Alteration of Ethanol Reward by Prior Mephedrone Exposure: The Role of Age and Matrix Metalloproteinase-9 (MMP-9)
Abstract
:1. Introduction
2. Results
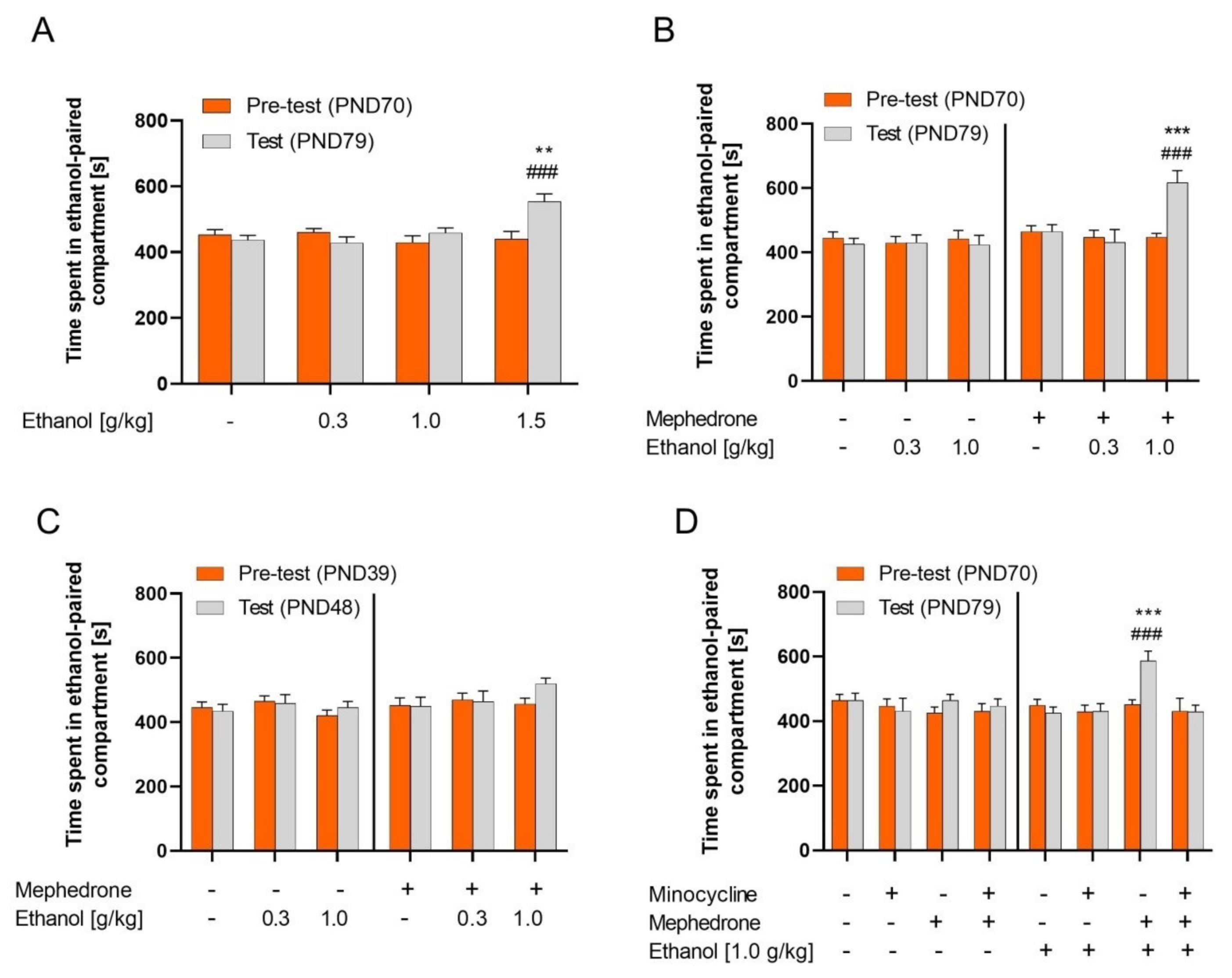
2.1. Ethanol CPP
2.2. Effect of Mephedrone Pretreatment on Ethanol CPP in Adult and Adolescent Rats
2.3. Influence of Minocycline Pretreatment on the Mephedrone Effect on the Ethanol CPP in Adult Rats
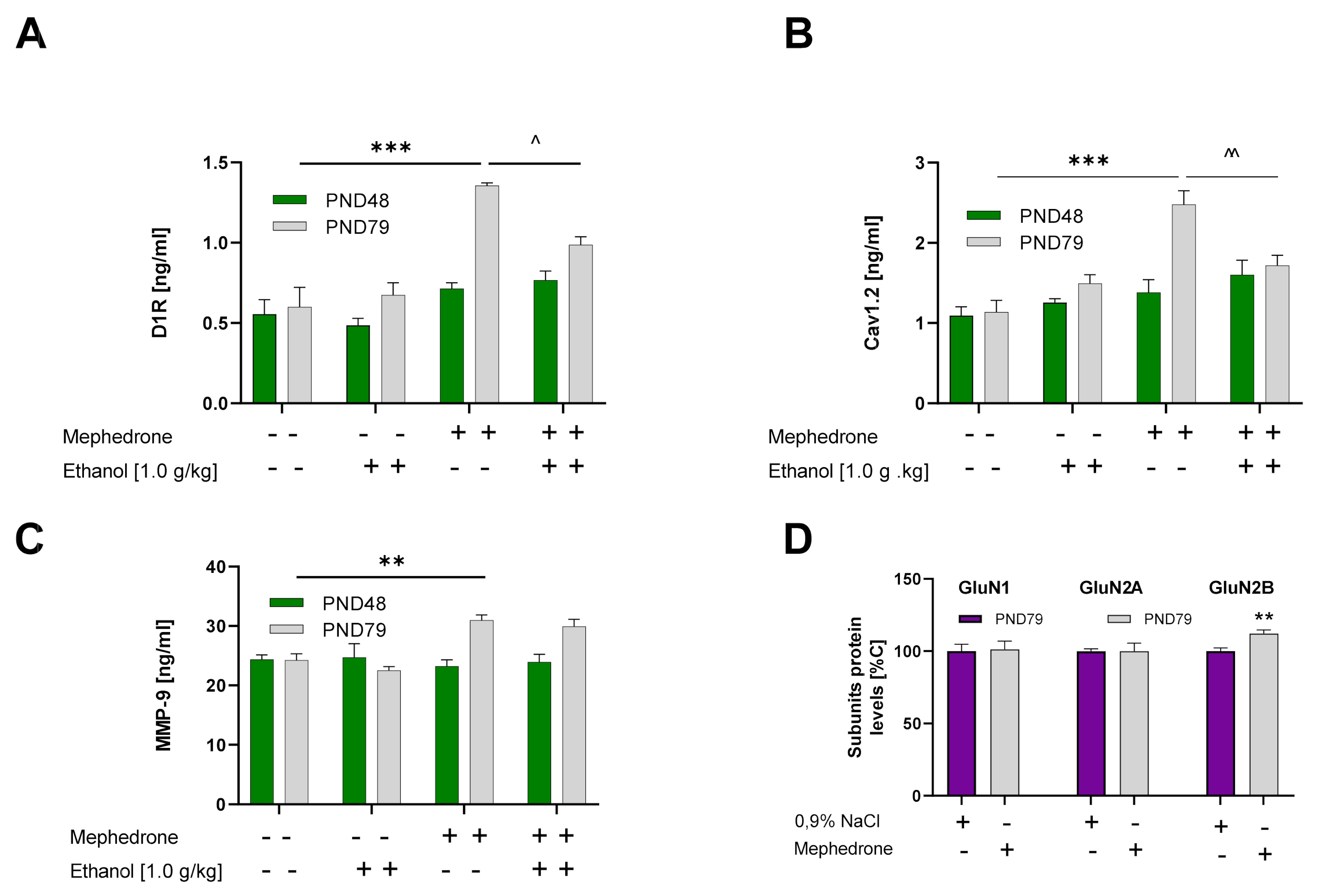
2.4. Biochemical Experiments
2.4.1. Effect of Mephedrone Pretreatment, Ethanol Treatment, and Age of Rats on D1R Expression in the vSTR
2.4.2. Effect of Mephedrone Pretreatment, Ethanol Treatment, and Age of Rats on Cav1.2 Expression in the vSTR
2.4.3. Effect of Mephedrone Pretreatment, Ethanol Treatment, and Age of Rats on MMP-9 Expression in the vSTR
2.4.4. Influence of Minocycline Pretreatment on the Mephedrone Effect on MMP-9 Expression in the vSTR
2.4.5. Effect of Mephedrone Treatment on the Expression of NMDAR Subunits (GluN1, GluN2A, and GluN2B) in the vSTR
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. CPP Apparatus
4.4. CPP Procedure
4.4.1. The Effect of Mephedrone Pretreatment on the Ethanol CPP
4.4.2. The Influence of Minocycline Pretreatment on the Mephedrone Effect on the Ethanol CPP
4.4.3. The Ethanol CPP
4.5. Biochemical Experiments
4.5.1. ELISA Assay
4.5.2. Western Blot
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schifano, F.; Albanese, A.; Fergus, S.; Stair, J.L.; Deluca, P.; Corazza, O.; Davey, Z.; Corkery, J.; Siemann, H.; Scherbaum, N.; et al. Mephedrone (4-methylmethcathinone; ‘meow meow’): Chemical, pharmacological and clinical issues. Psychopharmacology 2011, 214, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vardakou, I.; Pistos, C.; Spiliopoulou, C. Drugs for youth via Internet and the example of mephedrone. Toxicol. Lett. 2011, 201, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Bretteville-Jensen, A.L.; Tuv, S.S.; Bilgrei, O.R.; Fjeld, B.; Bachs, L. Synthetic cannabinoids and cathinones: Prevalence and markets. Forensic. Sci. Rev. 2013, 25, 7–26. [Google Scholar] [PubMed]
- Brandt, S.D.; Sumnall, H.R.; Measham, F.; Cole, J. Analyses of second-generation ‘legal highs’ in the UK: Initial findings. Drug Test. Anal. 2010, 2, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Hockenhull, J.; Murphy, K.G.; Paterson, S. Mephedrone use is increasing in London. Lancet 2016, 387, 1719–1720. [Google Scholar] [CrossRef] [Green Version]
- Angoa-Pérez, M.; Kane, M.J.; Francescutti, D.M.; Sykes, K.E.; Shah, M.M.; Mohammed, A.M.; Thomas, D.M.; Kuhn, D.M. Mephedrone, an abused psychoactive component of ‘bath salts’ and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J. Neurochem. 2012, 120, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, C.; McElrath, K. Simultaneous use of mephedrone and alcohol: A qualitative study of users’ experiences. J. Addict. Res. Ther. 2012, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Elliott, S.; Evans, J. A 3-year review of new psychoactive substances in casework. Forensic. Sci. Int. 2014, 243, 55–60. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; de Sousa Fernandes Perna, E.B.; Olesti, E.; Mateus, J.; Kuypers, K.P.; Theunissen, E.L.; Fonseca, F.; Torrens, M.; Ramaekers, J.G.; et al. Mephedrone and alcohol interactions in humans. Front. Pharmacol. 2020, 10, 1588. [Google Scholar] [CrossRef]
- Brunelle, C.; Barrett, S.P.; Pihl, R.O. Psychostimulant users are sensitive to the stimulant properties of alcohol as indexed by alcohol-induced cardiac reactivity. Psychol. Addict. Behav. 2006, 20, 478–483. [Google Scholar] [CrossRef]
- Salgado, S.; Kaplitt, M.G. The nucleus accumbens: A comprehensive review. Stereotact. Funct. Neurosurg. 2015, 93, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Higa, K.K.; Young, J.W.; Ji, B.; Nichols, D.E.; Geyer, M.A.; Zhou, X. Striatal dopamine D1 receptor suppression impairs reward-associative learning. Behav. Brain Res. 2017, 323, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.M.; Famous, K.R.; Sadri-Vakili, G.; Kumaresan, V.; Schmidt, H.D.; Bass, C.E.; Terwilliger, E.F.; Cha, J.H.; Pierce, R.C. CaMKII: A biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat. Neurosci. 2008, 11, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Simmler, L.D.; Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 2014, 79, 152–160. [Google Scholar] [CrossRef]
- Kehr, J.; Ichinose, F.; Yoshitake, S.; Goiny, M.; Sievertsson, T.; Nyberg, F.; Yoshitake, T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br. J. Pharmacol. 2011, 164, 1949–1958. [Google Scholar] [CrossRef] [Green Version]
- Baumann, M.H.; Ayestas, M.A.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 2012, 37, 1192–1203. [Google Scholar] [CrossRef]
- López-Arnau, R.; Martínez-Clemente, J.; Pubill, D.; Escubedo, E.; Camarasa, J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: Butylone, mephedrone and methylone. Br. J Pharmacol. 2012, 167, 407–420. [Google Scholar] [CrossRef] [Green Version]
- Gołembiowska, K.; Jurczak, A.; Kamińska, K.; Noworyta-Sokołowska, K.; Górska, A. Effect of some psychoactive drugs used as ‘legal highs’ on brain neurotransmitters. Neurotox. Res. 2016, 29, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Siggins, G.R.; Roberto, M.; Nie, Z. The tipsy terminal: Presynaptic effects of ethanol. Pharmacol. Ther. 2005, 107, 80–98. [Google Scholar] [CrossRef]
- Mitchell, J.M.; O’Neil, J.P.; Janabi, M.; Marks, S.M.; Jagust, W.J.; Fields, H.L. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci. Transl. Med. 2012, 4, 116ra6. [Google Scholar] [CrossRef] [Green Version]
- Riegert, C.; Wedekind, F.; Hamida, S.B.; Rutz, S.; Rothmaier, A.K.; Jones, B.C.; Cassel, J.C.; Jackisch, R. Effects of ethanol and 3,4-methylenedioxymethamphetamine (MDMA) alone or in combination on spontaneous and evoked overflow of dopamine, serotonin and acetylcholine in striatal slices of the rat brain. Int. J. Neuropsychopharmacol. 2008, 11, 743–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daws, L.C.; Montañez, S.; Munn, J.L.; Owens, W.A.; Baganz, N.L.; Boyce-Rustay, J.M.; Millstein, R.A.; Wiedholz, L.M.; Murphy, D.L.; Holmes, A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J. Neurosci. 2006, 26, 6431–6438. [Google Scholar] [CrossRef] [Green Version]
- Ethell, I.M.; Ethell, D.W. Matrix metalloproteinases in brain development and remodeling: Synaptic functions and targets. J. Neurosci. Res. 2007, 85, 2813–2823. [Google Scholar] [CrossRef]
- Wright, J.W.; Harding, J.W. The brain angiotensin system and extracellular matrix molecules in neural plasticity, learning, and memory. Prog. Neurobiol. 2004, 72, 263–293. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [PubMed] [Green Version]
- Reinhard, S.M.; Razak, K.; Ethell, I.M. A delicate balance: Role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front. Cell Neurosci. 2015, 9, 280. [Google Scholar] [CrossRef] [Green Version]
- Gore, S.V.; James, E.J.; Huang, L.C.; Park, J.J.; Berghella, A.; Thompson, A.C.; Cline, H.T.; Aizenman, C.D. Role of matrix metalloproteinase-9 in neurodevelopmental deficits and experience-dependent plasticity in Xenopus laevis. Elife 2021, 10, e62147. [Google Scholar] [CrossRef]
- Smith, A.C.; Kupchik, Y.M.; Scofield, M.D.; Gipson, C.D.; Wiggins, A.; Thomas, C.A.; Kalivas, P.W. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat. Neurosci. 2014, 17, 1655–1657. [Google Scholar] [CrossRef] [Green Version]
- Boguszewska-Czubara, A.; Kurzepa, J.; Biała, G.; Kaszubska, K.; Grot, K.; Tarkowski, P.; Kowalczyk, J.; Silvestro, S.; Faggio, C.; Budzyńska, B. Mephedrone impact on matrix metalloproteinases activity—Do they influence the memory processes? Curr. Mol. Pharmacol. 2019, 12, 115–121. [Google Scholar] [CrossRef]
- Grochecki, P.; Smaga, I.; Lopatynska-Mazurek, M.; Gibula-Tarlowska, E.; Kedzierska, E.; Listos, J.; Talarek, S.; Marszalek-Grabska, M.; Hubalewska-Mazgaj, M.; Korga-Plewko, A.; et al. Effects of mephedrone and amphetamine exposure during adolescence on spatial memory in adulthood: Behavioral and neurochemical analysis. Int. J. Mol. Sci. 2021, 22, 589. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Beroun, A.; Lebitko, T.; Markina, O.; Leski, S.; Meyza, K.; Grzywacz, A.; Samochowiec, J.; Samochowiec, A.; Radwanska, K.; et al. Matrix metalloproteinase-9 and synaptic plasticity in the central amygdala in control of alcohol-seeking behavior. Biol. Psychiatry 2017, 81, 907–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizoguchi, H.; Yamada, K.; Niwa, M.; Mouri, A.; Mizuno, T.; Noda, Y.; Nitta, A.; Itohara, S.; Banno, Y.; Nabeshima, T. Reduction of methamphetamine-induced sensitization and reward in matrix metalloproteinase-2 and -9-deficient mice. J. Neurochem. 2007, 100, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, H.; Yamada, K.; Mouri, A.; Niwa, M.; Mizuno, T.; Noda, Y.; Nitta, A.; Itohara, S.; Banno, Y.; Nabeshima, T. Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: Implications for dopamine receptor down-regulation and dopamine release. J. Neurochem. 2007, 102, 1548–1560. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Yamada, K.; Nabeshima, T. Neuropsychotoxicity of abused drugs: Involvement of matrix metalloproteinase-2 and -9 and tissue inhibitor of matrix metalloproteinase-2 in methamphetamine-induced behavioral sensitization and reward in rodents. J. Pharmacol. Sci. 2008, 106, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natarajan, R.; Harding, J.W.; Wright, J.W. A role for matrix metalloproteinases in nicotine-induced conditioned place preference and relapse in adolescent female rats. J. Exp. Neurosci. 2013, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.E.; Forquer, M.R.; Cocking, D.L.; Jansen, H.T.; Harding, J.W.; Sorg, B.A. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem. 2007, 14, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Spear, L.P. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol. Behav. 2015, 148, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Vetter-O’Hagen, C.S.; Spear, L.P. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev. Psychobiol. 2012, 54, 523–535. [Google Scholar] [CrossRef] [Green Version]
- Crews, F.; He, J.; Hodge, C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacol. Biochem. Behav. 2007, 86, 189–199. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Jacobus, J.; Tapert, S.F. The influence of substance use on adolescent brain development. Clin. EEG Neurosci. 2009, 40, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Mateos-García, A.; Roger-Sánchez, C.; Rodriguez-Arias, M.; Miñarro, J.; Aguilar, M.A.; Manzanedo, C.; Arenas, M.C. Higher sensitivity to the conditioned rewarding effects of cocaine and MDMA in High-Novelty-Seekers mice exposed to a cocaine binge during adolescence. Psychopharmacology 2015, 232, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Molet, J.; Bouaziz, E.; Hamon, M.; Lanfumey, L. Early exposure to ethanol differentially affects ethanol preference at adult age in two inbred mouse strains. Neuropharmacology 2012, 63, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Molet, J.; Hervé, D.; Thiébot, M.H.; Hamon, M.; Lanfumey, L. Juvenile ethanol exposure increases rewarding properties of cocaine and morphine in adult DBA/2J mice. Eur. Neuropsychopharmacol. 2013, 23, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Spear, L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef]
- Lee, C.Z.; Yao, J.S.; Huang, Y.; Zhai, W.; Liu, W.; Guglielmo, B.J.; Lin, E.; Yang, G.Y.; Young, W.L. Dose-response effect of tetracyclines on cerebral matrix metalloproteinase-9 after vascular endothelial growth factor hyperstimulation. J. Cereb. Blood Flow Metab. 2006, 26, 1157–1164. [Google Scholar] [CrossRef]
- Vandooren, J.; Knoops, S.; Aldinucci Buzzo, J.L.; Boon, L.; Martens, E.; Opdenakker, G.; Kolaczkowska, E. Differential inhibition of activity, activation and gene expression of MMP-9 in THP-1 cells by azithromycin and minocycline versus bortezomib: A comparative study. PLoS ONE 2017, 12, e0174853. [Google Scholar] [CrossRef] [Green Version]
- Modheji, M.; Olapour, S.; Khodayar, M.J.; Jalili, A.; Yaghooti, H. Minocycline is more potent than tetracycline and doxycycline in inhibiting MMP-9 in vitro. Jundishapur. J. Nat. Pharm. Prod. 2016, 11, e27377. [Google Scholar] [CrossRef]
- Pujara, M.S.; Philippi, C.L.; Motzkin, J.C.; Baskaya, M.K.; Koenigs, M. Ventromedial prefrontal cortex damage is associated with decreased ventral striatum volume and response to reward. J. Neurosci. 2016, 36, 5047–5054. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.P.; Magnuson, E.C.; Dahly, A.M.; Siegel, J.A. The effects of enriched environment on the behavioral and corticosterone response to methamphetamine in adolescent and adult mice. Dev. Psychobiol. 2018, 60, 664–673. [Google Scholar] [CrossRef]
- Good, R.L.; Liang, L.P.; Patel, M.; Radcliffe, R.A. Mouse strain- and age-dependent effects of binge methamphetamine on dopaminergic signaling. Neurotoxicology 2011, 32, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Stojakovic, A.; Ahmad, S.M.; Lutfy, K. Alterations of amphetamine reward by prior nicotine and alcohol treatment: The role of age and dopamine. Brain Sci. 2021, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Carrara-Nascimento, P.F.; Hoffmann, L.B.; Flório, J.C.; Planeta, C.S.; Camarini, R. Effects of ethanol exposure during adolescence or adulthood on locomotor sensitization and dopamine levels in the reward system. Front. Behav. Neurosci. 2020, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.C.; Sumnall, H.R.; O’Shea, E.; Marsden, C.A. Effects of MDMA exposure on the conditioned place preference produced by other drugs of abuse. Psychopharmacology 2003, 166, 383–390. [Google Scholar] [CrossRef]
- Self, D.W.; Choi, K.H. Extinction-induced neuroplasticity attenuates stress-induced cocaine seeking: A state-dependent learning hypothesis. Stress 2004, 7, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Tseng, A.; Marquez, P.; Hamid, A.; Lutfy, K. The role of endogenous dynorphin in ethanol-induced state-dependent CPP. Behav. Brain Res. 2012, 227, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Teixeira-Gomes, A.; Costa, V.M.; Feio-Azevedo, R.; Bastos Mde, L.; Carvalho, F.; Capela, J.P. The neurotoxicity of amphetamines during the adolescent period. Int. J. Dev. Neurosci. 2015, 41, 44–62. [Google Scholar] [CrossRef]
- Nestler, E.J. Molecular neurobiology of addiction. Am. J. Addict. 2001, 10, 201–217. [Google Scholar] [CrossRef]
- Bahi, A.; Dreyer, J.L. Involvement of nucleus accumbens dopamine D1 receptors in ethanol drinking, ethanol-induced conditioned place preference, and ethanol-induced psychomotor sensitization in mice. Psychopharmacology 2012, 222, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Brown, S.; Shaikh, J.; Fishback, J.A.; Matsumoto, R.R. Relationship between methamphetamine exposure and matrix metalloproteinase 9 expression. Neuroreport 2008, 19, 1407–1409. [Google Scholar] [CrossRef] [Green Version]
- Yrjanheikki, J.; Keinanen, R.; Pellikka, M.; Hokfelt, T.; Koistinaho, J. Tetra-cyclines inhibit microglial activation and are neuroprotec- tive in global brain ischemia. Proc. Natl. Acad. Sci. USA 1998, 95, 15769–15774. [Google Scholar] [CrossRef] [Green Version]
- Yrjanheikki, J.; Tikka, T.; Keinanen, R.; Goldsteins, G.; Chan, P.H.; Koistinaho, J. A tetracycline derivative, minocycline, reduces inflam- mation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl. Acad. Sci. USA 1999, 96, 13496–13500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, T.A.; Kelly, K.J.; Mang, H.E.; Plotkin, Z.; Sandoval, R.M.; Dagher, P.C. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am. J. Physiol. Renal. Physiol. 2005, 288, F91–F97. [Google Scholar] [CrossRef] [PubMed]
- Paemen, L.; Martens, E.; Norga, K.; Masure, S.; Roets, E.; Hoogmartens, J.; Opdenakker, G. The gelatinase inhibitory activity of tetracy-clines and chemically modified tetracycline analogues as measured by a novel microtiter assay for inhibitors. Biochem. Pharmacol. 1996, 52, 105–111. [Google Scholar] [CrossRef]
- Xu, L.; Fagan, S.C.; Waller, J.L.; Edwards, D.; Borlongan, C.V.; Zheng, J.; Hill, W.D.; Feuerstein, G.; Hess, D.C. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion- reperfusion in rats. BMC Neurol. 2004, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stirling, D.P.; Koochesfahani, K.M.; Steeves, J.D.; Tetzlaff, W. Minocycline as a neuroprotective agent. Neuroscientist 2005, 11, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Zemke, D.; Majid, A. The potential of minocycline for neuroprotection in human neurologic disease. Clin. Neuropharmacol. 2004, 27, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K.; Kawasaki, S.; Kobori, T.; Fujita-Hamabe, W.; Mizoguchi, H.; Yamada, K.; Nabeshima, T.; Tokuyama, S. Involvement of matrix metalloproteinase-9 in the development of morphine tolerance. Eur. J. Pharmacol. 2012, 683, 86–92. [Google Scholar] [CrossRef]
- Yin, L.; Li, F.; Li, J.; Yang, X.; Xie, X.; Xue, L.; Li, Y.; Zhang, C. Chronic intermittent ethanol exposure induces upregulation of matrix metalloproteinase-9 in the rat medial prefrontal cortex and hippocampus. Neurochem. Res. 2019, 44, 1593–1601. [Google Scholar] [CrossRef]
- Wright, J.W.; Masino, A.J.; Reichert, J.R.; Turner, G.D.; Meighan, S.E.; Meighan, P.C.; Harding, J.W. Ethanol-induced impairment of spatial memory and brain matrix metalloproteinases. Brain Res. 2003, 963, 252–261. [Google Scholar] [CrossRef]
- Lasek, A.W. Effects of ethanol on brain extracellular matrix: Implications for alcohol use disorder. Alcohol. Clin. Exp. Res. 2016, 40, 2030–2042. [Google Scholar] [CrossRef] [Green Version]
- Bardo, M.T.; Bevins, R.A. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology 2000, 153, 31–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.W.; Nealey, K.A.; Wright, J.W.; Walker, B.M. Plasticity associated with escalated operant ethanol self-administration during acute withdrawal in ethanol-dependent rats requires intact matrix metalloproteinase systems. Neurobiol. Learn. Mem. 2011, 96, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, B.S.; Sirohi, S.; Walker, B.M. The role of matrix metalloproteinase-9 in negative reinforcement learning and plasticity in alcohol dependence. Addict. Biol. 2020, 25, e12715. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Kramár, E.A.; Meighan, S.E.; Harding, J.W. Extracellular matrix molecules, long-term potentiation, memory consolidation and the brain angiotensin system. Peptides 2002, 23, 221–246. [Google Scholar] [CrossRef]
- Nagy, V.; Bozdagi, O.; Matynia, A.; Balcerzyk, M.; Okulski, P.; Dzwonek, J.; Costa, R.M.; Silva, A.J.; Kaczmarek, L.; Huntley, G.W. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci. 2006, 26, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Meighan, P.C.; Meighan, S.E.; Davis, C.J.; Wright, J.W.; Harding, J.W. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J. Neurochem. 2007, 102, 2085–2096. [Google Scholar] [CrossRef]
- Tian, L.; Stefanidakis, M.; Ning, L.; Van Lint, P.; Nyman-Huttunen, H.; Libert, C.; Itohara, S.; Mishina, M.; Rauvala, H.; Gahmberg, C.G. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J. Cell Biol. 2007, 178, 687–700. [Google Scholar] [CrossRef] [Green Version]
- Conant, K.; Wang, Y.; Szklarczyk, A.; Dudak, A.; Mattson, M.P.; Lim, S.T. Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience 2010, 166, 508–521. [Google Scholar] [CrossRef] [Green Version]
- Rauch, U.; Zhou, X.H.; Roos, G. Extracellular matrix alterations in brains lacking four of its components. Biochem. Biophys. Res. Commun. 2005, 328, 608–617. [Google Scholar] [CrossRef] [Green Version]
- Mitlöhner, J.; Kaushik, R.; Niekisch, H.; Blondiaux, A.; Gee, C.E.; Happel, M.F.K.; Gundelfinger, E.; Dityatev, A.; Frischknecht, R.; Seidenbecher, C. Dopamine receptor activation modulates the integrity of the perisynaptic extracellular matrix at excitatory synapses. Cells 2020, 9, 260. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Partridge, J.; Berger, C.; Sepulveda-Rodriguez, A.; Vicini, S.; Conant, K. Dopamine increases NMDA-stimulated calcium flux in striatopallidal neurons through a matrix metalloproteinase-dependent mechanism. Eur. J. Neurosci. 2016, 43, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Becker, J.B. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J. Neurosci. 2003, 23, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; Prendergast, B.J.; Liang, J.W. Female rats are not more variable than male rats: A meta-analysis of neuroscience studies. Biol. Sex Differ. 2016, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- López-Arnau, R.; Martínez-Clemente, J.; Rodrigo, T.; Pubill, D.; Camarasa, J.; Escubedo, E. Neuronal changes and oxidative stress in adolescent rats after repeated exposure to mephedrone. Toxicol. Appl. Pharmacol. 2015, 286, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Sangobowale, M.A.; Grin’kina, N.M.; Whitney, K.; Nikulina, E.; St Laurent-Ariot, K.; Ho, J.S.; Bayzan, N.; Bergold, P.J. Minocycline plus N-acetylcysteine reduce behavioral deficits and improve histology with a clinically useful time window. J. Neurotrauma 2018, 35, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Gibula-Tarlowska, E.; Grochecki, P.; Silberring, J.; Kotlinska, J.H. The kisspeptin derivative kissorphin reduces the acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in rats. Alcohol 2019, 81, 11–19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochecki, P.; Smaga, I.; Marszalek-Grabska, M.; Lopatynska-Mazurek, M.; Slowik, T.; Gibula-Tarlowska, E.; Kedzierska, E.; Listos, J.; Filip, M.; Kotlinska, J.H. Alteration of Ethanol Reward by Prior Mephedrone Exposure: The Role of Age and Matrix Metalloproteinase-9 (MMP-9). Int. J. Mol. Sci. 2022, 23, 2122. https://doi.org/10.3390/ijms23042122
Grochecki P, Smaga I, Marszalek-Grabska M, Lopatynska-Mazurek M, Slowik T, Gibula-Tarlowska E, Kedzierska E, Listos J, Filip M, Kotlinska JH. Alteration of Ethanol Reward by Prior Mephedrone Exposure: The Role of Age and Matrix Metalloproteinase-9 (MMP-9). International Journal of Molecular Sciences. 2022; 23(4):2122. https://doi.org/10.3390/ijms23042122
Chicago/Turabian StyleGrochecki, Pawel, Irena Smaga, Marta Marszalek-Grabska, Malgorzata Lopatynska-Mazurek, Tymoteusz Slowik, Ewa Gibula-Tarlowska, Ewa Kedzierska, Joanna Listos, Malgorzata Filip, and Jolanta H. Kotlinska. 2022. "Alteration of Ethanol Reward by Prior Mephedrone Exposure: The Role of Age and Matrix Metalloproteinase-9 (MMP-9)" International Journal of Molecular Sciences 23, no. 4: 2122. https://doi.org/10.3390/ijms23042122





