Zebrafish as a Model to Study Vascular Elastic Fibers and Associated Pathologies
Abstract
1. Introduction
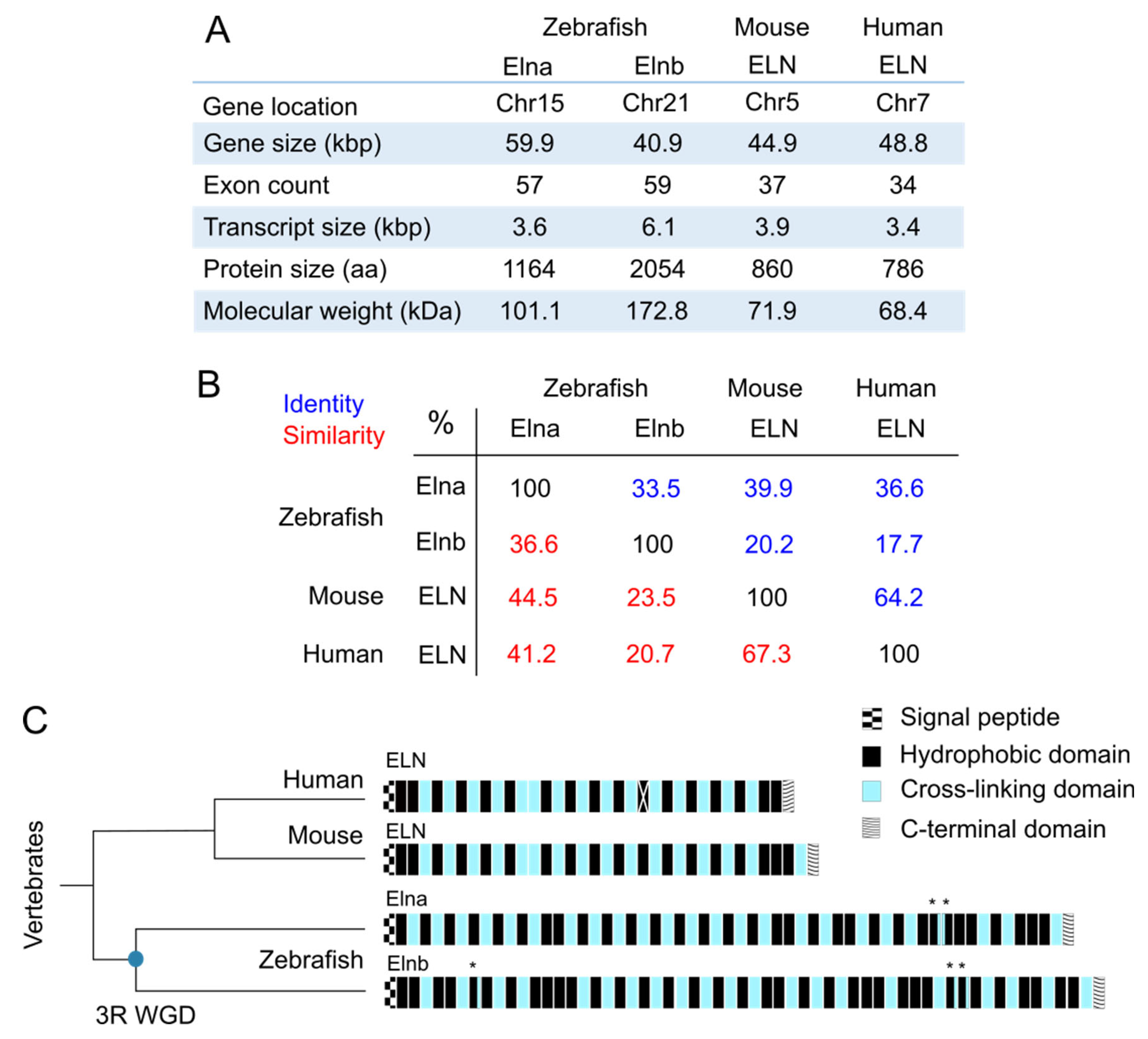
2. Tropoelastin in the Zebrafish
2.1. Sequences
2.2. Spatio-Temporal Expression
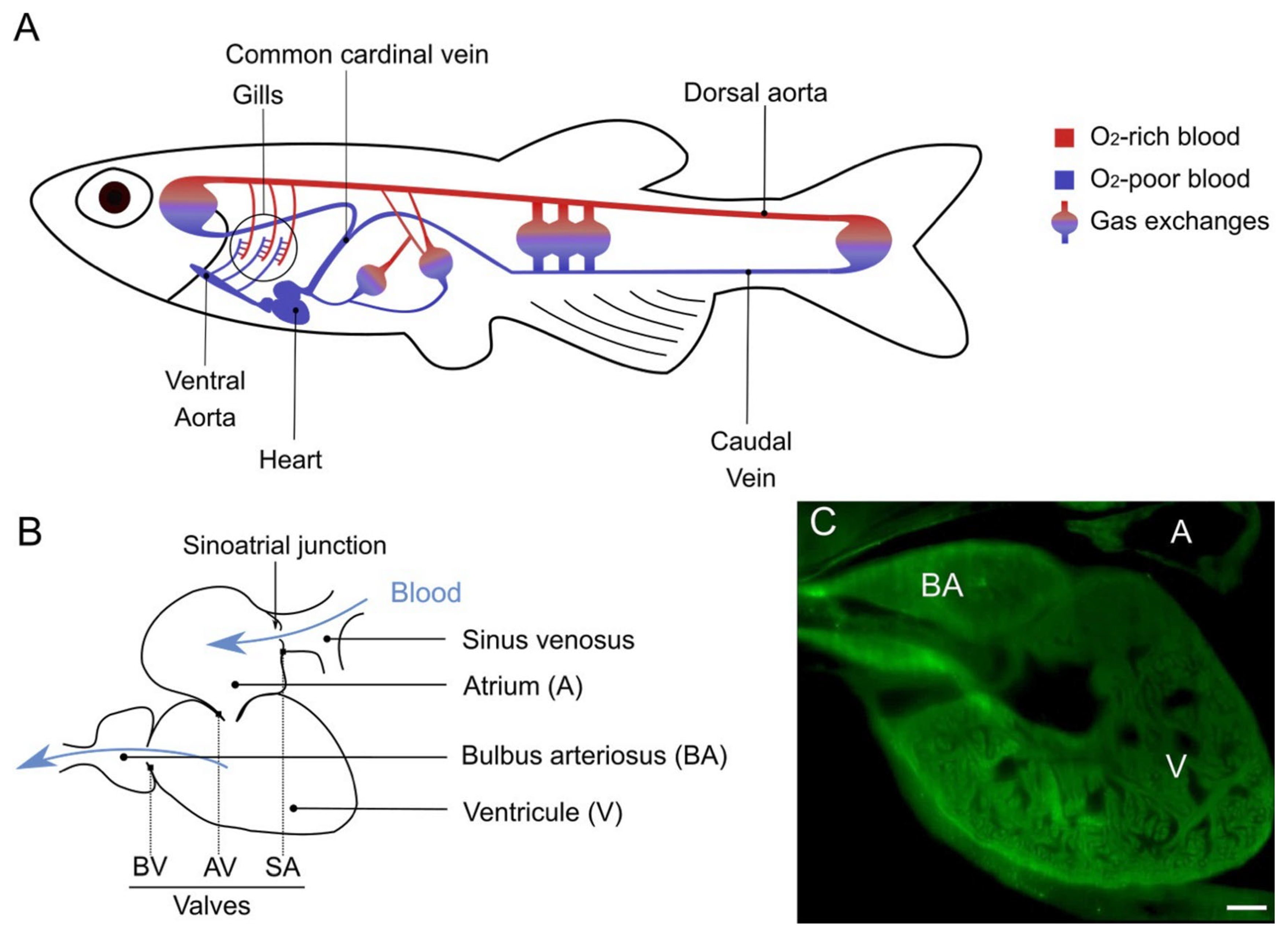
3. Zebrafish Cardiovascular System and Its Elastic Components
3.1. The Heart
3.1.1. The Sinus Venosus
3.1.2. The Atrium
3.1.3. The Ventricle
3.1.4. Cardiac Valves
3.1.5. The Bulbus Arteriosus
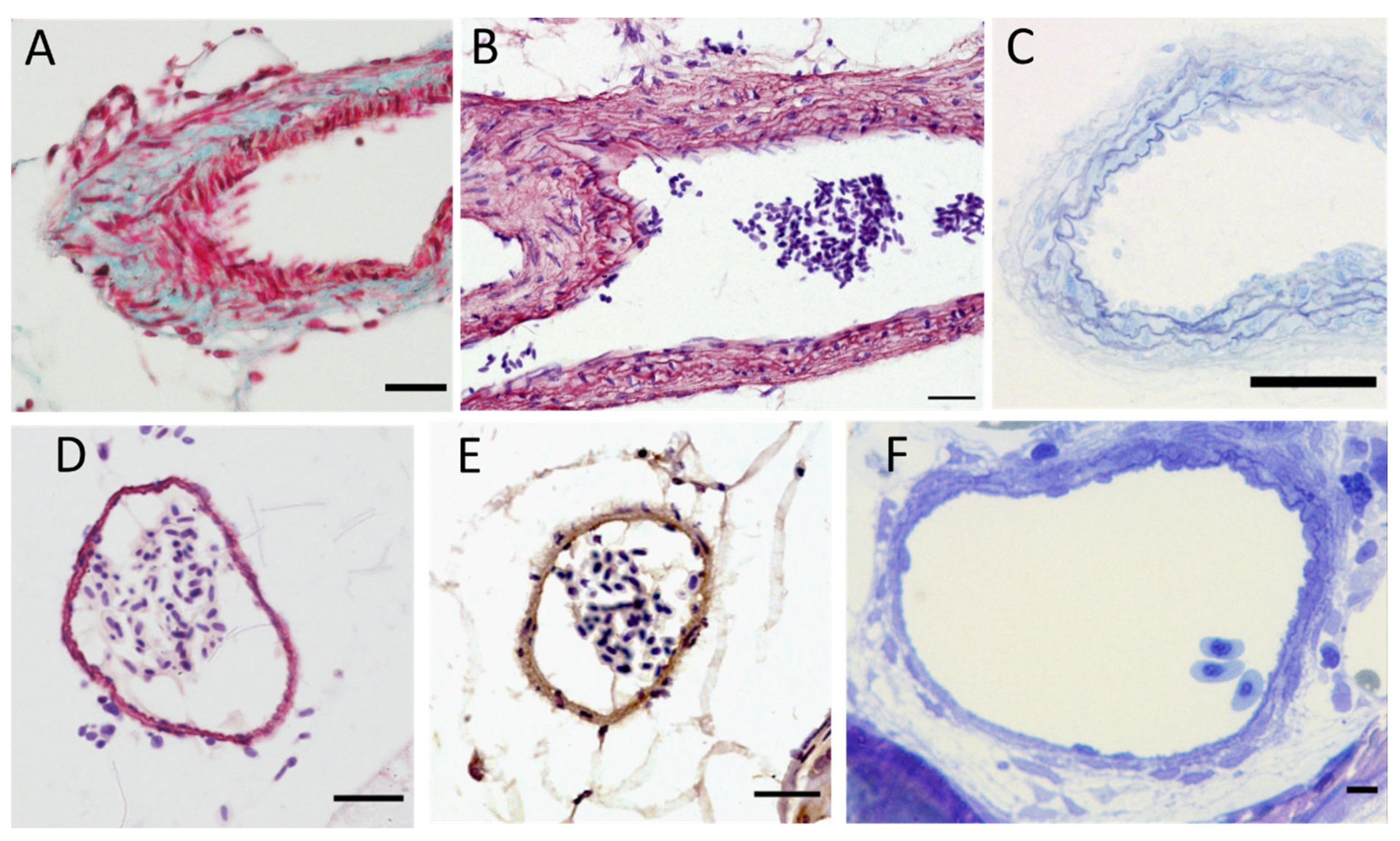
3.2. Arteries and Vessel Wall Organization
4. Vascular Elastic Fiber Pathologies and Associated Zebrafish Models
4.1. Elastin
4.2. Fibrillins
4.3. MAGPs
4.4. LTBPs
4.5. GLUT10
4.6. ADAMTSs
4.7. Fibulins
4.8. EMILINs
4.9. Ion Metabolism
4.9.1. Copper and Lysyl Oxidases
4.9.2. Calcium and Vascular Mineralization
4.10. Zebrafish Model Potential Contributions Depending on Developmental Stages
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wise, S.G.; Weiss, A.S. Tropoelastin. Int. J. Biochem. Cell Biol. 2009, 41, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Wagenseil, J.E.; Mecham, R.P. Vascular Extracellular Matrix and Arterial Mechanics. Physiol. Rev. 2009, 89, 957–989. [Google Scholar] [CrossRef] [PubMed]
- Koenigsberger, M.; Sauser, R.; Bény, J.-L.; Meister, J.-J. Effects of Arterial Wall Stress on Vasomotion. Biophys. J. 2006, 91, 1663–1674. [Google Scholar] [CrossRef]
- Mithieux, S.M.; Weiss, A.S. Elastin. Adv. Protein Chem. 2005, 70, 437–461. [Google Scholar] [CrossRef]
- Wise, S.G.; Yeo, G.C.; Hiob, M.A.; Rnjak-Kovacina, J.; Kaplan, D.L.; Ng, M.K.C.; Weiss, A.S. Tropoelastin: A Versatile, Bioactive Assembly Module. Acta Biomater. 2014, 10, 1532–1541. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Simpson, A.; Steer, R.; Cain, S.A.; Kielty, C.M. Elastic Fibres in Health and Disease. Expert Rev. Mol. Med. 2013, 15, e8. [Google Scholar] [CrossRef]
- Sherratt, M.J. Tissue Elasticity and the Ageing Elastic Fibre. Age 2009, 31, 305–325. [Google Scholar] [CrossRef]
- Wagenseil, J.E.; Mecham, R.P. Elastin in Large Artery Stiffness and Hypertension. J Cardiovasc. Transl. Res. 2012, 5, 264–273. [Google Scholar] [CrossRef]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The Behaviour and Ecology of the Zebrafish, Danio Rerio. Biol. Rev. Camb. Philos. Soc. 2008, 83, 13–34. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Bill, B.R.; Petzold, A.M.; Clark, K.J.; Schimmenti, L.A.; Ekker, S.C. A Primer for Morpholino Use in Zebrafish. Zebrafish 2009, 6, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Peterson, R.T.; Yeh, J.-R.J. Methods for Targeted Mutagenesis in Zebrafish Using TALENs. Methods 2014, 69, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.-R.J.; Joung, J.K. Efficient Genome Editing in Zebrafish Using a CRISPR-Cas System. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Sassen, W.A.; Köster, R.W. A Molecular Toolbox for Genetic Manipulation of Zebrafish. Adv. Genom. Genet. 2015, 5, 151–163. [Google Scholar] [CrossRef]
- Prykhozhij, S.V.; Fuller, C.; Steele, S.L.; Veinotte, C.J.; Razaghi, B.; Robitaille, J.M.; McMaster, C.R.; Shlien, A.; Malkin, D.; Berman, J.N. Optimized Knock-in of Point Mutations in Zebrafish Using CRISPR/Cas9. Nucleic Acids Res. 2018, 46, e102. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Han, B.; Li, L.; Li, M.; Lu, X.; Chen, C.; Lu, M.; Zhang, Y.; Jia, X.; et al. One-Step Efficient Generation of Dual-Function Conditional Knockout and Geno-Tagging Alleles in Zebrafish. eLife 2019, 8, e48081. [Google Scholar] [CrossRef]
- De Vrieze, E.; de Bruijn, S.E.; Reurink, J.; Broekman, S.; van de Riet, V.; Aben, M.; Kremer, H.; van Wijk, E. Efficient Generation of Knock-In Zebrafish Models for Inherited Disorders Using CRISPR-Cas9 Ribonucleoprotein Complexes. Int. J. Mol. Sci. 2021, 22, 9429. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Tarique, I.; Zhang, Y.; Lu, T.; Wang, J.; Chen, A.; Wen, F.; Zhang, Z.; Zhang, Y.; et al. A Time-Saving Strategy to Generate Double Maternal Mutants by an Oocyte-Specific Conditional Knockout System in Zebrafish. Biology 2021, 10, 777. [Google Scholar] [CrossRef]
- Rossi, A.; Kontarakis, Z.; Gerri, C.; Nolte, H.; Hölper, S.; Krüger, M.; Stainier, D.Y.R. Genetic Compensation Induced by Deleterious Mutations but Not Gene Knockdowns. Nature 2015, 524, 230–233. [Google Scholar] [CrossRef]
- Zebrafish Mutation Project–Wellcome Sanger Institute. Available online: https://www.sanger.ac.uk/resources/zebrafish/zmp/ (accessed on 24 December 2021).
- Kettleborough, R.N.W.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; de Bruijn, E.; van Eeden, F.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J.; et al. A Systematic Genome-Wide Analysis of Zebrafish Protein-Coding Gene Function. Nature 2013, 496, 494–497. [Google Scholar] [CrossRef]
- Meyer, A.; Schartl, M. Gene and Genome Duplications in Vertebrates: The One-to-Four (-to-Eight in Fish) Rule and the Evolution of Novel Gene Functions. Curr. Opin. Cell Biol. 1999, 11, 699–704. [Google Scholar] [CrossRef]
- Raldúa, D.; Piña, B. In Vivo Zebrafish Assays for Analyzing Drug Toxicity. Expert Opin. Drug Metab. Toxicol. 2014, 10, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Glaberman, S.; Padilla, S.; Barron, M.G. Evaluating the Zebrafish Embryo Toxicity Test for Pesticide Hazard Screening. Environ. Toxicol. Chem. 2017, 36, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish Embryos and Larvae as Alternative Animal Models for Toxicity Testing. Int. J. Mol. Sci. 2021, 22, 13417. [Google Scholar] [CrossRef]
- Wiley, D.S.; Redfield, S.E.; Zon, L.I. Chemical Screening in Zebrafish for Novel Biological and Therapeutic Discovery. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 138, pp. 651–679. ISBN 978-0-12-803473-6. [Google Scholar]
- Zon, L.I.; Peterson, R.T. In Vivo Drug Discovery in the Zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef]
- Wright, G.M.; Armstrong, L.A.; Jacques, A.M.; Youson, J.H. Trabecular, Nasal, Branchial, and Pericardial Cartilages in the Sea Lamprey, Petromyzon Marinus: Fine Structure and Immunohistochemical Detection of Elastin. Am. J. Anat. 1988, 182, 1–15. [Google Scholar] [CrossRef]
- Sage, E.H.; Gray, W.R. Evolution of Elastin Structure. Adv. Exp. Med. Biol. 1977, 79, 291–312. [Google Scholar] [CrossRef]
- Glasauer, S.M.K.; Neuhauss, S.C.F. Whole-Genome Duplication in Teleost Fishes and Its Evolutionary Consequences. Mol. Genet. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef]
- Pasquier, J.; Cabau, C.; Nguyen, T.; Jouanno, E.; Severac, D.; Braasch, I.; Journot, L.; Pontarotti, P.; Klopp, C.; Postlethwait, J.H.; et al. Gene Evolution and Gene Expression after Whole Genome Duplication in Fish: The PhyloFish Database. BMC Genom. 2016, 17, 368. [Google Scholar] [CrossRef]
- Schmelzer, C.E.H.; Duca, L. Elastic Fibers: Formation, Function, and Fate during Aging and Disease. FEBS J. 2021. [Google Scholar] [CrossRef]
- Sugitani, H.; Hirano, E.; Knutsen, R.H.; Shifren, A.; Wagenseil, J.E.; Ciliberto, C.; Kozel, B.A.; Urban, Z.; Davis, E.C.; Broekelmann, T.J.; et al. Alternative Splicing and Tissue-Specific Elastin Misassembly Act as Biological Modifiers of Human Elastin Gene Frameshift Mutations Associated with Dominant Cutis Laxa. J. Biol. Chem. 2012, 287, 22055–22067. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.I.S.; Miao, M.; Stahl, R.J.; Chan, E.; Parkinson, J.; Keeley, F.W. Sequences and Domain Structures of Mammalian, Avian, Amphibian and Teleost Tropoelastins: Clues to the Evolutionary History of Elastins. Matrix Biol. 2006, 25, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Ozsvar, J.; Yang, C.; Cain, S.A.; Baldock, C.; Tarakanova, A.; Weiss, A.S. Tropoelastin and Elastin Assembly. Front. Bioeng. Biotechnol. 2021, 9, 643110. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Ito, F.; Takeda, H.; Yano, T.; Okabe, M.; Kuraku, S.; Keeley, F.W.; Koshiba-Takeuchi, K. Evolution of the Fish Heart by Sub/Neofunctionalization of an Elastin Gene. Nat. Commun. 2016, 7, 10397. [Google Scholar] [CrossRef]
- Bax, D.V.; Rodgers, U.R.; Bilek, M.M.M.; Weiss, A.S. Cell Adhesion to Tropoelastin Is Mediated via the C-Terminal GRKRK Motif and Integrin AlphaVbeta3. J. Biol. Chem. 2009, 284, 28616–28623. [Google Scholar] [CrossRef]
- Nonaka, R.; Sato, F.; Wachi, H. Domain 36 of Tropoelastin in Elastic Fiber Formation. Biol. Pharm. Bull. 2014, 37, 698–702. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R.F. A Simple Method for Displaying the Hydropathic Character of a Protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Sage, H. Structure-Function Relationships in the Evolution of Elastin. J. Invest. Dermatol. 1982, 79, 146–153. [Google Scholar] [CrossRef][Green Version]
- Brown-Augsburger, P.; Tisdale, C.; Broekelmann, T.; Sloan, C.; Mecham, R.P. Identification of an Elastin Cross-Linking Domain That Joins Three Peptide Chains: Possible Role in Nucleated Assembly. J. Biol. Chem. 1995, 270, 17778–17783. [Google Scholar] [CrossRef]
- Sage, H.; Gray, W. Studies on the Evolution of Elastin—II. Histology. Comp. Biochem. Physiol. Part B Comp. Biochem. 1980, 66, 13–22. [Google Scholar] [CrossRef]
- Miao, M.; Bruce, A.E.E.; Bhanji, T.; Davis, E.C.; Keeley, F.W. Differential Expression of Two Tropoelastin Genes in Zebrafish. Matrix Biol. 2007, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, C.S.; Wang, X.; Sullivan, C.P.; Toselli, P.; Stone, P.J.; McLean, S.E.; Mecham, R.P.; Schreiber, B.M.; Sonenshein, G.E. B-Myb Represses Elastin Gene Expression in Aortic Smooth Muscle Cells. J. Biol. Chem. 2005, 280, 7694–7701. [Google Scholar] [CrossRef] [PubMed]
- Wagenseil, J.E.; Ciliberto, C.H.; Knutsen, R.H.; Levy, M.A.; Kovacs, A.; Mecham, R.P. The Importance of Elastin to Aortic Development in Mice. Am. J. Physiol.—Heart Circ. Physiol. 2010, 299, H257–H264. [Google Scholar] [CrossRef] [PubMed]
- Force, A.; Lynch, M.; Pickett, F.B.; Amores, A.; Yan, Y.L.; Postlethwait, J. Preservation of Duplicate Genes by Complementary, Degenerative Mutations. Genetics 1999, 151, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- Grimes, A.C.; Kirby, M.L. The Outflow Tract of the Heart in Fishes: Anatomy, Genes and Evolution. J. Fish Biol. 2009, 74, 983–1036. [Google Scholar] [CrossRef]
- Monahan-Earley, R.; Dvorak, A.M.; Aird, W.C. Evolutionary Origins of the Blood Vascular System and Endothelium. J. Thromb. Haemost. 2013, 11, 46–66. [Google Scholar] [CrossRef]
- Farrell, A.P.; Pieperhoff, S. Design and Physiology of the Heart | Cardiac Anatomy in Fishes. In Encyclopedia of Fish Physiology; Farrell, A.P., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 998–1005. ISBN 978-0-08-092323-9. [Google Scholar]
- Burton, D.; Burton, M. Essential Fish Biology: Diversity, Structure and Function; Oxford University Press: Oxford, UK, 2017; ISBN 978-0-19-878555-2. [Google Scholar]
- Hoage, T.; Ding, Y.; Xu, X. Quantifying Cardiac Functions in Embryonic and Adult Zebrafish. Methods Mol. Biol. 2012, 843, 11–20. [Google Scholar] [CrossRef]
- Driscoll, P. Gray’s Anatomy, 39th Edition. Emerg. Med. J. 2006, 23, 492. [Google Scholar] [CrossRef]
- Männer, J.; Yelbuz, T.M. Functional Morphology of the Cardiac Jelly in the Tubular Heart of Vertebrate Embryos. J. Cardiovasc. Dev. Dis. 2019, 6, 12. [Google Scholar] [CrossRef]
- Stainier, D.Y.R. Zebrafish Genetics and Vertebrate Heart Formation. Nat. Rev. Genet. 2001, 2, 39–48. [Google Scholar] [CrossRef]
- Derrick, C.J.; Sánchez-Posada, J.; Hussein, F.; Tessadori, F.; Pollitt, E.J.G.; Savage, A.M.; Wilkinson, R.N.; Chico, T.J.; van Eeden, F.J.; Bakkers, J.; et al. Asymmetric Hapln1a Drives Regionalized Cardiac ECM Expansion and Promotes Heart Morphogenesis in Zebrafish Development. Cardiovasc. Res. 2022, 118, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Scherz, P.J.; Huisken, J.; Sahai-Hernandez, P.; Stainier, D.Y.R. High-Speed Imaging of Developing Heart Valves Reveals Interplay of Morphogenesis and Function. Dev. Camb. Engl. 2008, 135, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, M.; Wirrig, E.; Phelps, A.; Wessels, A. Extracellular Matrix and Heart Development. Birt. Defects Res. A Clin. Mol. Teratol. 2011, 91, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yost, H.J.; Clark, E.B. Cardiac Morphology and Blood Pressure in the Adult Zebrafish. Anat. Rec. 2001, 264, 1–12. [Google Scholar] [CrossRef]
- Poon, K.L.; Brand, T. The Zebrafish Model System in Cardiovascular Research: A Tiny Fish with Mighty Prospects. Glob. Cardiol. Sci. Pract. 2013, 2013, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Boukens, B.; Wang, T.; Moorman, A.; Christoffels, V. Evolution of the Sinus Venosus from Fish to Human. J. Cardiovasc. Dev. Dis. 2014, 1, 14–28. [Google Scholar] [CrossRef]
- Tessadori, F.; van Weerd, J.H.; Burkhard, S.B.; Verkerk, A.O.; de Pater, E.; Boukens, B.J.; Vink, A.; Christoffels, V.M.; Bakkers, J. Identification and Functional Characterization of Cardiac Pacemaker Cells in Zebrafish. PLoS ONE 2012, 7, e47644. [Google Scholar] [CrossRef]
- Fishman, G.I. Understanding Conduction System Development: A Hop, Skip and Jump Away? Circ. Res. 2005, 96, 809–811. [Google Scholar] [CrossRef]
- Liu, J.; Bressan, M.; Hassel, D.; Huisken, J.; Staudt, D.; Kikuchi, K.; Poss, K.D.; Mikawa, T.; Stainier, D.Y.R. A Dual Role for ErbB2 Signaling in Cardiac Trabeculation. Development 2010, 137, 3867–3875. [Google Scholar] [CrossRef]
- Pieperhoff, S.; Bennett, W.; Farrell, A.P. The Intercellular Organization of the Two Muscular Systems in the Adult Salmonid Heart, the Compact and the Spongy Myocardium. J. Anat. 2009, 215, 536–547. [Google Scholar] [CrossRef]
- Patra, C.; Kontarakis, Z.; Kaur, H.; Rayrikar, A.; Mukherjee, D.; Stainier, D.Y.R. The Zebrafish Ventricle: A Hub of Cardiac Endothelial Cells for in Vitro Cell Behavior Studies. Sci. Rep. 2017, 7, 2687. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Sedmera, D.; Yost, H.J.; Clark, E.B. Structure and Function of the Developing Zebrafish Heart. Anat. Rec. 2000, 260, 148–157. [Google Scholar] [CrossRef]
- Bensimon-Brito, A.; Ramkumar, S.; Boezio, G.L.M.; Guenther, S.; Kuenne, C.; Helker, C.S.M.; Sánchez-Iranzo, H.; Iloska, D.; Piesker, J.; Pullamsetti, S.; et al. TGF-β Signaling Promotes Tissue Formation during Cardiac Valve Regeneration in Adult Zebrafish. Dev. Cell 2020, 52, 9–20.e7. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.; Yutzey, K.E. To EndoMT or Not to EndoMT: Zebrafish Heart Valve Development. Circ. Res. 2020, 126, 985–987. [Google Scholar] [CrossRef]
- Beis, D.; Bartman, T.; Jin, S.-W.; Scott, I.C.; D’Amico, L.A.; Ober, E.A.; Verkade, H.; Frantsve, J.; Field, H.A.; Wehman, A.; et al. Genetic and Cellular Analyses of Zebrafish Atrioventricular Cushion and Valve Development. Development 2005, 132, 4193–4204. [Google Scholar] [CrossRef]
- Grimes, A.C.; Stadt, H.A.; Shepherd, I.T.; Kirby, M.L. Solving an Enigma: Arterial Pole Development in the Zebrafish Heart. Dev. Biol. 2006, 290, 265–276. [Google Scholar] [CrossRef]
- Braun, M.H.; Brill, R.W.; Gosline, J.M.; Jones, D.R. Form and Function of the Bulbus Arteriosus in Yellowfin Tuna (Thunnus Albacares), Bigeye Tuna (Thunnus Obesus) and Blue Marlin (Makaira Nigricans): Static Properties. J. Exp. Biol. 2003, 206, 3311–3326. [Google Scholar] [CrossRef]
- Olson, K.R. Design and Physiology of Arteries and Veins | Anatomical Pathways and Patterns. In Encyclopedia of Fish Physiology; Farrell, A.P., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 1085–1094. ISBN 978-0-08-092323-9. [Google Scholar]
- Miano, J.M.; Georger, M.A.; Rich, A.; De Mesy Bentley, K.L. Ultrastructure of Zebrafish Dorsal Aortic Cells. Zebrafish 2006, 3, 455–463. [Google Scholar] [CrossRef]
- Faury, G. Function-Structure Relationship of Elastic Arteries in Evolution: From Microfibrils to Elastin and Elastic Fibres. Pathol. Biol. 2001, 49, 310–325. [Google Scholar] [CrossRef]
- Fritze, O.; Romero, B.; Schleicher, M.; Jacob, M.P.; Oh, D.-Y.; Starcher, B.; Schenke-Layland, K.; Bujan, J.; Stock, U.A. Age-Related Changes in the Elastic Tissue of the Human Aorta. J. Vasc. Res. 2012, 49, 77–86. [Google Scholar] [CrossRef]
- Chow, M.-J.; Turcotte, R.; Lin, C.P.; Zhang, Y. Arterial Extracellular Matrix: A Mechanobiological Study of the Contributions and Interactions of Elastin and Collagen. Biophys. J. 2014, 106, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.V.; Monzo, K.; Cha, Y.R.; Pan, W.; Weinstein, B.M. Vascular Development in the Zebrafish. Cold Spring Harb. Perspect. Med. 2012, 2, a006684. [Google Scholar] [CrossRef] [PubMed]
- Isogai, S.; Horiguchi, M.; Weinstein, B.M. The Vascular Anatomy of the Developing Zebrafish: An Atlas of Embryonic and Early Larval Development. Dev. Biol. 2001, 230, 278–301. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, J. Zebrafish as a Model to Study Cardiac Development and Human Cardiac Disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Schuermann, A.; Helker, C.S.M.; Herzog, W. Angiogenesis in Zebrafish. Semin. Cell Dev. Biol. 2014, 31, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.-L.; Harrison, M.R.; Tuan, T.-L.; Starnes, V.A. Heart Repair and Regeneration: Recent Insights from Zebrafish Studies. Wound Repair Regen. 2012, 20, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Morejón, A.; Mercader, N. Recent Insights into Zebrafish Cardiac Regeneration. Curr. Opin. Genet. Dev. 2020, 64, 37–43. [Google Scholar] [CrossRef]
- Ribeiro, A.O.; de Oliveira, A.C.; Costa, J.M.; Nachtigall, P.G.; Herkenhoff, M.E.; Campos, V.F.; Delella, F.K.; Pinhal, D. MicroRNA Roles in Regeneration: Multiple Lessons from Zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2021. [Google Scholar] [CrossRef]
- Penberthy, W.T.; Shafizadeh, E.; Lin, S. The Zebrafish as a Model for Human Disease. Front. Biosci. 2002, 7, D1439–D1453. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal Models of Human Disease: Zebrafish Swim into View. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Santoriello, C.; Zon, L.I. Hooked! Modeling Human Disease in Zebrafish. J. Clin. Investig. 2012, 122, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, R.N.; van Eeden, F.J.M. The Zebrafish as a Model of Vascular Development and Disease. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2014; Volume 124, pp. 93–122. ISBN 978-0-12-386930-2. [Google Scholar]
- Hogan, B.M.; Schulte-Merker, S. How to Plumb a Pisces: Understanding Vascular Development and Disease Using Zebrafish Embryos. Dev. Cell 2017, 42, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Bowley, G.; Kugler, E.; Wilkinson, R.; Lawrie, A.; van Eeden, F.; Chico, T.J.A.; Evans, P.C.; Noël, E.S.; Serbanovic-Canic, J. Zebrafish as a Tractable Model of Human Cardiovascular Disease. Br. J. Pharmacol. 2021, 179, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Chico, T.J.A.; Ingham, P.W.; Crossman, D.C. Modeling Cardiovascular Disease in the Zebrafish. Trends Cardiovasc. Med. 2008, 18, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Gabriela Espinosa, M.; Catalin Staiculescu, M.; Kim, J.; Marin, E.; Wagenseil, J.E. Elastic Fibers and Large Artery Mechanics in Animal Models of Development and Disease. J. Biomech. Eng. 2018, 140, 0208031–02080313. [Google Scholar] [CrossRef] [PubMed]
- Duque Lasio, M.L.; Kozel, B.A. Elastin-Driven Genetic Diseases. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 71–72, 144–160. [Google Scholar] [CrossRef]
- Davidson, J.M.; Zoia, O.; Liu, J.M. Modulation of Transforming Growth Factor-Beta 1 Stimulated Elastin and Collagen Production and Proliferation in Porcine Vascular Smooth Muscle Cells and Skin Fibroblasts by Basic Fibroblast Growth Factor, Transforming Growth Factor-Alpha, and Insulin-like Growth Factor-I. J. Cell. Physiol. 1993, 155, 149–156. [Google Scholar] [CrossRef]
- Suwanabol, P.A.; Seedial, S.M.; Shi, X.; Zhang, F.; Yamanouchi, D.; Roenneburg, D.; Liu, B.; Kent, K.C. TGF-β Increases Vascular Smooth Muscle Cell Proliferation through the Smad3 and ERK MAPK Pathways. J. Vasc. Surg. 2012, 56, 446–454.e1. [Google Scholar] [CrossRef]
- Judge, D.P.; Dietz, H.C. Marfan’s Syndrome. Lancet 2005, 366, 1965–1976. [Google Scholar] [CrossRef]
- Gansner, J.M.; Madsen, E.C.; Mecham, R.P.; Gitlin, J.D. Essential Role for Fibrillin-2 in Zebrafish Notochord and Vascular Morphogenesis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008, 237, 2844–2861. [Google Scholar] [CrossRef]
- Yin, X.; Hao, J.; Yao, Y. CRISPR/Cas9 in Zebrafish: An Attractive Model for FBN1 Genetic Defects in Humans. Mol. Genet. Genom. Med. 2021, 9, e1775. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Larson, J.D.; Ekker, S.C. Functional Analysis of Zebrafish Microfibril-Associated Glycoprotein-1 (Magp1) in Vivo Reveals Roles for Microfibrils in Vascular Development and Function. Blood 2006, 107, 4364–4374. [Google Scholar] [CrossRef] [PubMed]
- Craft, C.S.; Broekelmann, T.J.; Mecham, R.P. Microfibril-Associated Glycoproteins MAGP-1 and MAGP-2 in Disease. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 71–72, 100–111. [Google Scholar] [CrossRef]
- Craft, C.S. MAGP1, the Extracellular Matrix, and Metabolism. Adipocyte 2014, 4, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, Y.; Cederlund, M.L.; Cottell, D.C.; Bill, B.R.; Ekker, S.C.; Torres-Vazquez, J.; Weinstein, B.M.; Hyde, D.R.; Vihtelic, T.S.; Kennedy, B.N. Genetic Determinants of Hyaloid and Retinal Vasculature in Zebrafish. BMC Dev. Biol. 2007, 7, 114. [Google Scholar] [CrossRef]
- Robertson, I.B.; Horiguchi, M.; Zilberberg, L.; Dabovic, B.; Hadjiolova, K.; Rifkin, D.B. Latent TGF-β-Binding Proteins. Matrix Biol. J. Int. Soc. Matrix Biol. 2015, 47, 44–53. [Google Scholar] [CrossRef]
- Haji-Seyed-Javadi, R.; Jelodari-Mamaghani, S.; Paylakhi, S.H.; Yazdani, S.; Nilforushan, N.; Fan, J.-B.; Klotzle, B.; Mahmoudi, M.J.; Ebrahimian, M.J.; Chelich, N.; et al. LTBP2 Mutations Cause Weill-Marchesani and Weill-Marchesani-like Syndrome and Affect Disruptions in the Extracellular Matrix. Hum. Mutat. 2012, 33, 1182–1187. [Google Scholar] [CrossRef]
- Rifkin, D.B.; Rifkin, W.J.; Zilberberg, L. LTBPs in Biology and Medicine; LTBP Diseases. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 71–72, 90–99. [Google Scholar] [CrossRef]
- Callewaert, B.; Su, C.-T.; Van Damme, T.; Vlummens, P.; Malfait, F.; Vanakker, O.; Schulz, B.; Neal, M.M.; Davis, E.C.; Lee, J.G.H.; et al. Comprehensive Clinical and Molecular Analysis of 12 Families with Type 1 Recessive Cutis Laxa. Hum. Mutat. 2013, 34, 111–121. [Google Scholar] [CrossRef]
- Beyens, A.; Boel, A.; Symoens, S.; Callewaert, B. Cutis Laxa: A Comprehensive Overview of Clinical Characteristics and Pathophysiology. Clin. Genet. 2021, 99, 53–66. [Google Scholar] [CrossRef]
- Zhou, Y.; Cashman, T.J.; Nevis, K.R.; Obregon, P.; Carney, S.A.; Liu, Y.; Gu, A.; Mosimann, C.; Sondalle, S.; Peterson, R.E.; et al. Latent TGFβ Binding Protein 3 Identifies a Second Heart Field in Zebrafish. Nature 2011, 474, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Pottie, L.; Adamo, C.S.; Beyens, A.; Lütke, S.; Tapaneeyaphan, P.; Clercq, A.D.; Salmon, P.L.; Rycke, R.D.; Gezdirici, A.; Gulec, E.Y.; et al. Bi-Allelic Premature Truncating Variants in LTBP1 Cause Cutis Laxa Syndrome. Am. J. Hum. Genet. 2021, 108, 1095–1114. [Google Scholar] [CrossRef] [PubMed]
- Pletcher, B.A.; Fox, J.E.; Boxer, R.A.; Singh, S.; Blumenthal, D.; Cohen, T.; Brunson, S.; Tafreshi, P.; Kahn, E. Four Sibs with Arterial Tortuosity: Description and Review of the Literature. Am. J. Med. Genet. 1996, 66, 121–128. [Google Scholar] [CrossRef]
- Willaert, A.; Khatri, S.; Callewaert, B.L.; Coucke, P.J.; Crosby, S.D.; Lee, J.G.H.; Davis, E.C.; Shiva, S.; Tsang, M.; De Paepe, A.; et al. GLUT10 Is Required for the Development of the Cardiovascular System and the Notochord and Connects Mitochondrial Function to TGFβ Signaling. Hum. Mol. Genet. 2012, 21, 1248–1259. [Google Scholar] [CrossRef]
- Brunet, F.G.; Fraser, F.W.; Binder, M.J.; Smith, A.D.; Kintakas, C.; Dancevic, C.M.; Ward, A.C.; McCulloch, D.R. The Evolutionary Conservation of the A Disintegrin-like and Metalloproteinase Domain with Thrombospondin-1 Motif Metzincins across Vertebrate Species and Their Expression in Teleost Zebrafish. BMC Evol. Biol. 2015, 15, 22. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Hou, C.; Liu, Q.; Yang, S.; Zhang, Z.; Yang, W.; Yang, X. Internal Modulation of Proteolysis in Vascular Extracellular Matrix Remodeling: Role of ADAM Metallopeptidase with Thrombospondin Type 1 Motif 5 in the Development of Intracranial Aneurysm Rupture. Aging 2021, 13, 12800–12816. [Google Scholar] [CrossRef]
- Tsuda, T. Extracellular Interactions between Fibulins and Transforming Growth Factor (TGF)-β in Physiological and Pathological Conditions. Int. J. Mol. Sci. 2018, 19, 2787. [Google Scholar] [CrossRef]
- Cangemi, C.; Hansen, M.L.; Argraves, W.S.; Rasmussen, L.M. Fibulins and Their Role in Cardiovascular Biology and Disease. Adv. Clin. Chem. 2014, 67, 245–265. [Google Scholar] [CrossRef]
- Won, S.Y.; Kwon, S.; Jeong, H.S.; Chung, K.W.; Choi, B.; Chang, J.W.; Lee, J.E. Fibulin 5, a Human Wharton’s Jelly-derived Mesenchymal Stem Cells-secreted Paracrine Factor, Attenuates Peripheral Nervous System Myelination Defects through the Integrin-RAC1 Signaling Axis. Stem Cells 2020, 38, 1578–1593. [Google Scholar] [CrossRef]
- Russell, M.W.; Raeker, M.O.; Geisler, S.B.; Thomas, P.E.; Simmons, T.A.; Bernat, J.A.; Thorsson, T.; Innis, J.W. Functional Analysis of Candidate Genes in 2q13 Deletion Syndrome Implicates FBLN7 and TMEM87B Deficiency in Congenital Heart Defects and FBLN7 in Craniofacial Malformations. Hum. Mol. Genet. 2014, 23, 4272–4284. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Lardelli, M.; Ekblom, P. Sequence of Zebrafish Fibulin-1 and Its Expression in Developing Heart and Other Embryonic Organs. Dev. Genes Evol. 1997, 207, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Randell, A.; Daneshtalab, N. Elastin Microfibril Interface-Located Protein 1, Transforming Growth Factor Beta, and Implications on Cardiovascular Complications. J. Am. Soc. Hypertens. 2017, 11, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Milanetto, M.; Tiso, N.; Braghetta, P.; Volpin, D.; Argenton, F.; Bonaldo, P. Emilin Genes Are Duplicated and Dynamically Expressed during Zebrafish Embryonic Development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008, 237, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Munjal, C.; Opoka, A.M.; Osinska, H.; James, J.F.; Bressan, G.M.; Hinton, R.B. TGF-β Mediates Early Angiogenesis and Latent Fibrosis in an Emilin1-Deficient Mouse Model of Aortic Valve Disease. Dis. Model. Mech. 2014, 7, 987–996. [Google Scholar] [CrossRef]
- Iacomino, M.; Doliana, R.; Marchese, M.; Capuano, A.; Striano, P.; Spessotto, P.; Bosisio, G.; Iodice, R.; Manganelli, F.; Lanteri, P.; et al. Distal Motor Neuropathy Associated with Novel EMILIN1 Mutation. Neurobiol. Dis. 2020, 137, 104757. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xia, Z.; Wang, F. Zebrafish in the Sea of Mineral (Iron, Zinc, and Copper) Metabolism. Front. Pharmacol. 2014, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Hwang, P.-P. The Control of Calcium Metabolism in Zebrafish (Danio Rerio). Int. J. Mol. Sci. 2016, 17, 1783. [Google Scholar] [CrossRef]
- Rodriguez-Pascual, F.; Rosell-Garcia, T. Lysyl Oxidases: Functions and Disorders. J. Glaucoma 2018, 27 (Suppl. 1), S15–S19. [Google Scholar] [CrossRef]
- Ojha, R.; Prasad, A.N. Menkes Disease: What a Multidisciplinary Approach Can Do. J. Multidiscip. Healthc. 2016, 9, 371–385. [Google Scholar] [CrossRef]
- Vallet, S.D.; Ricard-Blum, S. Lysyl Oxidases: From Enzyme Activity to Extracellular Matrix Cross-Links. Essays Biochem. 2019, 63, 349–364. [Google Scholar] [CrossRef]
- Gansner, J.M.; Mendelsohn, B.A.; Hultman, K.A.; Johnson, S.L.; Gitlin, J.D. Essential Role of Lysyl Oxidases in Notochord Development. Dev. Biol. 2007, 307, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Regalado, E.S.; Gong, L.; Duan, X.; Santos-Cortez, R.L.P.; Arnaud, P.; Ren, Z.; Cai, B.; Hostetler, E.M.; Moran, R.; et al. LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections. Circ. Res. 2016, 118, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Bartlett, S.J.; Gansner, J.M.; Wilson, D.; He, L.; Gitlin, J.D.; Kelsh, R.N.; Dowden, J. Chemical Genetics Suggests a Critical Role for Lysyl Oxidase in Zebrafish Notochord Morphogenesis. Mol. Biosyst. 2007, 3, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, C.; Baas, D.; Gleyzal, C.; Le Guellec, D.; Sommer, P. Morpholino Knockdown of Lysyl Oxidase Impairs Zebrafish Development, and Reflects Some Aspects of Copper Metabolism Disorders. Matrix Biol. J. Int. Soc. Matrix Biol. 2008, 27, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, B.A.; Yin, C.; Johnson, S.L.; Wilm, T.P.; Solnica-Krezel, L.; Gitlin, J.D. Atp7a Determines a Hierarchy of Copper Metabolism Essential for Notochord Development. Cell Metab. 2006, 4, 155–162. [Google Scholar] [CrossRef]
- Madsen, E.C.; Morcos, P.A.; Mendelsohn, B.A.; Gitlin, J.D. In Vivo Correction of a Menkes Disease Model Using Antisense Oligonucleotides. Proc. Natl. Acad. Sci. USA 2008, 105, 3909–3914. [Google Scholar] [CrossRef]
- Madsen, E.C.; Gitlin, J.D. Zebrafish Mutants Calamity and Catastrophe Define Critical Pathways of Gene-Nutrient Interactions in Developmental Copper Metabolism. PLoS Genet. 2008, 4, e1000261. [Google Scholar] [CrossRef]
- Roach, E.S.; Islam, M.P. Pseudoxanthoma Elasticum. Handb. Clin. Neurol. 2015, 132, 215–221. [Google Scholar] [CrossRef]
- Van Gils, M.; Willaert, A.; De Vilder, E.Y.G.; Coucke, P.J.; Vanakker, O.M. Generation and Validation of a Complete Knockout Model of Abcc6a in Zebrafish. J. Invest. Dermatol. 2018, 138, 2333–2342. [Google Scholar] [CrossRef]
- Sun, J.; She, P.; Liu, X.; Gao, B.; Jin, D.; Zhong, T.P. Disruption of Abcc6 Transporter in Zebrafish Causes Ocular Calcification and Cardiac Fibrosis. Int. J. Mol. Sci. 2020, 22, 278. [Google Scholar] [CrossRef]
- Jansen, R.S.; Küçükosmanoglu, A.; de Haas, M.; Sapthu, S.; Otero, J.A.; Hegman, I.E.M.; Bergen, A.A.B.; Gorgels, T.G.M.F.; Borst, P.; van de Wetering, K. ABCC6 Prevents Ectopic Mineralization Seen in Pseudoxanthoma Elasticum by Inducing Cellular Nucleotide Release. Proc. Natl. Acad. Sci. USA 2013, 110, 20206–20211. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Baek, K.I.; Chang, C.-C.; Zhou, B.; Hsiai, T.K. Mechanosensitive Pathways Involved in Cardiovascular Development and Homeostasis in Zebrafish. J. Vasc. Res. 2019, 56, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Mackay, E.W.; Apschner, A.; Schulte-Merker, S. Vitamin K Reduces Hypermineralisation in Zebrafish Models of PXE and GACI. Development 2015, 142, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Pomozi, V.; Brampton, C.; Fülöp, K.; Chen, L.-H.; Apana, A.; Li, Q.; Uitto, J.; Le Saux, O.; Váradi, A. Analysis of Pseudoxanthoma Elasticum–Causing Missense Mutants of ABCC6 In Vivo; Pharmacological Correction of the Mislocalized Proteins. J. Invest. Dermatol. 2014, 134, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Apschner, A.; Huitema, L.F.A.; Ponsioen, B.; Peterson-Maduro, J.; Schulte-Merker, S. Pathological Mineralization in a Zebrafish Enpp1 Mutant Exhibits Features of Generalized Arterial Calcification of Infancy (GACI) and Pseudoxanthoma Elasticum (PXE). Dis. Model. Mech. 2014, 7, 811–822. [Google Scholar] [CrossRef]
- Ribas, L.; Piferrer, F. The Zebrafish (Danio Rerio) as a Model Organism, with Emphasis on Applications for Finfish Aquaculture Research. Rev. Aquac. 2014, 6, 209–240. [Google Scholar] [CrossRef]
- Lawson, N.D.; Weinstein, B.M. Arteries and Veins: Making a Difference with Zebrafish. Nat. Rev. Genet. 2002, 3, 674–682. [Google Scholar] [CrossRef]
- Jin, S.-W.; Beis, D.; Mitchell, T.; Chen, J.-N.; Stainier, D.Y.R. Cellular and Molecular Analyses of Vascular Tube and Lumen Formation in Zebrafish. Development 2005, 132, 5199–5209. [Google Scholar] [CrossRef]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent Adult Zebrafish as a Tool for in Vivo Transplantation Analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef]
- Jung, H.M.; Isogai, S.; Kamei, M.; Castranova, D.; Gore, A.V.; Weinstein, B.M. Imaging Blood Vessels and Lymphatic Vessels in the Zebrafish. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 133, pp. 69–103. ISBN 978-0-12-803475-0. [Google Scholar]
- Boselli, F.; Vermot, J. Live Imaging and Modeling for Shear Stress Quantification in the Embryonic Zebrafish Heart. Methods 2016, 94, 129–134. [Google Scholar] [CrossRef]
- Yalcin, H.C.; Amindari, A.; Butcher, J.T.; Althani, A.; Yacoub, M. Heart Function and Hemodynamic Analysis for Zebrafish Embryos. Dev. Dyn. 2017, 246, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-Y.; Kwon, H.-B.; Ahn, J.-C.; Kang, D.; Kwon, S.-H.; Park, J.A.; Kim, K.-W. Functional and Developmental Analysis of the Blood-Brain Barrier in Zebrafish. Brain Res. Bull. 2008, 75, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.R.M.; Bussmann, J.; Huang, Y.; Zhao, L.; Osorio, A.; Burns, C.G.; Burns, C.E.; Sucov, H.M.; Siekmann, A.F.; Lien, C.-L. Chemokine-Guided Angiogenesis Directs Coronary Vasculature Formation in Zebrafish. Dev. Cell 2015, 33, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.; Vásquez, I.C. Cardiac and Somatic Parameters in Zebrafish: Tools for the Evaluation of Cardiovascular Function. Fish Physiol. Biochem. 2016, 42, 569–577. [Google Scholar] [CrossRef]
- Fei, P.; Lee, J.; Packard, R.R.S.; Sereti, K.-I.; Xu, H.; Ma, J.; Ding, Y.; Kang, H.; Chen, H.; Sung, K.; et al. Cardiac Light-Sheet Fluorescent Microscopy for Multi-Scale and Rapid Imaging of Architecture and Function. Sci. Rep. 2016, 6, 22489. [Google Scholar] [CrossRef]
- Scott, A.; Sueiro Ballesteros, L.; Bradshaw, M.; Tsuji, C.; Power, A.; Lorriman, J.; Love, J.; Paul, D.; Herman, A.; Emanueli, C.; et al. In Vivo Characterization of Endogenous Cardiovascular Extracellular Vesicles in Larval and Adult Zebrafish. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2454–2468. [Google Scholar] [CrossRef]
- Li, D.Y.; Brooke, B.; Davis, E.C.; Mecham, R.P.; Sorensen, L.K.; Boak, B.B.; Eichwald, E.; Keating, M.T. Elastin Is an Essential Determinant of Arterial Morphogenesis. Nature 1998, 393, 276–280. [Google Scholar] [CrossRef]
- Goldner, J. A Modification of the Masson Trichrome Technique for Routine Laboratory Purposes. Am. J. Pathol. 1938, 14, 237–243. [Google Scholar]
- Shikata, T.; Uzawa, T.; Yoshiwara, N.; Akatsuka, T.; Yamazaki, S. Staining Methods of Australia Antigen in Paraffin Section--Detection of Cytoplasmic Inclusion Bodies. Jpn. J. Exp. Med. 1974, 44, 25–36. [Google Scholar]
- Henwood, A.F. Shikata’s Orcein Stain—A Routine Stain for Liver Biopsies. Aust. J. Med. Lab. Sci. 1983, 4, 76–80. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoareau, M.; El Kholti, N.; Debret, R.; Lambert, E. Zebrafish as a Model to Study Vascular Elastic Fibers and Associated Pathologies. Int. J. Mol. Sci. 2022, 23, 2102. https://doi.org/10.3390/ijms23042102
Hoareau M, El Kholti N, Debret R, Lambert E. Zebrafish as a Model to Study Vascular Elastic Fibers and Associated Pathologies. International Journal of Molecular Sciences. 2022; 23(4):2102. https://doi.org/10.3390/ijms23042102
Chicago/Turabian StyleHoareau, Marie, Naïma El Kholti, Romain Debret, and Elise Lambert. 2022. "Zebrafish as a Model to Study Vascular Elastic Fibers and Associated Pathologies" International Journal of Molecular Sciences 23, no. 4: 2102. https://doi.org/10.3390/ijms23042102
APA StyleHoareau, M., El Kholti, N., Debret, R., & Lambert, E. (2022). Zebrafish as a Model to Study Vascular Elastic Fibers and Associated Pathologies. International Journal of Molecular Sciences, 23(4), 2102. https://doi.org/10.3390/ijms23042102






