Matrix Metalloproteinase-10 in Kidney Injury Repair and Disease
Abstract
1. Introduction
2. Biology of MMP-10
2.1. Structure of MMP-10
2.2. Transcriptional Regulation of MMP-10
2.3. Posttranscriptional Activation of MMP-10
2.4. Inhibitors of MMP-10
3. MMP-10 Expression in the Kidney
3.1. AKI
3.2. CKD
3.3. RCC
4. Role of MMP-10 in Kidney Disease
4.1. Acute Kidney Injury
4.2. Diabetic Kidney Disease
4.3. Nondiabetic Glomerular Disease
4.4. Alport Glomerular Disease
4.5. Kidney Cancer
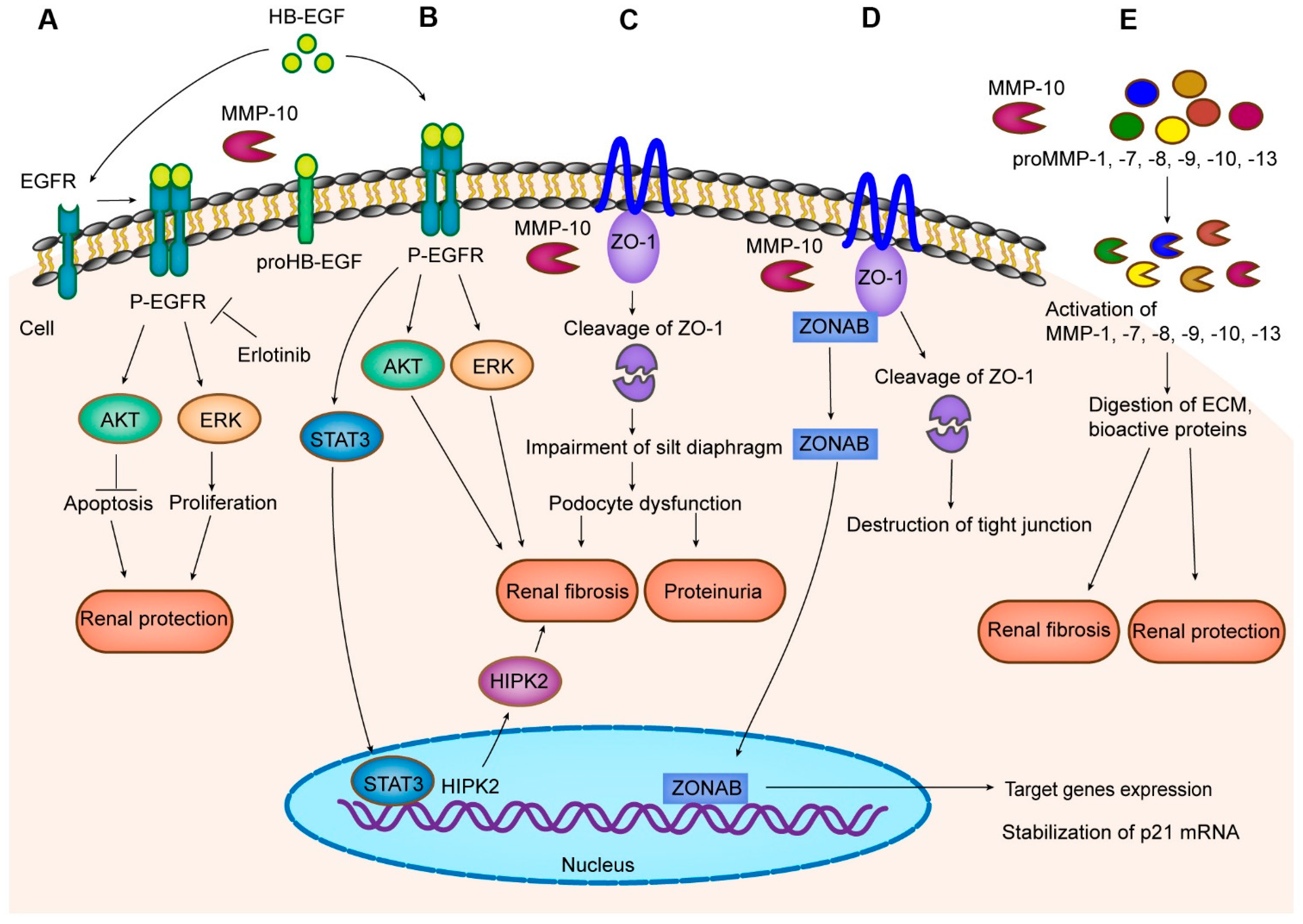
5. Mechanism of MMP-10 Action in Kidney Disease
5.1. HB-EGF
5.2. ZO-1
5.3. Other MMPs
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wozniak, J.; Floege, J.; Ostendorf, T.; Ludwig, A. Key metalloproteinase-mediated pathways in the kidney. Nat. Rev. Nephrol. 2021, 17, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.J.; Liu, Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol. Renal. Physiol. 2012, 302, F1351–F1361. [Google Scholar] [CrossRef] [PubMed]
- Saunders, W.B.; Bayless, K.J.; Davis, G.E. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J. Cell Sci. 2005, 118, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Irigoyen, O.; Carotti, S.; Latasa, M.U.; Uriarte, I.; Fernandez-Barrena, M.G.; Elizalde, M.; Urtasun, R.; Vespasiani-Gentilucci, U.; Morini, S.; Banales, J.M.; et al. Matrix metalloproteinase-10 expression is induced during hepatic injury and plays a fundamental role in liver tissue repair. Liver Int. 2014, 34, e257–e270. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, W.; Ali, M.A.M.; Schulz, R. Multifunctional intracellular matrix metalloproteinases: Implications in disease. FEBS J. 2021, 288, 7162–7182. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.P.; Holcomb, J.; Al-Ramahi, I.; de Haro, M.; Gafni, J.; Zhang, N.; Kim, E.; Sanhueza, M.; Torcassi, C.; Kwak, S.; et al. Matrix metalloproteinases are modifiers of huntingtin proteolysis and toxicity in Huntington’s disease. Neuron 2010, 67, 199–212. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Wang, C.; Sun, X.L.; Hu, C.X.; Liu, J.X.; Hong, X.; Shen, W.W.; Nie, J.; Hou, F.F.; Zhou, L.L.; et al. Identification of matrix metalloproteinase-10 as a key mediator of podocyte injury and proteinuria. Kidney Int. 2021, 100, 837–849. [Google Scholar] [CrossRef]
- McMahan, R.S.; Birkland, T.P.; Smigiel, K.S.; Vandivort, T.C.; Rohani, M.G.; Manicone, A.M.; McGuire, J.K.; Gharib, S.A.; Parks, W.C. Stromelysin-2 (MMP10) Moderates Inflammation by Controlling Macrophage Activation. J. Immunol. 2016, 197, 899–909. [Google Scholar] [CrossRef]
- Koller, F.L.; Dozier, E.A.; Nam, K.T.; Swee, M.; Birkland, T.P.; Parks, W.C.; Fingleton, B. Lack of MMP10 exacerbates experimental colitis and promotes development of inflammation-associated colonic dysplasia. Lab. Investig. 2012, 92, 1749–1759. [Google Scholar] [CrossRef]
- Lv, Y.P.; Cheng, P.; Zhang, J.Y.; Mao, F.Y.; Teng, Y.S.; Liu, Y.G.; Kong, H.; Wu, X.L.; Hao, C.J.; Han, B.; et al. Helicobacter pylori-induced matrix metallopeptidase-10 promotes gastric bacterial colonization and gastritis. Sci. Adv. 2019, 5, eaau6547. [Google Scholar] [CrossRef]
- Back, M.; Ketelhuth, D.F.; Agewall, S. Matrix metalloproteinases in atherothrombosis. Prog. Cardiovasc. Dis. 2010, 52, 410–428. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zuo, Y.; Ren, Q.; Sun, X.; Zhou, S.; Liao, J.; Hong, X.; Miao, J.; Zhou, L.; Liu, Y. Matrix metalloproteinase-10 protects against acute kidney injury by augmenting epidermal growth factor receptor signaling. Cell Death Dis. 2021, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Schlage, P.; Kockmann, T.; Sabino, F.; Kizhakkedathu, J.N.; Auf dem Keller, U. Matrix metalloproteinase 10 degradomics in keratinocytes and epidermal tissue identifies bioactive substrates with pleiotropic functions. Mol. Cell Proteom. 2015, 14, 3234–3246. [Google Scholar] [CrossRef]
- Barksby, H.E.; Milner, J.M.; Patterson, A.M.; Peake, N.J.; Hui, W.; Robson, T.; Lakey, R.; Middleton, J.; Cawston, T.E.; Richards, C.D.; et al. Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: Implications for cartilage degradation in arthritis. Arthritis Rheum. 2006, 54, 3244–3253. [Google Scholar] [CrossRef]
- Zakiyanov, O.; Kalousova, M.; Zima, T.; Tesar, V. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in kidney disease. Adv. Clin. Chem. 2021, 105, 141–212. [Google Scholar] [PubMed]
- Windsor, L.J.; Grenett, H.; Birkedal-Hansen, B.; Bodden, M.K.; Engler, J.A.; Birkedal-Hansen, H. Cell type-specific regulation of SL-1 and SL-2 genes. Induction of the SL-2 gene but not the SL-1 gene by human keratinocytes in response to cytokines and phorbolesters. J. Biol. Chem. 1993, 268, 17341–17347. [Google Scholar] [CrossRef]
- Muller, D.; Quantin, B.; Gesnel, M.C.; Millon-Collard, R.; Abecassis, J.; Breathnach, R. The collagenase gene family in humans consists of at least four members. Biochem. J. 1988, 253, 187–192. [Google Scholar] [CrossRef]
- Tallant, C.; Marrero, A.; Gomis-Rüth, F.X. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim. Biophys. Acta 2010, 1803, 20–28. [Google Scholar] [CrossRef]
- Kohrmann, A.; Kammerer, U.; Kapp, M.; Dietl, J.; Anacker, J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer 2009, 9, 188. [Google Scholar] [CrossRef]
- Kirita, Y.; Wu, H.; Uchimura, K.; Wilson, P.C.; Humphreys, B.D. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc. Natl. Acad. Sci. USA 2020, 117, 15874–15883. [Google Scholar] [CrossRef]
- Nakamura, H.; Fujii, Y.; Ohuchi, E.; Yamamoto, E.; Okada, Y. Activation of the precursor of human stromelysin 2 and its interactions with other matrix metalloproteinases. Eur. J. Biochem. 1998, 253, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Van Wart, H.E.; Birkedal-Hansen, H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA 1990, 87, 5578–5582. [Google Scholar] [CrossRef]
- Bertini, I.; Calderone, V.; Fragai, M.; Luchinat, C.; Mangani, S.; Terni, B. Crystal structure of the catalytic domain of human matrix metalloproteinase 10. J. Mol. Biol. 2004, 336, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, A.; Beltrán-Debón, R.; Mulero, M.; Pujadas, G.; Garcia-Vallvé, S. Understanding the variability of the S1’ pocket to improve matrix metalloproteinase inhibitor selectivity profiles. Drug Discov. Today 2020, 25, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Banci, L.; Bertini, I.; Luchinat, C.; Rosato, A. Bioinformatic comparison of structures and homology-models of matrix metalloproteinases. J. Proteome Res. 2004, 3, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Boyd, D.D. Regulation of matrix metalloproteinase gene expression. J. Cell. Physiol. 2007, 211, 19–26. [Google Scholar] [CrossRef]
- Murray, M.Y.; Birkland, T.P.; Howe, J.D.; Rowan, A.D.; Fidock, M.; Parks, W.C.; Gavrilovic, J. Macrophage migration and invasion is regulated by MMP10 expression. PLoS ONE 2013, 8, e63555. [Google Scholar]
- Benbow, U.; Brinckerhoff, C.E. The AP-1 site and MMP gene regulation: What is all the fuss about? Matrix Biol. 1997, 15, 519–526. [Google Scholar] [CrossRef]
- Couillard, J.; Demers, M.; Lavoie, G.; St-Pierre, Y. The role of DNA hypomethylation in the control of stromelysin gene expression. Biochem. Biophys. Res. Commun. 2006, 342, 1233–1239. [Google Scholar] [CrossRef]
- Mezzano, S.A.; Barría, M.; Droguett, M.A.; Burgos, M.E.; Ardiles, L.G.; Flores, C.; Egido, J. Tubular NF-kappaB and AP-1 activation in human proteinuric renal disease. Kidney Int. 2001, 60, 1366–1377. [Google Scholar] [CrossRef]
- Sundqvist, A.; Zieba, A.; Vasilaki, E.; Herrera Hidalgo, C.; Söderberg, O.; Koinuma, D.; Miyazono, K.; Heldin, C.H.; Landegren, U.; Ten Dijke, P.; et al. Specific interactions between Smad proteins and AP-1 components determine TGFβ-induced breast cancer cell invasion. Oncogene 2013, 32, 3606–3615. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, F.; Miyoshi, H.; Nose, K.; Shibanuma, M. Transcriptional induction of MMP-10 by TGF-beta, mediated by activation of MEF2A and downregulation of class IIa HDACs. Oncogene 2010, 29, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Delimont, D.; Dufek, B.M.; Meehan, D.T.; Zallocchi, M.; Gratton, M.A.; Phillips, G.; Cosgrove, D. Laminin alpha2-mediated focal adhesion kinase activation triggers Alport glomerular pathogenesis. PLoS ONE 2014, 9, e99083. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhu, H.; Gao, Z.; Li, J.; Zhuang, J.; Dong, Y.; Shen, B.; Li, M.; Zhou, H.; Guo, H.; et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J. Biol. Chem. 2018, 293, 6693–6706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, X.; Lu, M.; Wu, Q.; Yuan, Q.; Hu, C.; Miao, J.; Zhang, Y.; Li, H.; Hou, F.F.; et al. Wnt/β-catenin links oxidative stress to podocyte injury and proteinuria. Kidney Int. 2019, 95, 830–845. [Google Scholar] [CrossRef]
- Tan, R.J.; Zhou, D.; Zhou, L.; Liu, Y. Wnt/β-catenin signaling and kidney fibrosis. Kidney Int. Suppl. 2014, 4, 84–90. [Google Scholar] [CrossRef]
- Zhou, D.; Tan, R.J.; Fu, H.; Liu, Y. Wnt/β-catenin signaling in kidney injury and repair: A double-edged sword. Lab. Investig. 2016, 96, 156–167. [Google Scholar] [CrossRef]
- Deraz, E.M.; Kudo, Y.; Yoshida, M.; Obayashi, M.; Tsunematsu, T.; Tani, H.; Siriwardena, S.B.; Keikhaee, M.R.; Qi, G.; Iizuka, S.; et al. MMP-10/stromelysin-2 promotes invasion of head and neck cancer. PLoS ONE 2011, 6, e25438. [Google Scholar] [CrossRef]
- Jackson, H.W.; Defamie, V.; Waterhouse, P.; Khokha, R. TIMPs: Versatile extracellular regulators in cancer. Nat. Rev. Cancer 2017, 17, 38–53. [Google Scholar] [CrossRef]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 233. [Google Scholar] [CrossRef]
- Batra, J.; Robinson, J.; Soares, A.S.; Fields, A.P.; Radisky, D.C.; Radisky, E.S. Matrix metalloproteinase-10 (MMP-10) interaction with tissue inhibitors of metalloproteinases TIMP-1 and TIMP-2: Binding studies and crystal structure. J. Biol. Chem. 2012, 287, 15935–15946. [Google Scholar] [CrossRef] [PubMed]
- Batra, J.; Soares, A.S.; Mehner, C.; Radisky, E.S. Matrix metalloproteinase-10/TIMP-2 structure and analyses define conserved core interactions and diverse exosite interactions in MMP/TIMP complexes. PLoS ONE 2013, 8, e75836. [Google Scholar] [CrossRef] [PubMed]
- Saunders, W.B.; Bohnsack, B.L.; Faske, J.B.; Anthis, N.J.; Bayless, K.J.; Hirschi, K.K.; Davis, G.E. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J. Cell Biol. 2006, 175, 179–191. [Google Scholar] [CrossRef] [PubMed]
- El Ashry, E.S.H.; Awad, L.F.; Teleb, M.; Ibrahim, N.A.; Abu-Serie, M.M.; Abd Al Moaty, M.N. Structure-based design and optimization of pyrimidine- and 1,2,4-triazolo[4,3-a]pyrimidine-based matrix metalloproteinase-10/13 inhibitors via Dimroth rearrangement towards targeted polypharmacology. Bioorg. Chem. 2020, 96, 103616. [Google Scholar] [CrossRef]
- Senn, N.; Ott, M.; Lanz, J.; Riedl, R. Targeted polypharmacology: Discovery of a highly potent non-hydroxamate dual matrix metalloproteinase (MMP)-10/-13 inhibitor. J. Med. Chem. 2017, 60, 9585–9598. [Google Scholar] [CrossRef]
- Nam, D.H.; Rodriguez, C.; Remacle, A.G.; Strongin, A.Y.; Ge, X. Active-site MMP-selective antibody inhibitors discovered from convex paratope synthetic libraries. Proc. Natl. Acad. Sci. USA 2016, 113, 14970–14975. [Google Scholar] [CrossRef]
- Razai, A.S.; Eckelman, B.P.; Salvesen, G.S. Selective inhibition of matrix metalloproteinase 10 (MMP10) with a single-domain antibody. J. Biol. Chem. 2020, 295, 2464–2472. [Google Scholar] [CrossRef]
- Miyata, Y.; Iwata, T.; Maruta, S.; Kanda, S.; Nishikido, M.; Koga, S.; Kanetake, H. Expression of matrix metalloproteinase-10 in renal cell carcinoma and its prognostic role. Eur. Urol. 2007, 52, 791–797. [Google Scholar] [CrossRef]
- Toni, M.; Hermida, J.; Goni, M.J.; Fernandez, P.; Parks, W.C.; Toledo, E.; Montes, R.; Diez, N. Matrix metalloproteinase-10 plays an active role in microvascular complications in type 1 diabetic patients. Diabetologia 2013, 56, 2743–2752. [Google Scholar] [CrossRef]
- Mora-Gutierrez, J.M.; Rodriguez, J.A.; Fernandez-Seara, M.A.; Orbe, J.; Escalada, F.J.; Soler, M.J.; Slon Roblero, M.F.; Riera, M.; Paramo, J.A.; Garcia-Fernandez, N. MMP-10 is increased in early stage diabetic kidney disease and can be reduced by renin-angiotensin system blockade. Sci. Rep. 2020, 10, 26. [Google Scholar] [CrossRef]
- Su, M.; Hu, X.; Lin, J.; Zhang, L.; Sun, W.; Zhang, J.; Tian, Y.; Qiu, W. Identification of candidate genes involved in renal ischemia/reperfusion injury. DNA Cell Biol. 2019, 38, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.; Dufek, B.; Meehan, D.T.; Delimont, D.; Hartnett, M.; Samuelson, G.; Gratton, M.A.; Phillips, G.; MacKenna, D.A.; Bain, G. Lysyl oxidase like-2 contributes to renal fibrosis in Col4alpha3/Alport mice. Kidney Int. 2018, 94, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Naudin, C.; Smith, B.; Bond, D.R.; Dun, M.D.; Scott, R.J.; Ashman, L.K.; Weidenhofer, J.; Roselli, S. Characterization of the early molecular changes in the glomeruli of Cd151 (-/-) mice highlights induction of mindin and MMP-10. Sci. Rep. 2017, 7, 15987. [Google Scholar] [CrossRef] [PubMed]
- Meehan, D.T.; Delimont, D.; Cheung, L.; Zallocchi, M.; Sansom, S.C.; Holzclaw, J.D.; Rao, V.; Cosgrove, D. Biomechanical strain causes maladaptive gene regulation, contributing to Alport glomerular disease. Kidney Int. 2009, 76, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Grgic, I.; Hofmeister, A.F.; Genovese, G.; Bernhardy, A.J.; Sun, H.; Maarouf, O.H.; Bijol, V.; Pollak, M.R.; Humphreys, B.D. Discovery of new glomerular disease-relevant genes by translational profiling of podocytes in vivo. Kidney Int. 2014, 86, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, Y.; Lin, L.; Zhou, L.; Igarashi, P.; Liu, Y. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int. 2012, 82, 537–547. [Google Scholar] [CrossRef]
- Gobin, E.; Bagwell, K.; Wagner, J.; Mysona, D.; Sandirasegarane, S.; Smith, N.; Bai, S.; Sharma, A.; Schleifer, R.; She, J.X. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer 2019, 19, 581. [Google Scholar] [CrossRef]
- De Peralta-Venturina, M.; Moch, H.; Amin, M.; Tamboli, P.; Hailemariam, S.; Mihatsch, M.; Javidan, J.; Stricker, H.; Ro, J.Y.; Amin, M.B. Sarcomatoid differentiation in renal cell carcinoma: A study of 101 cases. Am. J. Surg. Pathol. 2001, 25, 275–284. [Google Scholar] [CrossRef]
- Lin, T.C.; Yeh, Y.M.; Fan, W.L.; Chang, Y.C.; Lin, W.M.; Yang, T.Y.; Hsiao, M. Ghrelin upregulates oncogenic Aurora A to promote renal cell carcinoma invasion. Cancers 2019, 11, 303. [Google Scholar] [CrossRef]
- Petrella, B.L.; Vincenti, M.P. Interleukin-1β mediates metalloproteinase-dependent renal cell carcinoma tumor cell invasion through the activation of CCAAT enhancer binding protein β. Cancer Med. 2012, 1, 17–27. [Google Scholar] [CrossRef]
- Saini, S.; Liu, J.; Yamamura, S.; Majid, S.; Kawakami, K.; Hirata, H.; Dahiya, R. Functional significance of secreted Frizzled-related protein 1 in metastatic renal cell carcinomas. Cancer Res. 2009, 69, 6815–6822. [Google Scholar] [CrossRef] [PubMed]
- Guvercin, G.; Karakus, V.; Aksit, M.; Dere, Y.; Aktar, M.; Alpay, H.; Bozkaya, G.; Tatar, E. Matrix metalloproteinase-9, 10, and stress hyperglycaemia in acute kidney injury. Eur. J. Clin. Investig. 2018, 48, e12963. [Google Scholar] [CrossRef] [PubMed]
- Friese, R.S.; Rao, F.; Khandrika, S.; Thomas, B.; Ziegler, M.G.; Schmid-Schönbein, G.W.; O’Connor, D.T. Matrix metalloproteinases: Discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin. Exp. Hypertens. 2009, 31, 521–533. [Google Scholar] [CrossRef]
- Gonsalez, S.R.; Cortes, A.L.; Silva, R.C.D.; Lowe, J.; Prieto, M.C.; Silva Lara, L.D. Acute kidney injury overview: From basic findings to new prevention and therapy strategies. Pharmacol. Ther. 2019, 200, 1–12. [Google Scholar] [CrossRef]
- Xu, X.; Nie, S.; Liu, Z.; Chen, C.; Xu, G.; Zha, Y.; Qian, J.; Liu, B.; Han, S.; Xu, A.; et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin. J. Am. Soc. Nephrol. 2015, 10, 1510–1518. [Google Scholar] [CrossRef]
- Anderson, S.; Eldadah, B.; Halter, J.B.; Hazzard, W.R.; Himmelfarb, J.; Horne, F.M.; Kimmel, P.L.; Molitoris, B.A.; Murthy, M.; O’Hare, A.M.; et al. Acute kidney injury in older adults. J. Am. Soc. Nephrol. 2011, 22, 28–38. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D.A. Renal aging: Causes and consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, M.; Wang, X. The impact of transient and persistent acute kidney injury on short-term outcomes in very elderly patients. Clin. Interv. Aging 2017, 12, 1013–1020. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Reyes, R.; Rodriguez, J.A.; Orbe, J.; Arnau, M.R.; Evora, C.; Delgado, A. Combined sustained release of BMP2 and MMP10 accelerates bone formation and mineralization of calvaria critical size defect in mice. Drug Deliv. 2018, 25, 750–756. [Google Scholar] [CrossRef]
- Valdes-Fernandez, J.; Lopez-Martinez, T.; Ripalda-Cemborain, P.; Calvo, I.A.; Saez, B.; Romero-Torrecilla, J.A.; Aldazabal, J.; Muinos-Lopez, E.; Montiel, V.; Orbe, J.; et al. Molecular and cellular mechanisms of delayed fracture healing in Mmp10 (Stromelysin 2) Knockout mice. J. Bone Miner. Res. 2021, 36, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Helve, J.; Sund, R.; Arffman, M.; Harjutsalo, V.; Groop, P.H.; Gronhagen-Riska, C.; Finne, P. Incidence of end-stage renal disease in patients with type 1 diabetes. Diabetes Care 2018, 41, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Huang, Y.; Huang, Y.M.; Fan, R.R.; Sui, Y.; Zhao, H.L. Global trend of diabetes mortality attributed to vascular complications, 2000–2016. Cardiovasc Diabetol. 2020, 19, 182. [Google Scholar] [CrossRef] [PubMed]
- Peeters, S.A.; Engelen, L.; Buijs, J.; Chaturvedi, N.; Fuller, J.H.; Schalkwijk, C.G.; Stehouwer, C.D.; Group, E.P.C.S. Plasma levels of matrix metalloproteinase-2, -3, -10, and tissue inhibitor of metalloproteinase-1 are associated with vascular complications in patients with type 1 diabetes: The EURODIAB Prospective Complications Study. Cardiovasc. Diabetol. 2015, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Coll, B.; Rodriguez, J.A.; Craver, L.; Orbe, J.; Martinez-Alonso, M.; Ortiz, A.; Diez, J.; Beloqui, O.; Borras, M.; Valdivielso, J.M.; et al. Serum levels of matrix metalloproteinase-10 are associated with the severity of atherosclerosis in patients with chronic kidney disease. Kidney Int. 2010, 78, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Ding, H.; Han, J.; Arriens, C.; Wei, C.; Han, W.; Pedroza, C.; Jiang, S.; Anolik, J.; Petri, M.; et al. Antibody-array-based proteomic screening of serum markers in systemic lupus erythematosus: A discovery study. J. Proteome Res. 2016, 15, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Petrackova, A.; Smrzova, A.; Gajdos, P.; Schubertova, M.; Schneiderova, P.; Kromer, P.; Snasel, V.; Skacelova, M.; Mrazek, F.; Zadrazil, J.; et al. Serum protein pattern associated with organ damage and lupus nephritis in systemic lupus erythematosus revealed by PEA immunoassay. Clin. Proteom. 2017, 14, 32. [Google Scholar] [CrossRef]
- Purroy, A.; Roncal, C.; Orbe, J.; Meilhac, O.; Belzunce, M.; Zalba, G.; Villa-Bellosta, R.; Andres, V.; Parks, W.C.; Paramo, J.A.; et al. Matrix metalloproteinase-10 deficiency delays atherosclerosis progression and plaque calcification. Atherosclerosis 2018, 278, 124–134. [Google Scholar] [CrossRef]
- Matilla, L.; Roncal, C.; Ibarrola, J.; Arrieta, V.; Garcia-Pena, A.; Fernandez-Celis, A.; Navarro, A.; Alvarez, V.; Gainza, A.; Orbe, J.; et al. A role for MMP-10 (matrix Metalloproteinase-10) in calcific aortic valve stenosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1370–1382. [Google Scholar] [CrossRef]
- Chang, S.; Young, B.D.; Li, S.; Qi, X.; Richardson, J.A.; Olson, E.N. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell 2006, 126, 321–334. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Orbe, J.; Martinez de Lizarrondo, S.; Calvayrac, O.; Rodriguez, C.; Martinez-Gonzalez, J.; Paramo, J.A. Metalloproteinases and atherothrombosis: MMP-10 mediates vascular remodeling promoted by inflammatory stimuli. Front. Biosci. 2008, 13, 2916–2921. [Google Scholar] [CrossRef] [PubMed]
- Orbe, J.; Montero, I.; Rodriguez, J.A.; Beloqui, O.; Roncal, C.; Paramo, J.A. Independent association of matrix metalloproteinase-10, cardiovascular risk factors and subclinical atherosclerosis. J. Thromb. Haemost. 2007, 5, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kashtan, C.E. Alport syndrome: Achieving early diagnosis and treatment. Am. J. Kidney Dis. 2021, 77, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.W.; Morais, M.; Lennon, R. Complexities of the glomerular basement membrane. Nat. Rev. Nephrol. 2021, 17, 112–127. [Google Scholar] [CrossRef]
- Ding, F.; Wickman, L.; Wang, S.Q.; Zhang, Y.; Wang, F.; Afshinnia, F.; Hodgin, J.; Ding, J.; Wiggins, R.C. Accelerated podocyte detachment and progressive podocyte loss from glomeruli with age in Alport Syndrome. Kidney Int. 2017, 92, 1515–1525. [Google Scholar] [CrossRef]
- Funk, S.D.; Lin, M.H.; Miner, J.H. Alport syndrome and Pierson syndrome: Diseases of the glomerular basement membrane. Matrix Biol. 2018, 71, 250–261. [Google Scholar] [CrossRef]
- Cosgrove, D.; Liu, S. Collagen IV diseases: A focus on the glomerular basement membrane in Alport syndrome. Matrix Biol. 2017, 57, 45–54. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhu, Y.; Xie, K.L.; Peng, Y.P.; Tao, J.Q.; Tang, J.; Li, Z.; Xu, Z.K.; Dai, C.C.; Qian, Z.Y.; et al. Yin Yang-1 suppresses invasion and metastasis of pancreatic ductal adenocarcinoma by downregulating MMP10 in a MUC4/ErbB2/p38/MEF2C-dependent mechanism. Mol. Cancer 2014, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Justilien, V.; Regala, R.P.; Tseng, I.C.; Walsh, M.P.; Batra, J.; Radisky, E.S.; Murray, N.R.; Fields, A.P. Matrix metalloproteinase-10 is required for lung cancer stem cell maintenance, tumor initiation and metastatic potential. PLoS ONE 2012, 7, e35040. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Gardi, N.; Desai, S.; Chandrani, P.; Joshi, A.; Dharavath, B.; Arora, P.; Bal, M.; Nair, S.; Dutt, A. Genomic characterization of tobacco/nut chewing HPV-negative early stage tongue tumors identify MMP10 asa candidate to predict metastases. Oral Oncol. 2017, 73, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Sakai, M.; Cheng, H.F.; Yasuda, T.; Coffey, R.J., Jr.; Harris, R.C. Induction of heparin-binding epidermal growth factor-like growth factor mRNA in rat kidney after acute injury. J. Clin. Investig. 1995, 96, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Kinsey, G.R.; Rasbach, K.; Schnellmann, R.G. Heparin-binding epidermal growth factor and Src family kinases in proliferation of renal epithelial cells. Am. J. Physiol. Renal Physiol. 2008, 294, F459–F468. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, N.; Bayliss, G.; Zhuang, S. EGFR activity is required for renal tubular cell dedifferentiation and proliferation in a murine model of folic acid-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 2013, 304, F356–F366. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.K.; Harris, R.C. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int. 2012, 82, 45–52. [Google Scholar] [CrossRef]
- Chen, J.; You, H.; Li, Y.; Xu, Y.; He, Q.; Harris, R.C. EGF receptor-dependent YAP activation is important for renal recovery from AKI. J. Am. Soc. Nephrol. 2018, 29, 2372–2385. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Iyoda, M.; Tachibana, S.; Matsumoto, K.; Wada, Y.; Suzuki, T.; Iseri, K.; Saito, T.; Fukuda-Hihara, K.; Shibata, T. Erlotinib attenuates the progression of chronic kidney disease in rats with remnant kidney. Nephrol. Dial. Transplant. 2018, 33, 598–606. [Google Scholar] [CrossRef]
- Tang, J.; Liu, N.; Zhuang, S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int. 2013, 83, 804–810. [Google Scholar] [CrossRef]
- Harskamp, L.R.; Gansevoort, R.T.; van Goor, H.; Meijer, E. The epidermal growth factor receptor pathway in chronic kidney diseases. Nat. Rev. Nephrol. 2016, 12, 496–506. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Overstreet, J.M.; Chung, S.; Niu, A.; Fan, X.; Wang, S.; Wang, Y.; Zhang, M.Z.; Harris, R.C. Inhibition of Epidermal Growth Factor Receptor Activation Is Associated with Improved Diabetic Nephropathy and Insulin Resistance in Type 2 Diabetes. Diabetes 2018, 67, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Mulder, G.M.; Nijboer, W.N.; Seelen, M.A.; Sandovici, M.; Bos, E.M.; Melenhorst, W.B.; Trzpis, M.; Kloosterhuis, N.J.; Visser, L.; Henning, R.H.; et al. Heparin binding epidermal growth factor in renal ischaemia/reperfusion injury. J. Pathol. 2010, 221, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kefaloyianni, E.; Keerthi Raja, M.R.; Schumacher, J.; Muthu, M.L.; Krishnadoss, V.; Waikar, S.S.; Herrlich, A. Proximal tubule-derived amphiregulin amplifies and integrates profibrotic EGF receptor signals in kidney fibrosis. J. Am. Soc. Nephrol. 2019, 30, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Guo, J.K.; Pang, M.; Tolbert, E.; Ponnusamy, M.; Gong, R.; Bayliss, G.; Dworkin, L.D.; Yan, H.; Zhuang, S. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 854–867. [Google Scholar] [CrossRef]
- Overstreet, J.M.; Wang, Y.; Wang, X.; Niu, A.; Gewin, L.S.; Yao, B.; Harris, R.C.; Zhang, M.Z. Selective activation of epidermal growth factor receptor in renal proximal tubule induces tubulointerstitial fibrosis. FASEB J. 2017, 31, 4407–4421. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Zhang, F.; Wu, L.; Dong, Z.; Zhang, D. EGFR drives the progression of AKI to CKD through HIPK2 overexpression. Theranostics 2019, 9, 2712–2726. [Google Scholar] [CrossRef]
- Carrasco-Rando, M.; Prieto-Sanchez, S.; Culi, J.; Tutor, A.S.; Ruiz-Gomez, M. A specific isoform of Pyd/ZO-1 mediates junctional remodeling and formation of slit diaphragms. J. Cell Biol. 2019, 218, 2294–2308. [Google Scholar] [CrossRef]
- Itoh, M.; Nakadate, K.; Matsusaka, T.; Hunziker, W.; Sugimoto, H. Effects of the differential expression of ZO-1 and ZO-2 on podocyte structure and function. Genes Cells 2018, 23, 546–556. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Zhang, X.; Chen, Q.; Bai, X.; Hong, X.; Zhou, L.; Liu, Y. Role of miRNA-671-5p in mediating Wnt/β-catenin-triggered podocyte injury. Front. Pharmacol. 2022, 12, 784489. [Google Scholar] [CrossRef]
- Sagar, A.; Arif, E.; Solanki, A.K.; Srivastava, P.; Janech, M.G.; Kim, S.H.; Lipschutz, J.H.; Ashish, S.-H.K.; Nihalani, D. Targeting Neph1 and ZO-1 protein-protein interaction in podocytes prevents podocyte injury and preserves glomerular filtration function. Sci. Rep. 2017, 7, 12047. [Google Scholar] [CrossRef]
- Itoh, M.; Nakadate, K.; Horibata, Y.; Matsusaka, T.; Xu, J.; Hunziker, W.; Sugimoto, H. The structural and functional organization of the podocyte filtration slits is regulated by Tjp1/ZO-1. PLoS ONE 2014, 9, e106621. [Google Scholar] [CrossRef] [PubMed]
- Balda, M.S.; Matter, K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000, 19, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, G.H. Roles of claudin-2, ZO-1 and occludin in leaky HK-2 cells. PLoS ONE 2017, 12, e0189221. [Google Scholar] [CrossRef] [PubMed]
- Amoozadeh, Y.; Anwer, S.; Dan, Q.; Venugopal, S.; Shi, Y.; Branchard, E.; Liedtke, E.; Ailenberg, M.; Rotstein, O.D.; Kapus, A.; et al. Cell confluence regulates claudin-2 expression: Possible role for ZO-1 and Rac. Am. J. Physiol. Cell Physiol. 2018, 314, C366–C378. [Google Scholar] [CrossRef] [PubMed]
- Festa, B.P.; Chen, Z.; Berquez, M.; Debaix, H.; Tokonami, N.; Prange, J.A.; Hoek, G.V.; Alessio, C.; Raimondi, A.; Nevo, N.; et al. Impaired autophagy bridges lysosomal storage disease and epithelial dysfunction in the kidney. Nat. Commun. 2018, 9, 161. [Google Scholar] [CrossRef]
- Balda, M.S.; Garrett, M.D.; Matter, K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 2003, 160, 423–432. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, Z.; Shao, Y.; Yang, Z.; Zhou, S.; Zou, X.; Zhou, Y.; Tan, G. Bradykinin potentially stimulates cell proliferation in rabbit corneal endothelial cells through the ZO1/ZONAB pathway. Int. J. Mol. Med. 2018, 42, 71–80. [Google Scholar] [PubMed]
- Ruan, Y.C.; Wang, Y.; Da Silva, N.; Kim, B.; Diao, R.Y.; Hill, E.; Brown, D.; Chan, H.C.; Breton, S. CFTR interacts with ZO-1 to regulate tight junction assembly and epithelial differentiation through the ZONAB pathway. J. Cell Sci. 2014, 127, 4396–4408. [Google Scholar] [PubMed]
- Xu, X.; Zhang, X.; Gao, L.; Liu, C.; You, K. Neonatal hyperoxia downregulates claudin-4, occludin, and ZO-1 expression in rat kidney accompanied by impaired proximal tubular development. Oxid. Med. Cell Longev. 2020, 2020, 2641461. [Google Scholar] [CrossRef]
- Gonzalez-Mariscal, L.; Namorado, M.C.; Martin, D.; Luna, J.; Alarcon, L.; Islas, S.; Valencia, L.; Muriel, P.; Ponce, L.; Reyes, J.L. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int. 2000, 57, 2386–2402. [Google Scholar] [CrossRef]
- Lima, W.R.; Parreira, K.S.; Devuyst, O.; Caplanusi, A.; N’Kuli, F.; Marien, B.; Van Der Smissen, P.; Alves, P.M.; Verroust, P.; Christensen, E.I.; et al. ZONAB promotes proliferation and represses differentiation of proximal tubule epithelial cells. J. Am. Soc. Nephrol. 2010, 21, 478–488. [Google Scholar] [CrossRef]
- Nie, M.; Balda, M.S.; Matter, K. Stress- and Rho-activated ZO-1-associated nucleic acid binding protein binding to p21 mRNA mediates stabilization, translation, and cell survival. Proc. Natl. Acad. Sci. USA 2012, 109, 10897–10902. [Google Scholar] [CrossRef] [PubMed]
- Kitada, K.; Nakano, D.; Ohsaki, H.; Hitomi, H.; Minamino, T.; Yatabe, J.; Felder, R.A.; Mori, H.; Masaki, T.; Kobori, H.; et al. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J. Diabetes Complicat. 2014, 28, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Raggi, C.; Luciani, A.; Nevo, N.; Antignac, C.; Terryn, S.; Devuyst, O. Dedifferentiation and aberrations of the endolysosomal compartment characterize the early stage of nephropathic cystinosis. Hum. Mol. Genet. 2014, 23, 2266–2278. [Google Scholar] [CrossRef] [PubMed]
- Schlage, P.; Egli, F.E.; Nanni, P.; Wang, L.W.; Kizhakkedathu, J.N.; Apte, S.S.; Auf dem Keller, U. Time-resolved analysis of the matrix metalloproteinase 10 substrate degradome. Mol. Cell. Proteom. 2014, 13, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Yazgan, B.; Avci, F.; Memi, G.; Tastekin, E. Inflammatory response and matrix metalloproteinases in chronic kidney failure: Modulation by adropin and spexin. Exp. Biol. Med. 2021, 246, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tan, R.J.; Liu, Y. The many faces of matrix metalloproteinase-7 in kidney diseases. Biomolecules 2020, 10, 960. [Google Scholar] [CrossRef]
- Djuric, T.; Zivkovic, M.; Milosevic, B.; Andjelevski, M.; Cvetkovic, M.; Kostic, M.; Stankovic, A. MMP-1 and -3 haplotype is associated with congenital anomalies of the kidney and urinary tract. Pediatr. Nephrol. 2014, 29, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Salinas, H.; Meza-Rios, A.; Garcia-Banuelos, J.; Sandoval-Rodriguez, A.; Sanchez-Orozco, L.; Garcia-Benavides, L.; De la Rosa-Bibiano, R.; Monroy Ramirez, H.C.; Gutierrez-Cuevas, J.; Santos-Garcia, A.; et al. Fibrosis regression is induced by AdhMMP8 in a murine model of chronic kidney injury. PLoS ONE 2020, 15, e0243307. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, J.; Rudemiller, N.P.; Griffiths, R.; Wen, Y.; Lu, X.; Privratsky, J.R.; Gunn, M.D.; Crowley, S.D. Twist1 in infiltrating macrophages attenuates kidney fibrosis via matrix metallopeptidase 13-mediated matrix degradation. J. Am. Soc. Nephrol. 2019, 30, 1674–1685. [Google Scholar] [CrossRef]
- Chen, L.; Cao, G.; Wang, M.; Feng, Y.L.; Chen, D.Q.; Vaziri, N.D.; Zhuang, S.; Zhao, Y.Y. The matrix metalloproteinase-13 inhibitor poricoic acid ZI ameliorates renal fibrosis by mitigating epithelial-mesenchymal transition. Mol. Nutr. Food Res. 2019, 63, e1900132. [Google Scholar] [CrossRef] [PubMed]

| Disease Model | Location | Expression | Ref. |
|---|---|---|---|
| Ischemic AKI 1 | Renal tubular epithelia | Increase | [12,51] |
| Cisplatin-induced AKI | - | Increase | [12] |
| Rhabdomyolysis-associated AKI | - | Increase | [12] |
| ADR nephropathy 2 | Glomerular podocyte | Increase | [7] |
| 5/6 nephrectomy | - | Increase | [7] |
| T1DM 3 | - | Increase | [49] |
| T2DM 4 | Glomerular podocyte, juxtaglomerular apparatus | Increase | [7,50] |
| Alport syndrome | Glomerular podocyte | Increase | [33,52,53,54] |
| FSGS 5 | Glomerular podocyte | Increase | [7,55] |
| IgA nephropathy | Glomerular podocyte | Increase | [7] |
| Renal cell carcinoma | Tubular cancer cells, sarcomatous cancer cell | Increase | [48] |
| Disease Model | Intervention | Role | Ref. |
|---|---|---|---|
| IRI-induced AKI 1 | Overexpression Knockdown | Promote tubular cell proliferation and inhibit apoptosis Aggravate AKI by promoting tubular cell apoptosis and inhibiting proliferation | [12] |
| Cisplatin-induced AKI | Overexpression | Alleviate tubular injury following nephrotoxic AKI | [12] |
| Non-diabetic geriatric AKI | - | Serve as a predictor for emergency renal replacement therapy (RRT) | [62] |
| DKD 2 | Knockout | Protect against DKD, improve renal function, reduce mesangial expansion and macrophage influx | [49] |
| ADR-induced CKD 3 | Overexpression Knockdown | Exacerbate podocyte injury and proteinuria Mitigate glomerular sclerosis and proteinuria | [7] |
| Hypertensive nephropathy | - | Contribute to the development of renal injury | [63] |
| RCC 4 | Knockdown | Repress RCC invasion | [48,59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Liu, Y. Matrix Metalloproteinase-10 in Kidney Injury Repair and Disease. Int. J. Mol. Sci. 2022, 23, 2131. https://doi.org/10.3390/ijms23042131
Sun X, Liu Y. Matrix Metalloproteinase-10 in Kidney Injury Repair and Disease. International Journal of Molecular Sciences. 2022; 23(4):2131. https://doi.org/10.3390/ijms23042131
Chicago/Turabian StyleSun, Xiaoli, and Youhua Liu. 2022. "Matrix Metalloproteinase-10 in Kidney Injury Repair and Disease" International Journal of Molecular Sciences 23, no. 4: 2131. https://doi.org/10.3390/ijms23042131
APA StyleSun, X., & Liu, Y. (2022). Matrix Metalloproteinase-10 in Kidney Injury Repair and Disease. International Journal of Molecular Sciences, 23(4), 2131. https://doi.org/10.3390/ijms23042131






