A. Production of tumour necrosis factor-alpha (TNF-alpha) and interleukin-1 (IL-1) in patients with AIDS. Enhanced level of TNF- alpha is related to a higher cytotoxic activity. Clin. Exp. Immunol. 1989, 78, 329–333. [Google Scholar]
- Fuchs, D.; Hausen, A.; Reibnegger, G.; Werner, E.R.; Werner-Felmayer, G.; Dierich, M.P.; Wachter, H. Interferon-gamma concentrations are increased in sera from individuals infected with human immunodeficiency virus type 1. J. Acquir. Immune Defic. Syndr. 1989, 2, 158–162. [Google Scholar]
- Emilie, D.; Peuchmaur, M.; Maillot, M.C.; Crevon, M.C.; Brousse, N.; Delfraissy, J.F.; Dormont, J.; Galanaud, P. Production of interleukins in human immunodeficiency virus-1-replicating lymph nodes. J. Clin. Investig. 1990, 86, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Vyakarnam, A.; McKeating, J.; Meager, A.; Beverley, P.C. Tumour necrosis factors (alpha, beta) induced by HIV-1 in peripheral blood mononuclear cells potentiate virus replication. AIDS 1990, 4, 21–27. [Google Scholar] [CrossRef]
- Rizzardini, G.; Piconi, S.; Ruzzante, S.; Fusi, M.L.; Lukwiya, M.; Declich, S.; Tamburini, M.; Villa, M.L.; Fabiani, M.; Milazzo, F.; et al. Immunological activation markers in the serum of African and European HIV-seropositive and seronegative individuals. AIDS 1996, 10, 1535–1542. [Google Scholar] [CrossRef]
- Fagiolo, U.; Cossarizza, A.; Scala, E.; Fanales-Belasio, E.; Ortolani, C.; Cozzi, E.; Monti, D.; Franceschi, C.; Paganelli, R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur. J. Immunol. 1993, 23, 2375–2378. [Google Scholar] [CrossRef] [PubMed]
- Sirianni, M.C.; Vincenzi, L.; Fiorelli, V.; Topino, S.; Scala, E.; Uccini, S.; Angeloni, A.; Faggioni, A.; Cerimele, D.; Cottoni, F.; et al. g-Interferon production in peripheral blood mononuclear cells (PBMC) and tumour infiltrating lymphocytes from Kaposi’s sarcoma patients: Correlation with the presence of human herpesvirus-8 in PBMC and lesional macrophages. Blood 1998, 91, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, V.; Gendelman, R.; Sirianni, M.C.; Chang, H.K.; Colombini, S.; Markham, P.D.; Monini, P.; Sonnabend, J.; Pintus, A.; Gallo, R.C.; et al. g-Interferon produced by CD81 T cells infiltrating Kaposi’s sarcoma induces spindle cells with angiogenic phenotype and synergy with HIV-1 Tat protein: An immune response to HHV-8 infection? Blood 1998, 91, 956–967. [Google Scholar] [PubMed]
- Monini, P.; Colombini, S.; Sturzl, M.; Goletti, D.; Cafaro, A.; Sgadari, C.; Buttò, S.; Franco, M.; Leone, P.; Fais, S.; et al. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi’s sarcoma. Blood 1999, 93, 4044–4058. [Google Scholar]
- Ensoli, B.; Stürzl, M.; Monini, P. Reactivation and role of HHV-8 in Kaposi’s sarcoma initiation. Adv. Cancer Res. 2001, 81, 161–200. [Google Scholar] [CrossRef]
- Real, F.; Krown, S.E. Spontaneous regression of Kaposi’s sarcoma in patients with AIDS. N. Engl. J. Med. 1985, 313, 1659. [Google Scholar] [CrossRef]
- Brooks, J.J. Kaposi’s sarcoma: A reversible hyperplasia. Lancet 1986, 328, 1309–1311. [Google Scholar] [CrossRef]
- Saikevyc, I.A.; Mayer, M.; White, R.L.; Ho, R.C. Cytogenetic study of Kaposi’s sarcoma associated with acquired immunodeficiency syndrome. Arch. Pathol. Lab. Med. 1988, 112, 825–828. [Google Scholar]
- Dictor, M.; Ferno, M.; Baldentorp, B. Flow cytometric DNA content in Kaposis’s sarcoma by histologic stage: Comparison with angiosarcoma. Anal. Quant. Cytol. Histol. 1991, 13, 201. [Google Scholar]
- Rabkin, C.S.; Bedi, G.; Musaba, E.; Sunkutu, R.; Mwansa, N.; Sidransky, D.; Biggar, R. AIDS-related Kaposi’s Sarcoma is a clonal neoplasm. Clin. Cancer Res. 1995, 1, 257–260. [Google Scholar]
- Rabkin, C.S.; Janz, S.; Lash, A.; Coleman, A.E.; Musaba, E.; Liotta, L.; Biggar, R.J.; Zhuang, Z. Monoclonal origin of multicentric Kaposi’s sarcoma lesions. N. Engl. J. Med. 1997, 336, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Ensoli, B.; Buonaguro, L.; Barillari, G.; Fiorelli, V.; Gendelman, R.; Morgan, R.A.; Wingfield, P.; Gallo, R.C. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 1993, 67, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.C.; Samaniego, F.; Nair, B.C.; Buonaguro, L.; Ensoli, B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 1997, 11, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Ensoli, B.; Barillari, G.; Salahuddin, S.Z.; Gallo, R.C.; Wong-Staal, F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of AIDS patients. Nature 1990, 345, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Barillari, G.; Gendelman, R.; Gallo, R.C.; Ensoli, B. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi’s sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc. Natl. Acad. Sci. USA 1993, 90, 7941–7945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albini, A.; Barillari, G.; Benelli, R.; Gallo, R.C.; Ensoli, B. Angiogenic properties of human immunodeficiency virus type 1 Tat protein. Proc. Natl. Acad. Sci. USA 1995, 92, 4838–4842. [Google Scholar] [CrossRef] [Green Version]
- Aoki, Y.; Feldman, G.M.; Tosato, G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 2003, 101, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Foulke, J.S., Jr.; Barabitskaya, O.; Kim, J.; Nair, B.C.; Hone, D.; Smart, J.; Feldman, R.A.; Reitz, M. Human herpesvirus 8-encoded vGPCR activates nuclear factor of activated T cells and collaborates with human immunodeficiency virus type 1 Tat. J. Virol. 2003, 77, 5759–5773. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.G.; Pati, S.; Sadowska, M.; Charurat, M.; Reitz, M. Tumorigenesis by human herpesvirus 8 vGPCR is accelerated by human immunodeficiency virus type 1 Tat. J. Virol. 2004, 78, 9336–9342. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Xue, M.; Qin, D.; Zhu, X.; Wang, C.; Zhu, J.; Hao, T.; Cheng, L.; Chen, X.; Bai, Z.; et al. HIV-1 Tat promotes Kaposi’s sarcoma-associated herpesvirus (KSHV) vIL-6-induced angiogenesis and tumorigenesis by regulating PI3K/PTEN/AKT/GSK-3β signaling pathway. PLoS ONE 2013, 8, e53145. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Hu, M.; Hao, T.; Li, W.; Xue, X.; Xue, M.; Zhu, X.; Zhou, F.; Qin, D.; Yan, Q.; et al. MiRNA-891a-5p mediates HIV-1 Tat and KSHV Orf-K1 synergistic induction of angiogenesis by activating NF-κB signaling. Nucleic Acids Res. 2015, 43, 9362–9378. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cheng, L.; Jia, X.; Zeng, Y.; Yao, S.; Lv, Z.; Qin, D.; Fang, X.; Lei, Y.; Lu, C. Human immunodeficiency virus type 1 Tat accelerates Kaposi sarcoma-associated herpesvirus Kaposin A-mediated tumorigenesis of transformed fibroblasts in vitro as well as in nude and immunocompetent mice. Neoplasia 2009, 11, 1272–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, J.; Hinrichs, S.H.; Reynolds, R.K.; Luciw, P.A.; Jay, G. The HIV tat gene induces dermal lesions resembling Kaposi’s sarcoma in transgenic mice. Nature 1988, 335, 606–611. [Google Scholar] [CrossRef]
- Corallini, A.; Altavilla, G.; Pozzi, L.; Bignozzi, F.; Negrini, M.; Rimessi, P.; Gualandi, F.; Barbanti-Brodano, G. Systemic expression of HIV-1 tat gene in transgenic mice induces endothelial proliferation and tumors of different histotypes. Cancer Res. 1993, 53, 5569–5575. [Google Scholar] [PubMed]
- Altavilla, G.; Trabanelli, C.; Merlin, M.; Caputo, A.; Lanfredi, M.; Barbanti-Brodano, G.; Corallini, A. Morphological, histochemical, immunohistochemical, and ultrastructural characterization of tumors and dysplastic and non-neoplastic lesions arising in BK virus/tat transgenic mice. Am. J. Pathol. 1999, 154, 1231–1244. [Google Scholar] [CrossRef] [Green Version]
- Cavallaro, U.; Gasparini, G.; Soria, M.R.; Maier, J.A. Spindle cells isolated from Kaposi’s sarcoma-like lesions of BKV/tat-transgenic mice co-express markers of different cell types. AIDS 1996, 10, 1211–1219. [Google Scholar] [CrossRef]
- Ensoli, B.; Nakamura, S.; Salahuddin, S.Z.; Biberfeld, P.; Larsson, L.; Beaver, B.; Wong-Staal, F.; Gallo, R.C. AIDS-Kaposi’s sarcoma derived cells express cytokines with autocrine and paracrine growth effects. Science 1989, 243, 223–226. [Google Scholar] [CrossRef]
- Fiorelli, V.; Gendelman, R.; Samaniego, F.; Markham, P.D.; Ensoli, B. Cytokines from activated T cells induce normal endothelial cells to acquire the phenotypic and functional features of AIDS-Kaposi’s sarcoma spindle cells. J. Clin. Investig. 1995, 95, 1723–1734. [Google Scholar] [CrossRef] [Green Version]
- Samaniego, F.; Markham, P.D.; Gendelman, R.; Gallo, R.C.; Ensoli, B. Inflammatory cytokines induce endothelial cells to produce and release basic fibroblast growth factor and to promote Kaposi’s sarcoma-like lesions in nude mice. J. Immunol. 1997, 158, 1887–1894. [Google Scholar]
- Samaniego, F.; Markham, P.; Gallo, R.C.; Ensoli, B. Inflammatory cytokines induce AIDS-Kaposi’s sarcoma-derived spindle cells to produce and release basic fibroblast growth factor and enhance Kaposi’s sarcoma-like lesion formation in nude mice. J. Immunol. 1995, 154, 3582–3592. [Google Scholar]
- Faris, M.; Ensoli, B.; Kokot, N.; Nel, A.E. Inflammatory cytokines induce the expression of basic fibroblast growth factor (bFGF) isoforms required for the growth of Kaposi’s sarcoma and endothelial cells through the activation of AP-1 response elements in the bFGF promoter. AIDS 1998, 12, 19–27. [Google Scholar] [CrossRef]
- Cornali, E.; Zietz, C.; Benelli, R.; Weninger, W.; Masiello, L.; Breier, G.; Tschachler, E.; Albini, A.; Stürzl, M. Vascular endothelial growth factor regulates angiogenesis and vascular permeability in Kaposi’s sarcoma. Am. J. Pathol. 1996, 149, 1851–1869. [Google Scholar] [PubMed]
- Samaniego, F.; Markham, P.D.; Gendelman, R.; Watanabe, Y.; Kao, V.; Kowalski, K.; Sonnabend, J.A.; Pintus, A.; Gallo, R.C.; Ensoli, B. Vascular endothelial growth factor and basic fibroblast growth factor present in Kaposi’s sarcoma are induced by inflammatory cytokines and synergize to induce vascular permeability and KS lesion development. Am. J. Pathol. 1998, 152, 1433–1443. [Google Scholar] [PubMed]
- Barillari, G.; Sgadari, C.; Palladino, C.; Gendelman, R.; Caputo, A.; Bohan Morris, C.; Nair, B.C.; Markham, P.; Nel, A.; Stürzl, M.; et al. Inflammatory cytokines synergize with the HIV-1 Tat protein to promote angiogenesis and Kaposi’s sarcoma via induction of basic fibroblast growth factor and the alpha v beta 3 integrin. J. Immunol. 1999, 163, 1929–1935. [Google Scholar] [PubMed]
- Barillari, G.; Sgadari, C.; Fiorelli, V.; Samaniego, F.; Colombini, S.; Manzari, V.; Modesti, A.; Nair, B.C.; Cafaro, A.; Stürzl, M.; et al. The Tat protein of human immunodeficiency virus type-1 promotes vascular cell growth and locomotion by engaging alpha5beta1 and alphavbeta3 integrins by mobilizing sequestered basic fibroblast growth factor. Blood 1999, 94, 663–672. [Google Scholar] [PubMed]
- Ensoli, B.; Gendelman, R.; Markham, P.; Fiorelli, V.; Colombini, S.; Raffeld, M.; Cafaro, A.; Chang, H.K.; Brady, J.N.; Gallo, R.C. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi’s sarcoma. Nature 1994, 371, 674–680. [Google Scholar] [CrossRef]
- Fiorelli, V.; Barillari, G.; Toschi, E.; Sgadari, C.; Monini, P.; Stürzl, M.; Ensoli, B. IFN-gamma induces endothelial cells to proliferate and to invade the extracellular matrix in response to the HIV-1 Tat protein: Implications for AIDS-Kaposi’s sarcoma pathogenesis. J. Immunol. 1999, 162, 1165–1170. [Google Scholar]
- Barillari, G.; Buonaguro, L.; Fiorelli, V.; Hoffman, J.; Michaels, F.; Gallo, R.C.; Ensoli, B. Effects of cytokines from activated immune cells on vascular cell growth and HIV-1 gene expression. Implications for AIDS-Kaposi’s sarcoma pathogenesis. J. Immunol. 1992, 149, 3727–3734. [Google Scholar]
- Monini, P.; Cafaro, A.; Srivastava, I.K.; Moretti, S.; Sharma, V.A.; Andreini, C.; Chiozzini, C.; Ferrantelli, F.; Pavone Cossut, M.R.; Tripiciano, A.; et al. HIV-1 tat promotes integrin-mediated HIV transmission to dendritic cells by binding Env spikes and competes neutralization by anti-HIV antibodies. PLoS ONE 2012, 7, e48781. [Google Scholar] [CrossRef]
- Cafaro, A.; Barillari, G.; Moretti, S.; Palladino, C.; Tripiciano, A.; Falchi, M.; Picconi, O.; Pavone Cossut, M.R.; Campagna, M.; Arancio, A.; et al. HIV-1 Tat Protein Enters Dysfunctional Endothelial Cells via Integrins and Renders Them Permissive to Virus Replication. Int. J. Mol. Sci. 2020, 22, 317. [Google Scholar] [CrossRef]
- Veettil, M.V.; Sadagopan, S.; Sharma-Walia, N.; Wang, F.Z.; Raghu, H.; Varga, L.; Chandran, B. Kaposi’s sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J. Virol. 2008, 82, 12126–12144. [Google Scholar] [PubMed] [Green Version]
- Akula, S.M.; Pramod, N.P.; Wang, F.Z.; Chandran, B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 2002, 108, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Ben Haij, N.; Leghmari, K.; Planès, R.; Thieblemont, N.; Bahraoui, E. HIV-1 Tat protein binds to TLR4-MD2 and signals to induce TNF-alpha and IL-10. Retrovirology 2013, 10, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planès, R.; Ben Haij, N.; Leghmari, K.; Serrero, M.; BenMohamed, L.; Bahraoui, E. HIV-1 Tat Protein Activates both the MyD88 and TRIF Pathways to Induce Tumor Necrosis Factor Alpha and Interleukin-10 in Human Monocytes. J. Virol. 2016, 90, 5886–5898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Haij, N.; Planes, R.; Leghmari, K.; Serrero, M.; Delobel, P.; Izopet, J.; BenMohamed, L.; Bahraoui, E. HIV-1 Tat protein induces production of proinflammatory cytokines by human dendritic cells and monocytes/macrophages through engagement of TLR4-MD2-CD14 complex and activation of NF-kappaB pathway. PLoS ONE 2015, 10, e0129425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planes, R.; Bahraoui, E. HIV-1 Tat protein induces the production of IDO in human monocyte derived-dendritic cells through a direct mechanism: Effect on T cells proliferation. PLoS ONE 2013, 8, e74551. [Google Scholar] [CrossRef]
- Planes, R.; BenMohamed, L.; Leghmari, K.; Delobel, P.; Izopet, J.; Bahraoui, E. HIV-1 Tat protein induces PD-L1 (B7-H1) expression on dendritic cells through tumor necrosis factor alpha- and Toll-like receptor 4-mediated mechanisms. J. Virol. 2014, 88, 6672–6689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima Machado, P.R.; Silva Farias, K.J.; Genre, J.; Freire Oliveira, C.J.; Matta Guedes, P.M.; Lopes da Fonseca, B.A. Disseminated Kaposi’s Sarcoma in Patients with HIV Infection Correlates to High Serum Levels of IL-10. Viral Immunol. 2014, 27, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Jary, A.; Veyri, M.; Gothland, A.; Leducq, V.; Calvez, V.; Marcelin, A.G. Kaposi’s Sarcoma-Associated Herpesvirus, the Etiological Agent of All Epidemiological Forms of Kaposi’s Sarcoma. Cancers 2021, 13, 6208. [Google Scholar] [CrossRef]
- Possati, L.; Campioni, D.; Sola, F.; Leone, L.; Ferrante, L.; Trabanelli, C.; Ciomei, M.; Montesi, M.; Rocchetti, R.; Talevi, S.; et al. Antiangiogenic, antitumoural and antimetastatic effects of two distamycin A derivatives with anti-HIV-1 Tat activity in a Kaposi’s sarcoma-like murine model. Clin. Exp. Metastasis 1999, 17, 575–582. [Google Scholar] [CrossRef]
- Sgadari, C.; Barillari, G.; Toschi, E.; Carlei, D.; Bacigalupo, I.; Baccarini, S.; Palladino, C.; Leone, P.; Bugarini, R.; Malavasi, L.; et al. HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi’s sarcoma. Nat. Med. 2002, 8, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Toschi, E.; Sgadari, C.; Malavasi, L.; Bacigalupo, I.; Chiozzini, C.; Carlei, D.; Compagnoni, D.; Bellino, S.; Bugarini, R.; Falchi, M.; et al. Human immunodeficiency virus protease inhibitors reduce the growth of human tumors via a proteasome-independent block of angiogenesis and matrix metalloproteinases. Int. J. Cancer 2011, 128, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Monini, P.; Sgadari, C.; Toschi, E.; Barillari, G.; Ensoli, B. Antitumor effects of antiretroviral therapy. Nat. Rev. Cancer 2004, 4, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Monini, P.; Sgadari, C.; Grosso, M.G.; Bellino, S.; Di Biagio, A.; Toschi, E.; Bacigalupo, I.; Sabbatucci, M.; Cencioni, G.; Salvi, E.; et al. Clinical course of classic Kaposi’s sarcoma in HIV-negative patients treated with the HIV protease inhibitor indinavir. AIDS 2009, 23, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Hampson, L.; Maranga, I.O.; Masinde, M.S.; Oliver, A.W.; Batman, G.; He, X.; Desai, M.; Okemwa, P.M.; Stringfellow, H.; Martin-Hirsch, P.; et al. A Single-Arm, Proof-Of-Concept Trial of Lopimune (Lopinavir/Ritonavir) as a Treatment for HPV-Related Pre-Invasive Cervical Disease. PLoS ONE 2016, 11, e0147917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgadari, C.; Bacigalupo, I.; Barillari, G.; Ensoli, B. Pharmacological management of Kaposi’s sarcoma. Expert Opin. Pharmacother. 2011, 12, 1669–1690. [Google Scholar] [CrossRef] [PubMed]
- Ensoli, B.; Moretti, S.; Borsetti, A.; Maggiorella, M.T.; Buttò, S.; Picconi, O.; Tripiciano, A.; Sgadari, C.; Monini, P.; Cafaro, A. New insights into pathogenesis point to HIV-1 Tat as a key vaccine target. Arch. Virol. 2021, 166, 2955–2974. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brocca-Cofano, E.; Sgadari, C.; Picconi, O.; Palladino, C.; Caputo, A.; Ensoli, B. Kaposi’s Sarcoma Lesion Progression in BKV-Tat Transgenic Mice Is Increased by Inflammatory Cytokines and Blocked by Treatment with Anti-Tat Antibodies. Int. J. Mol. Sci. 2022, 23, 2081. https://doi.org/10.3390/ijms23042081
Brocca-Cofano E, Sgadari C, Picconi O, Palladino C, Caputo A, Ensoli B. Kaposi’s Sarcoma Lesion Progression in BKV-Tat Transgenic Mice Is Increased by Inflammatory Cytokines and Blocked by Treatment with Anti-Tat Antibodies. International Journal of Molecular Sciences. 2022; 23(4):2081. https://doi.org/10.3390/ijms23042081
Chicago/Turabian StyleBrocca-Cofano, Egidio, Cecilia Sgadari, Orietta Picconi, Clelia Palladino, Antonella Caputo, and Barbara Ensoli. 2022. "Kaposi’s Sarcoma Lesion Progression in BKV-Tat Transgenic Mice Is Increased by Inflammatory Cytokines and Blocked by Treatment with Anti-Tat Antibodies" International Journal of Molecular Sciences 23, no. 4: 2081. https://doi.org/10.3390/ijms23042081
APA StyleBrocca-Cofano, E., Sgadari, C., Picconi, O., Palladino, C., Caputo, A., & Ensoli, B. (2022). Kaposi’s Sarcoma Lesion Progression in BKV-Tat Transgenic Mice Is Increased by Inflammatory Cytokines and Blocked by Treatment with Anti-Tat Antibodies. International Journal of Molecular Sciences, 23(4), 2081. https://doi.org/10.3390/ijms23042081






