Abstract
Copy number variations (CNVs) are the predominant class of structural genomic variations involved in the processes of evolutionary adaptation, genomic disorders, and disease progression. Compared with single-nucleotide variants, there have been challenges associated with the detection of CNVs owing to their diverse sizes. However, the field has seen significant progress in the past 20–30 years. This has been made possible due to the rapid development of molecular diagnostic methods which ensure a more detailed view of the genome structure, further complemented by recent advances in computational methods. Here, we review the major approaches that have been used to routinely detect CNVs, ranging from cytogenetics to the latest sequencing technologies, and then cover their specific features.
1. Introduction
Ever since Joe Hin Tjio and Albert Levan first identified the exact number of chromosomes in human cells in 1956, there have been massive advances in the understanding of the human genome and its structure [1]. In earlier times, due to the low resolution of methods for genetic material analysis, any form of chromosomal rearrangement was associated with disease or an abnormal condition. With advancements in the field after the completion of the human genome project, the use of comparative genomic hybridization (CGH) arrays has led to the discovery of an abundance of copy number variations (CNV), a structural variation of the DNA sequence which has a >50 bp multiplication and deletions of a particular segment of DNA, in the human genome [2,3]. It was shown that CNVs are widespread in human populations and comprise about 5–10% of the genome [4]. There is growing evidence showing the role of CNVs in causing various disorders. The list of diseases for which association with copy number variations has been established includes schizophrenia, type I diabetes, autism, cardiovascular diseases, congenital abnormalities, and neurodegenerative diseases [5]. However, due to the wide range of possible lengths and non-trivial estimation of the effect of CNVs on phenotype, there are difficulties in accurately screening and detecting CNVs. Due to limited resolution, modern technologies do not allow for complete CNV profile descriptions. However, each new approach to CNV detection has introduced new, valuable improvements. For example, chromosomal microarray analysis has allowed for large-scale genome studies with high resolution; whole-genome sequencing has provided the tool for the identification of all types of structural variations starting from 50 bp; and emerging sequencing technologies have opened up possibilities for the investigation of regions that were poorly accessible due to short reads. An enormous amount of copy number analysis methods using various types of data have been elaborated, and most of them have been described in detail in early reviews dedicated to a particular single technology or several CNV detection technologies [6,7,8,9]. Recently, the ELIXIR human CNV Community was initiated and focuses primarily on detection, annotation, and variant interpretation issues, which will ultimately help in developing an optimal protocol for CNV identification [10].
Hence, the aim of this review is to highlight the major contributions made in the field to detect CNVs to date and describe their main principles and challenges. We will also describe the most widely used computational approaches and discuss burning issues in this research field.
2. Methods of Cytogenetics
The first approaches to CNV detection were based on the analysis of metaphase plates in cells. During metaphase in cell division, condensed chromosomes align along the cell equator, facilitating convenient viewing of chromosome structure through light microscopy. Until the early 1970s, the only method for chromosome staining was Giemsa staining. In such an approach, all chromosomes are stained evenly length-wise, allowing for changes in karyotype concerning chromosome number, shape, and size to be visualized. Giemsa staining was used to detect many chromosome aneuploidies, such as Down’s syndrome [11], Klinefelter’s syndrome [12], and Edwards syndrome [13], as well as the structural abnormalities of chromosomes in cancer cells [14].
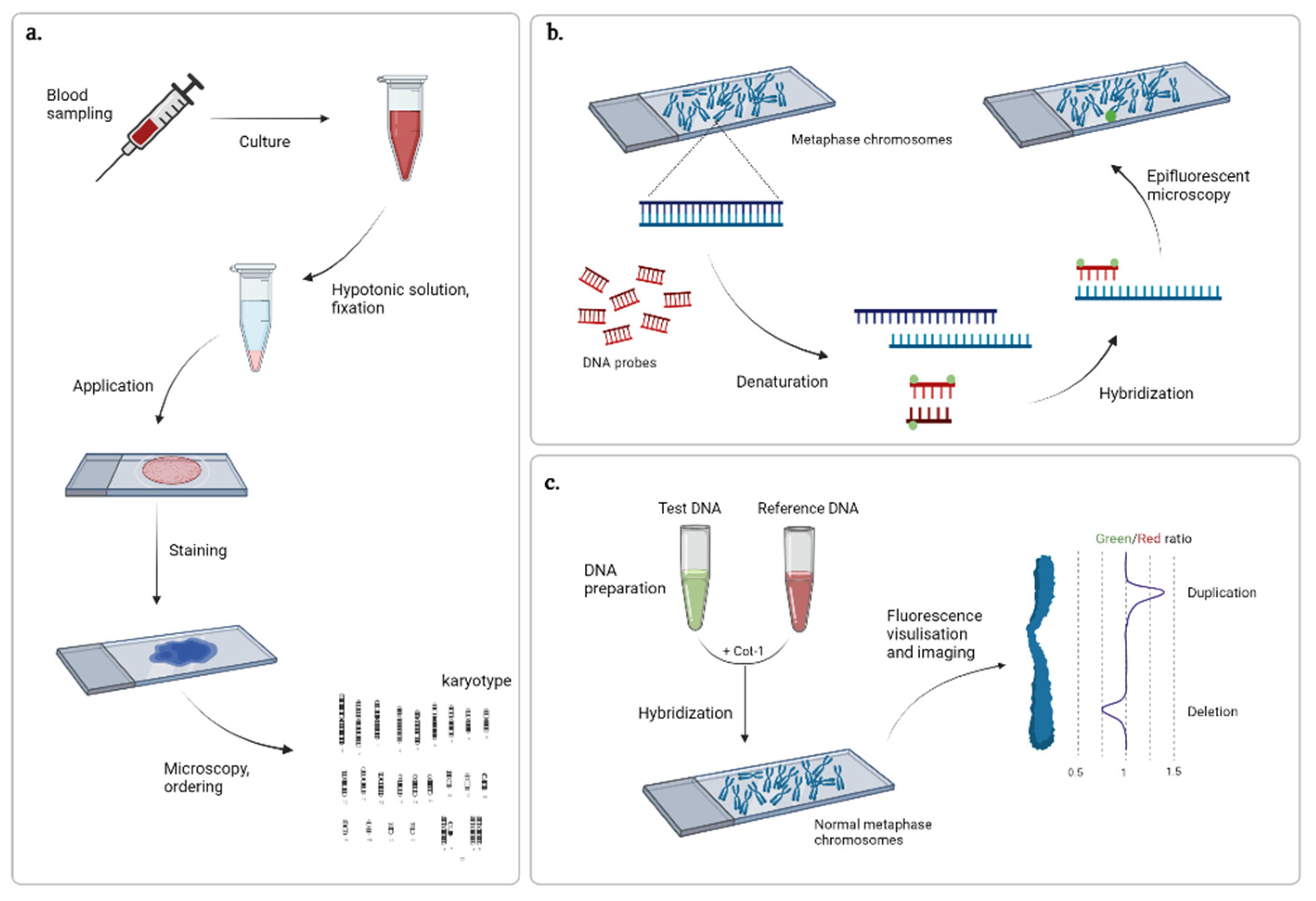
After the advent of Giemsa staining, new methods of differential staining that allowed for visualization of certain chromosomal structures, e.g., C-banding for centromere or T-banding for telomere regions staining, as well as producing chromosome-specific sequences of dark and light bands along the length (R, Q, and G-banding) were developed. The most common was the G-banding technique, a method that implies preliminary treatment of chromosomes with trypsin for DNA denaturing and further staining of renatured chromosomes with Giemsa dye [15]. Pairs of homologous chromosomes are arranged according to their number (sex chromosomes on the end) with their short shoulders upwards, ensuring the centromeres are horizontally aligned [16] (Figure 1a). The technique has the potential to elucidate rather large abnormalities exceeding 5 Mb in length and is used today in cases of suspected or existing congenital pathologies, as well as family planning.
Figure 1.
Cytogenetic techniques: (a) karyotyping, (b) FISH, and (c) comparative genome hybridization. Created with BioRender.com (accessed on 9 February 2022).
More detailed analyses of the genome became possible upon the development of DNA hybridization techniques. At first, tritium-labeled RNA probes were used to search for a specific DNA sequence and the hybrids were detected by autoradiography [17]. Later, the protocol was modified with fluorescent labels replacing the radioactive ones, thus improving safety and decreasing labor consumption [18,19]. Due to its targeted nature, fluorescence in situ hybridization (FISH) (Figure 1b) remains one of the most commonly used techniques to verify variations previously identified by real-time qPCR and multiplex ligation-dependent probe amplification (MLPA). However, its principle has been refined for use in other techniques, such as spectral karyotyping and multicolor FISH, offering new approaches to the visualization and analysis of chromosomes with the use of various combinations of fluorochromes and light filters [20,21].
For whole-genome analysis, comparative genome hybridization was developed in 1992 [22]. In contrast to FISH, where metaphase chromosomes obtained from a patient and DNA probes are complementary to the sequence of interest, the method proposed by Kallioniemi and colleagues isolated and hybridized differentially labeled genomic DNA from the tumor and normal tissue of a patient and utilized chromosome samples from peripheral blood lymphocytes of a healthy volunteer as a reference (Figure 1c). Further refinement of the protocol and development of specialized software for image analysis made the technique more accessible to many laboratories [23]. It has been widely used for the cytogenetic analysis of solid tumors [24]. However, utilization of metaphase chromosomes considerably limits the method resolution (the average size of detected CNVs is 5–10 Mb). Therefore, further research has been aimed at alternative representations of the cytogenetic map.
3. Chromosome Microarray Analysis (CMA)
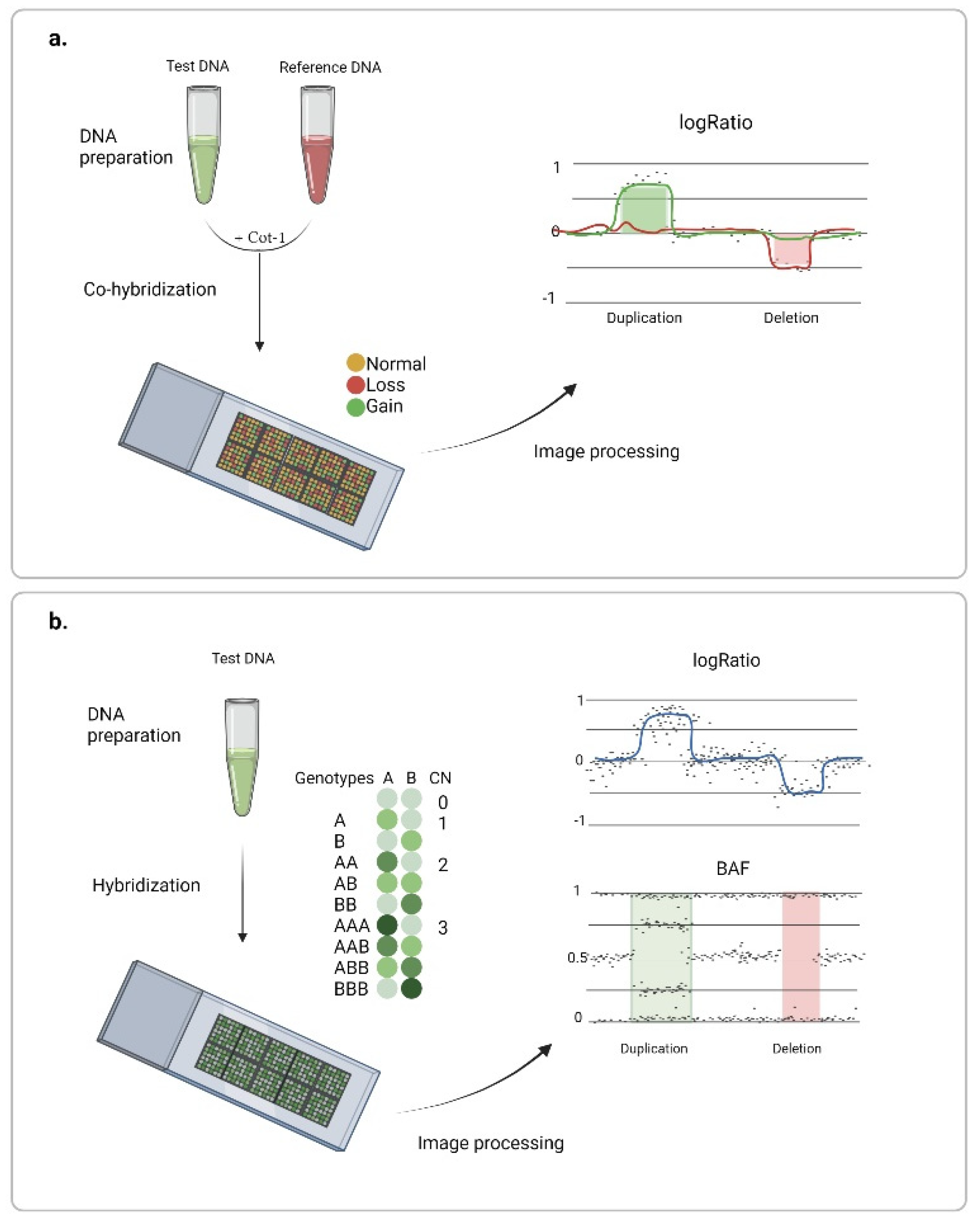
The application of microarrays instead of metaphase chromosomes has provided a solution for large-scale genome studies. Hybridization occurs on multiple DNA probes attached to a solid surface, and physical position on a chip and specific nucleotide sequences of the probes are pre-determined, which allows visualization of relative fluorescence intensities of test and control samples along the genome. For example, in 1997, the matrix-based comparative genomic hybridization method was introduced [25], and soon after, array-based comparative genomic hybridization (aCGH) was introduced [26] (Figure 2a). Resolution of the method depends directly on the probe type, number, and distribution over the genome. At that time, a large set of bacterial artificial chromosome (BAC) clones 80–200 kb long spanning the genome in a fairly complete manner was created in the course of the human genome project [27]. This genomic library has been used for the construction of most CGH arrays capable of identifying variations exceeding 1 Mb [28,29]. In general, any nucleotide sequence can be used as a probe. Later, cDNA- [30,31] and PCR amplicon-based [32] arrays have been successfully used for DNA copy number analysis.

Figure 2.
Chromosome microarray analysis: (a) array-based comparative genomic hybridization, and (b) DNA arrays for genotyping. Created with BioRender.com (accessed on 9 February 2022).
Further improvement of the method occurred upon the deciphering of the whole-genome sequence. This development led to the application of oligonucleotide probes (8–25 bp) that had been previously used for gene expression studies [33,34]. Oligonucleotide probes simplify the process of chip development in regard to both design customization and reproducibility. In addition, these microarrays provide better signal-to-noise ratio and event (CNV) confirmation with several probes due to a more complete genome coverage [35]. Array capacity has also improved, as the mechanical application of DNA onto a chip limited by approximately 50,000 probes has been replaced with oligonucleotide synthesis directly on the glass surface. Commercial technology proposed by Agilent—one of the leading comparative chromosomal analysis technologies today—synthesizes 60-nucleotide probes by an ink-jet technology [36]. Several chip formats have been developed (1 × 1 M, 2 × 400 K, 4 × 180 K, and 8 × 60 K) for whole-genome studies or for the targeted analysis of regions that have been strongly associated with various diseases (Table 1). A higher density of probes is typically used for regions of interest with the distance between adjacent probes as little as several hundreds of nucleotides, and coverage of the rest of the genome provided by evenly spread backbone probes.

Table 1.
Modern platforms for chromosomal microarray analysis.
Another type of microarray used to search for regions of chromosome imbalance has been developed for genotyping and genome-wide association studies. Here, only fluorescently labeled DNA of the sample is applied to the chip, and copy number analysis is performed based on the rate of target DNA hybridization to allele-specific probes (Figure 2b). Such a technique was first demonstrated in 2004 using the Affymetrix chips [37]. Soon, Illumina introduced an alternative platform, Infinium BeadChip, in which after DNA hybridization occurs on complementary probes, SNPs are evaluated based on the brightness and color of fluorescent nucleotides attached to the probe. The platform could detect deletions and duplications of fragments shorter than 100 kb [38]. However, earlier versions of the chips limited the scope of the search for variation to the regions covering common polymorphisms. Later, the introduction of non-polymorphous probes allowed for more even genome coverage [39]. Today, a wide choice of DNA arrays equipped with 300,000 to several million probes is available on the market (Table 1), including arrays with the possibility of partial or complete customized designs. High genome coverage with short probes provides for higher resolution and more accurate determination of the breakpoints, which makes this type of array a convenient tool when searching for rare and short (starting from 500 bp) variations. Another advantage of the technology is the possibility of identifying mosaicism, loss of heterozygosity (LOH), and uniparent disomies. Soon, the inherent superiority of CMA replaced G-karyotyping as a first-line test in clinical diagnostics of multiple congenital anomalies [40].
Not all chip platforms are equally fit for CNV detection. Although for any type of microarray (CGH, SNP) there is a dependence of the number of identified variations on the total number of probes, the design plays an equally important role [41]. Haraksingh and colleagues demonstrated that a suboptimal number of backbone probes or lack of probes in the intergenic regions results in a disability to identify large, potentially biologically important variations. Further, the comparison of existing platforms shows that additional enrichment with exome variants is not always optimal for CNV detection, as is the case with the Illumina Infinium Omni lineage. However, despite all platform-specific features, the choice of detection algorithm and settings wields the most influence on the results [41,42].
The aim of any algorithm is the analysis of signal intensity along the genome (logR) and elucidating any significant deviations. For this purpose, both simple thresholds, e.g., change of logR by 0.2–0.3 [43,44,45] or by more than 3–4 standard deviations [46,47], and more complex statistical models can be used. Data segmentation methods have found wide applications. For example, Olshen et al. adopted the method of binary segmentation to determine all sites dividing the genome into fragments of the same copy number [48]. In the modified version, two points are determined at each stage, limiting a region so that the t-statistics for the difference in mean probe intensity inside and outside the region are the highest. If the differences are statistically significant, the region is divided, and the procedure is repeated for each of the new three regions, if possible. An alternative method is based on a local genetic search. In this technique, the focus is on the optimization of a certain set of points chosen randomly by means of minimization of the negative logarithmic function of likeness and penalty for the high rate of genome fragmentation [49]. Later on, other methods for change-point detection have been suggested, e.g., ones that are based on the techniques of adaptive weight smoothing [50], fused lasso [51,52], and local search [52,53].
A large class of algorithms utilize hidden Markov models (HMM), allowing for the description of a system with unknown variables based on the observed ones. Changes in the number of copies (loss, gain, or maintenance of genetic material) [54] or absolute number of copies as such [55] are considered hidden states, while their most probable sequence is determined using the dynamic programming after preliminary optimization of the model parameters (the initial probabilities of the states, transition probabilities, and emission probabilities). HMMs are convenient as they work with several variables, as in the case of DNA microarrays. In the latter case, in addition to normalized probe intensities, the ratio of intensities between polymorphous alleles is also under investigation. Additional parameters, such as distance between the probes [56] and the population frequencies of SNPs [57], can be taken into account as well.
Today, multiple ways for microarray data to be processed have been proposed (Table 2). They differ by the number and size of CNVs detected and false-discovery rate [7,42,58,59,60]. As a result, it is recommended to use several algorithms to improve efficiency. Regardless, CMA remains one of the most in-demand methods for CNV detection in both research and clinical diagnostics due to its reliability, flexibility, and relatively low cost. To search for clinically important variants, aCGH or DNA microarrays developed specifically for cytogenetic studies (e.g., Affimetrix Cytoscan HD) and capable of highly reliable identification of variations exceeding 25 kb are preferred.

Table 2.
The most widely used algorithms for CNV detection that use microarray data.
4. Sequencing Data
Methods of CNV detection gained momentum with the rise of high-throughput sequencing (new generation sequencing, or NGS) technology. Based on the analysis of millions of short readings, this was quickly deemed a revolutionary technique due to its high productivity, reproducibility, and accuracy. Data generated from this technique can also be used repeatedly in various types of studies, and this research domain has expanded considerably concerning some genomic variations that were previously difficult to detect. Firstly, the microarray technique had limitations in accessing balanced rearrangements. Secondly, as a consequence of multiple sequencing of random fragments, variation size is not strictly limited as is the case in CMA, where variation less than the distance between two probes cannot be resolved.
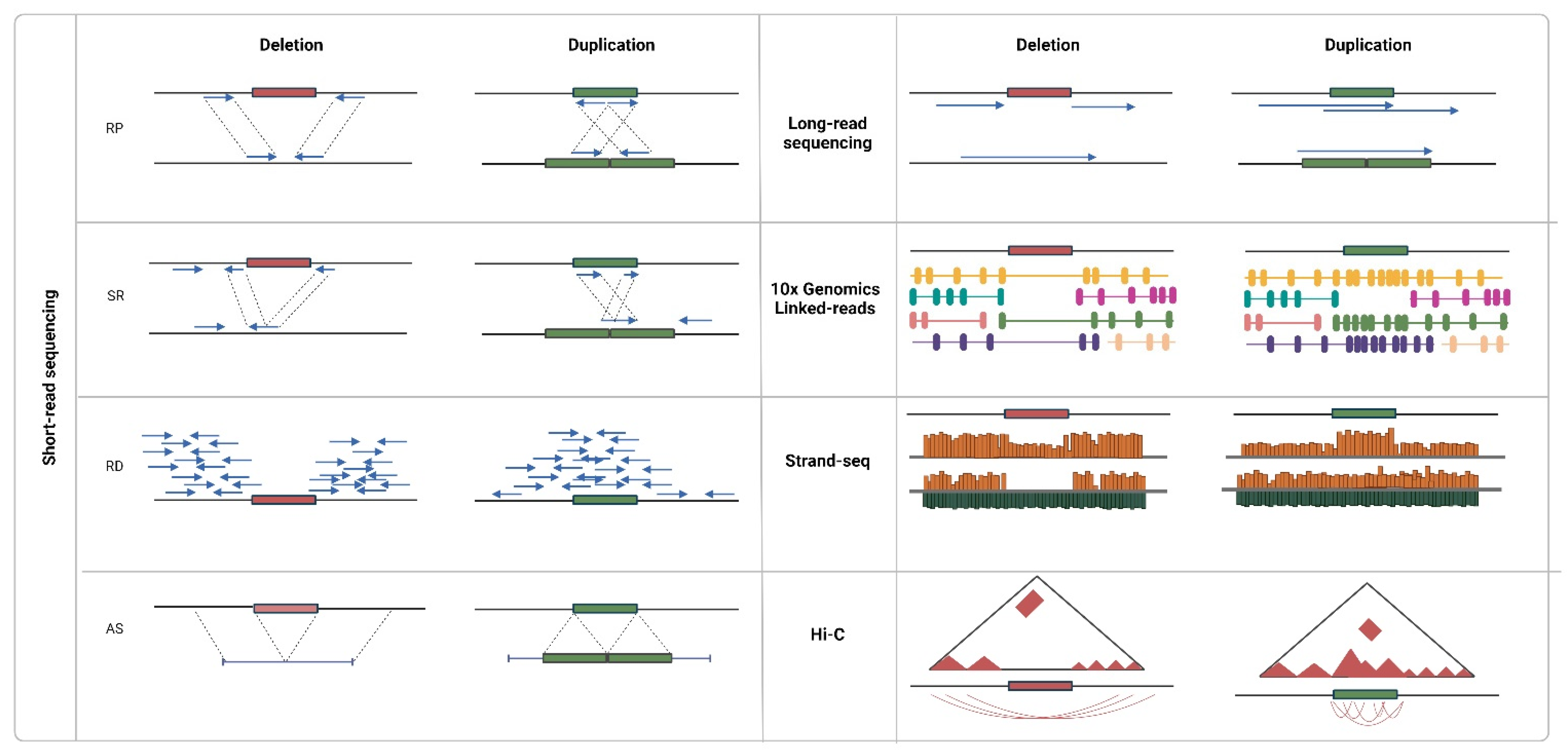
Detection algorithms are readily being developed in several directions since NGS data can be described by various signatures (Figure 3). One of the first approaches was based on the read pair concept (RP). It implies the presence of aberrations under the condition that distances between the mapped reads on a reference genome reads and/or their orientation is different from the expected ones [65,66]. To search for clusters of such abnormal RPs, two strategies were proposed: in the first case, the distance between the paired reads (insert size) is considered known and constant [67,68], and at least two discordant pairs are required to form a cluster; in the second case, the distribution of insert size for each region over the whole genome is taken into account [69,70]. In the latest studies, concordant read pairs are also taken into account, implying that smaller variations can thus be traced [71].

Figure 3.
Approaches to CNV detection using sequencing data. Created with BioRender.com (accessed on 9 February 2022).
The split-read (SR) approach also originated from the incoherence property; however, in this case, read is not mapped onto the genome at all or is only partially mapped. The following repeated alignment of the read parts can indicate possible coordinates of the start and end of the variation [72]. The SR approach is suitable for both single and paired reads, but the latter ones impose additional limitations, which accelerate the search. For example, in the Pindel algorithm [73], alignment of the 3′ end of unmapped paired-ends is performed within the double insert size from the 3′ end of mapped paired-ends. The SR approach exists as a rare method to identify deletions 50–100 bp long. Due to its sensitivity to the quality of alignment, it is intended for studies of unique regions of the genome.
In contrast to previous methods indicating only the presence of a variation, the next approach was intended to evaluate the number of copies. The read-depth (RD) approach is based on the assumption that region coverage correlates with the number of its copies. Not limited by either read length or insert size, the approach is suitable for the identification of preferably large variations. The standard detection procedure consists of four stages: mapping of reads and calculation of coverage; normalization; segmentation; and evaluation of the copy number. In the first stage, a genome, or a sliding window, is usually used. The window size can be both chosen voluntarily (for example, 100 nucleotides was considered sufficient for the identification of small variations and accurate search for the breakpoints [74]) or selected based on the data and the desired confidence level of the CNV event [75,76]. Presumably, the number of reads per window is distributed normally; however, in reality, the coverage is shifted in the function of the GC content of the regions [77] and depends on mappability [50]. To take these factors into account, mean normalization methods [78,79,80], as well as various regression models [81,82,83,84], have been proposed. Segmentation and evaluation of copy numbers are performed by most CMA methods, including HMM, mixed Gaussian models, LASSO regression, and CBS.
The RD approach is applicable to the data of whole-exome or targeted sequencing [85]. Although identification of most variations does not seem possible, this type of analysis is convenient in the primary search for patterns specific to a disorder. In addition to the higher coverage of target regions, one should take into account that during genomic library preparation, the efficiency of the enrichment of target regions varies and some regions are over or under-represented. To describe exome data, various models have been proposed, including Gaussian [86], Poissonian [87], beta-binomial [88], and negative binomial [89] distributions (Table 3). In addition, the discrete structure of the data, with rare exceptions, does not allow an analysis of the exact breakpoints of the CNV; however, the analysis can be expanded by using the information on non-target regions that make up 30–40% of sequencing data and providing low genome coverage [78,90]. Another important issue considered in the framing of the problem is the choice of reference samples which are used at the stage of normalization with questions, such as how many reference samples are necessary for efficient detection and are all of them equally useful at being vitally important. The most frequently used strategies include having all available samples sequenced on the same platform with the same chemistry, having all samples in a single sequencing run, or using a set of the most coverage-correlated samples [91].

Table 3.
The most widely used algorithms for whole-exome and targeted data sequencing.
Recently, the team behind the GATK (Genome Analysis Toolkit), which is known as the most popular tool for analyzing short genomic variations, has also proposed a pipeline to call rare and common germline copy number variants. It uses negative-binomial factor analysis and HMM, and requires at least one-hundred samples to build a model (https://github.com/broadinstitute/gatk/blob/master/docs/CNV/germline-cnv-caller-model.pdf, accessed on 9 February 2022). A somatic mode is also available.
The last approach to detect CNVs using NGS data implies that first, DNA fragments are assembled from overlapping reads de novo, and then the contigs are aligned onto a reference genome (AS). The approach does not require high coverage and is potentially fit for the identification of all types of structural variations, especially new ones. The assembly is performed using graph models (overlapping graphs built by the overlap layout consensus (OLC) method and de Bruijn graph [96] are most often used). Searches for variations can be performed without a reference genome; in such a case, the graph is constructed for several samples and is then analyzed for bifurcations and copy number [97,98].
Despite progress, each of the four described approaches alone is not able to identify the whole range of variations; therefore, the next step was the development of combined methods (Table 4). The most frequently used combinations were RP plus SR or RP plus RD, which are capable of the identification of variations of different lengths and more accurate prediction of breakpoints. Later, algorithms started to take into account the advantages of all three signatures [99,100,101,102,103], which allows a decreased number of false-positive identifications.

Table 4.
Combinations of approaches to the analysis of whole-genome sequencing and the most frequently implemented algorithms.
Often, CNV detection is narrowed down to classification problems solved by methods of machine learning. Along with various signatures, a number of additional factors, for example, mapping quality (MAPQ) or nucleotide content, are considered [108]. In addition, it is possible to take into account specific features of the formed validation sets, which typically contain intermediate-sized variants. For example, a one-class model trained on a representative set of regions with normal copy numbers searches for regions unlike those in the set, thus covering variations of varying type and size [109]. The latest developments include the DeepSV algorithm based on the analysis of mapped read images [110].
Integration of the data can proceed not only at the level of the signatures, but also at the level of the variants predicted by multiple individual algorithms. Today, the so-called ensemble-based approach is not standardized in any way. Various methods are used for combining and evaluating the variants, including coordinate overlapping, distance between the breakpoints, signature prioritization, agreement of the algorithms, number of confirmations of the event, confidence intervals of the breakpoints, FDR cutoff, and metaheuristics [111,112,113]. Despite the improvement in the accuracy of predictions, all these methods are limited by the characteristics of the input data (short read or insert size), and they do not allow a comprehensive analysis of complex genome regions.
On the contrary, long reads (above 5 kb) produced by third-generation sequencing machines can be used to solve the problem, despite their lack of accuracy. The Pacbio platform performs real-time sequencing of a single molecule through the synthesis of a new strand using a polymerase bound to the well bottom and registration of fluorescence of each newly added nucleotide. In turn, the Oxford nanopore technology is based on the evaluation of change in an electrical current induced by a single-stranded DNA molecule passing through a nanopore. In either case, to detect variations, signatures inside the reads completely covering variations and signatures indicating the presence of a variation based on discrepancies between the reads (orientation, size, or location) are analyzed [114,115] Moreover, calling efficiency depends more on coverage than on the read length or error rate [116].
As an alternative to long reads, the sequencing of bound molecules can be used. For example, using additional barcodes to indicate association to a single DNA molecule, synthetic long reads can be efficient in the detection of large variations. The identification of variants proceeds based on the evaluation of the density of molecule coverage by paired reads, the excessive increase or decrease of which implies a CNV event in a certain region [117,118]. In Strand-seq technology, DNA strands are sequenced independently, and large deletions or duplications are identified based on the evaluation of coverage rate [119]. The size of predicted variations is often limited, which is due to the considerable decrease of coverage resulting from the many purification steps during library preparation. Another method, Hi-C, has been developed to study the 3D structure of chromatin, in particular, to determine the nucleotide sequences that are separately located in the genome but still interact with each other. The matrix of contacts between any two loci can be transformed into the coverage profile with a certain resolution; then, signature-typical analysis methods are applied (normalization, segmentation, and copy number evaluation) [119,120].
In addition to the sequence-based methods mentioned above, optical mapping that aims to determine the physical location of specific sequence motifs or enzymes has great potential for CNV calling. The method first demonstrated in 1993 on the example of construction restriction maps of Saccharomyces cerevisiae chromosomes has undergone some modifications [121]. Today’s workflow includes the isolation of high molecular weight DNA, labeling of specific sequences across the entire genome, single-molecule DNA linearization in nanochannels, and imaging using high-resolution fluorescent microscopy. The data obtained can be used for genome assembly improvement [122], haplotype phasing [123], and searches for large structural variations [124]. A CNV event is defined by changes in the density or the distance between restriction sites compared with a reference map obtained from the in silico digestion of the reference sequence. Errors most often occur due to excessive or insufficient stretching of the molecule in the channel, non-specific enzymatic cuts, and incomplete enzyme digestion [125].
5. Conclusions
Despite considerable progress, the identification of copy number variations remains a difficult task. Each of the proposed approaches, from cytogenetics to emerging sequencing technologies, capable of the analysis of the so-called dark genome regions has its own limitations. In an attempt to neutralize the latter, over one-hundred methods of analysis have been developed. Particular attention is paid to the development of algorithms aimed at exome and targeted sequencing as optimal tools for applied methods of genome analysis in regard to information load and cost [85], as well as integration of data of any kind, including the use of ensemble models [126,127,128]. Therefore, researchers face a huge solution space from which they usually choose established algorithms, even though they may be less ideal than newer approaches. Algorithms are mainly shaped to detect certain types of variations or length ranges, which should be considered when choosing the approach [129].
Evaluations of the efficiency of existing CNV detection methods and understanding their advantages and limitations are complicated by the lack of a comprehensive validation set. The only available approach for the description of many genomic variations today is the integration of the results of several platforms, as proposed by Zook et al., for simple deletions and insertions [130]. Expansion of the set of platforms used for the analysis and improvement of their accuracy, as well as the development of protocols for integration of the whole bulk of information, are the key problems in this area of research. The design of a variation profile is necessary not only to be used as a reference in the choice of appropriate detection method, but also for basic research focuses, such as the study of the role and function of CNVs, evaluation of their effect on pathogenesis, and many others.
Author Contributions
Conceptualization, V.G.; writing—original draft preparation, V.G.; writing —review and editing, E.S. and G.A.; visualization, V.G.; supervision, G.A.; funding acquisition, G.A. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by grant 075-15-2019-1669 from the Ministry of Science and Higher Education of the Russian Federation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The original figures were created with BioRender.com (accessed on 9 February 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hollox, E.J.; Zuccherato, L.W.; Tucci, S. Genome Structural Variation in Human Evolution. Trends Genet. 2022, 38, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Iafrate, A.J.; Feuk, L.; Rivera, M.N.; Listewnik, M.L.; Donahoe, P.K.; Qi, Y.; Scherer, S.W.; Lee, C. Detection of Large-Scale Variation in the Human Genome. Nat. Genet. 2004, 36, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Redon, R.; Ishikawa, S.; Fitch, K.R.; Feuk, L.; Perry, G.H.; Andrews, T.D.; Fiegler, H.; Shapero, M.H.; Carson, A.R.; Chen, W.; et al. Global Variation in Copy Number in the Human Genome. Nature 2006, 444, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Zarrei, M.; MacDonald, J.R.; Merico, D.; Scherer, S.W. A Copy Number Variation Map of the Human Genome. Nat. Rev. Genet. 2015, 16, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, T.H. Copy Number Variation Disorders. Curr. Genet. Med. Rep. 2017, 5, 183. [Google Scholar] [CrossRef]
- Roca, I.; González-Castro, L.; Fernández, H.; Couce, M.L.; Fernández-Marmiesse, A. Free-Access Copy-Number Variant Detection Tools for Targeted next-Generation Sequencing Data. Mutat. Res./Rev. Mutat. Res. 2019, 779, 114–125. [Google Scholar] [CrossRef]
- Seiser, E.L.; Innocenti, F. Hidden Markov Model-Based CNV Detection Algorithms for Illumina Genotyping Microarrays. Cancer Inform. 2015, 13, CIN-S16345. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.P. Methods and Strategies for Analyzing Copy Number Variation Using DNA Microarrays. Nat. Genet. 2007, 39. [Google Scholar] [CrossRef]
- Mahmoud, M.; Gobet, N.; Cruz-Dávalos, D.I.; Mounier, N.; Dessimoz, C.; Sedlazeck, F.J. Structural Variant Calling: The Long and the Short of It. Genome Biol. 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Salgado, D.; Armean, I.M.; Baudis, M.; Beltran, S.; Capella-Gutierrez, S.; Carvalho-Silva, D.; Del Angel, V.D.; Dopazo, J.; Furlong, L.I.; Gao, B.; et al. The ELIXIR Human Copy Number Variations Community: Building Bioinformatics Infrastructure for Research. F1000Research 2020, 9, 1229. [Google Scholar] [CrossRef]
- Roizen, N.J.; Patterson, D. Down’s Syndrome. Lancet 2003, 361, 1281–1289. [Google Scholar] [CrossRef]
- Lanfranco, F.; Kamischke, A.; Zitzmann, M.; Nieschlag, E. Klinefelter’s Syndrome. Lancet 2004, 364, 273–283. [Google Scholar] [CrossRef]
- Cereda, A.; Carey, J.C. The Trisomy 18 Syndrome. Orphanet J. Rare Dis. 2012, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Nowell, P.C. The Minute Chromosome (Phl) in Chronic Granulocytic Leukemia. Blut 1962, 8, 65–66. [Google Scholar] [CrossRef]
- Bayani, J.; Squire, J.A. Traditional Banding of Chromosomes for Cytogenetic Analysis. Curr. Protoc. Cell Biol. 2004, 22, 22.3.1–22.3.7. [Google Scholar] [CrossRef]
- Swansbury, J. Introduction to the Analysis of the Human G-Banded Karyotype. Methods Mol. Biol. 2003, 220, 259–269. [Google Scholar] [PubMed]
- Gall, J.G.; Pardue, M.L. Formation and Detection of RNA-DNA Hybrid Molecules in Cytological Preparations. Proc. Natl. Acad. Sci. USA 1969, 63, 378–383. [Google Scholar] [CrossRef]
- Rudkin, G.T.; Stollar, B.D. High Resolution Detection of DNA-RNA Hybrids in Situ by Indirect Immunofluorescence. Nature 1977, 265, 472–473. [Google Scholar] [CrossRef]
- Bauman, J.G.; Wiegant, J.; Borst, P.; van Duijn, P. A New Method for Fluorescence Microscopical Localization of Specific DNA Sequences by in Situ Hybridization of Fluorochromelabelled RNA. Exp. Cell Res. 1980, 128. [Google Scholar] [CrossRef]
- Schrock, E.; du Manoir, S.; Veldman, T.; Schoell, B.; Wienberg, J.; Ferguson-Smith, M.A.; Ning, Y.; Ledbetter, D.H.; Bar-Am, I.; Soenksen, D.; et al. Multicolor Spectral Karyotyping of Human Chromosomes. Science 1996, 273, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Speicher, M.R.; Ballard, S.G.; Ward, D.C. Karyotyping Human Chromosomes by Combinatorial Multi-Fluor FISH. Nat. Genet. 1996, 12, 368–375. [Google Scholar] [CrossRef]
- Kallioniemi, A.; Kallioniemi, O.P.; Sudar, D.; Rutovitz, D.; Gray, J.W.; Waldman, F.; Pinkel, D. Comparative Genomic Hybridization for Molecular Cytogenetic Analysis of Solid Tumors. Science 1992, 258, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Kallioniemi, O.P.; Kallioniemi, A.; Piper, J.; Isola, J.; Waldman, F.M.; Gray, J.W.; Pinkel, D. Optimizing Comparative Genomic Hybridization for Analysis of DNA Sequence Copy Number Changes in Solid Tumors. Genes Chromosomes Cancer 1994, 10, 231–243. [Google Scholar] [CrossRef]
- Gebhart, E. Comparative Genomic Hybridization (CGH): Ten Years of Substantial Progress in Human Solid Tumor Molecular Cytogenetics. Cytogenet. Genome Res. 2004, 104, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Solinas-Toldo, S.; Lampel, S.; Stilgenbauer, S.; Nickolenko, J.; Benner, A.; Döhner, H.; Cremer, T.; Lichter, P. Matrix-Based Comparative Genomic Hybridization: Biochips to Screen for Genomic Imbalances. Genes Chromosomes Cancer 1997, 20, 399–407. [Google Scholar] [CrossRef]
- Pinkel, D.; Segraves, R.; Sudar, D.; Clark, S.; Poole, I.; Kowbel, D.; Collins, C.; Kuo, W.-L.; Chen, C.; Zhai, Y.; et al. High Resolution Analysis of DNA Copy Number Variation Using Comparative Genomic Hybridization to Microarrays. Nat. Genet. 1998, 20, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Osoegawa, K.; Mammoser, A.G.; Wu, C.; Frengen, E.; Zeng, C.; Catanese, J.J.; de Jong, P.J. A Bacterial Artificial Chromosome Library for Sequencing the Complete Human Genome. Genome Res. 2001, 11, 483. [Google Scholar] [CrossRef]
- Cowell, J.K.; Nowak, N.J. High-Resolution Analysis of Genetic Events in Cancer Cells Using Bacterial Artificial Chromosome Arrays and Comparative Genome Hybridization. Adv. Cancer Res. 2003, 90, 91–125. [Google Scholar] [CrossRef]
- Malan, V.; Chevallier, S.; Soler, G.; Coubes, C.; Lacombe, D.; Pasquier, L.; Soulier, J.; Morichon-Delvallez, N.; Turleau, C.; Munnich, A.; et al. Array-Based Comparative Genomic Hybridization Identifies a High Frequency of Copy Number Variations in Patients with Syndromic Overgrowth. Eur. J. Hum. Genet. 2009, 18, 227–232. [Google Scholar] [CrossRef]
- Pollack, J.R.; Perou, C.M.; Alizadeh, A.A.; Eisen, M.B.; Pergamenschikov, A.; Williams, C.F.; Jeffrey, S.S.; Botstein, D.; Brown, P.O. Genome-Wide Analysis of DNA Copy-Number Changes Using cDNA Microarrays. Nat. Genet. 1999, 23, 41–46. [Google Scholar] [CrossRef]
- Bashyam; Bair, R.; Kim, Y.H.; Wang, P.; Hernandez-Boussard, T.; Karikari, C.A.; Tibshirani, R.; Maitra, A.; Pollack, J.R. Array-Based Comparative Genomic Hybridization Identifies Localized DNA Amplifications and Homozygous Deletions in Pancreatic Cancer. Neoplasia 2005, 7, 556-IN16. [Google Scholar] [CrossRef] [PubMed]
- Dhami, P.; Coffey, A.J.; Abbs, S.; Vermeesch, J.R.; Dumanski, J.P.; Woodward, K.J.; Andrews, R.M.; Langford, C.; Vetrie, D. Exon Array CGH: Detection of Copy-Number Changes at the Resolution of Individual Exons in the Human Genome. Am. J. Hum. Genet. 2005, 76, 750. [Google Scholar] [CrossRef] [PubMed]
- Schena, M.; Shalon, D.; Heller, R.; Chai, A.; Brown, P.O.; Davis, R.W. Parallel Human Genome Analysis: Microarray-Based Expression Monitoring of 1000 Genes. Proc. Natl. Acad. Sci. USA 1996, 93, 10614–10619. [Google Scholar] [CrossRef] [PubMed]
- DeRisi, J.; Penland, L.; Brown, P.O.; Bittner, M.L.; Meltzer, P.S.; Ray, M.; Chen, Y.; Su, Y.A.; Trent, J.M. Use of a cDNA Microarray to Analyse Gene Expression Patterns in Human Cancer. Nat. Genet. 1996, 14, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Lucito, R.; Healy, J.; Alexander, J.; Reiner, A.; Esposito, D.; Chi, M.; Rodgers, L.; Brady, A.; Sebat, J.; Troge, J.; et al. Representational Oligonucleotide Microarray Analysis: A High-Resolution Method to Detect Genome Copy Number Variation. Genome Res. 2003, 13, 2291–2305. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.T.; Scheffer, A.; Ben-Dor, A.; Sampas, N.; Lipson, D.; Kincaid, R.; Tsang, P.; Curry, B.; Baird, K.; Meltzer, P.S.; et al. Comparative Genomic Hybridization Using Oligonucleotide Microarrays and Total Genomic DNA. Proc. Natl. Acad. Sci. USA 2004, 101, 17765–17770. [Google Scholar] [CrossRef]
- Bignell, G.R.; Huang, J.; Greshock, J.; Watt, S.; Butler, A.; West, S.; Grigorova, M.; Jones, K.W.; Wei, W.; Stratton, M.R.; et al. High-Resolution Analysis of DNA Copy Number Using Oligonucleotide Microarrays. Genome Res. 2004, 14, 287–295. [Google Scholar] [CrossRef]
- Peiffer, D.A.; Le, J.M.; Steemers, F.J.; Chang, W.; Jenniges, T.; Garcia, F.; Haden, K.; Li, J.; Shaw, C.A.; Belmont, J.; et al. High-Resolution Genomic Profiling of Chromosomal Aberrations Using Infinium Whole-Genome Genotyping. Genome Res. 2006, 16, 1136–1148. [Google Scholar] [CrossRef]
- Shen, F.; Huang, J.; Fitch, K.R.; Truong, V.B.; Kirby, A.; Chen, W.; Zhang, J.; Liu, G.; McCarroll, S.A.; Jones, K.W.; et al. Improved Detection of Global Copy Number Variation Using High Density, Non-Polymorphic Oligonucleotide Probes. BMC Genet. 2008, 9, 27. [Google Scholar] [CrossRef]
- Miller, D.T.; Adam, M.P.; Aradhya, S.; Biesecker, L.G.; Brothman, A.R.; Carter, N.P.; Church, D.M.; Crolla, J.A.; Eichler, E.E.; Epstein, C.J.; et al. Consensus Statement: Chromosomal Microarray Is a First-Tier Clinical Diagnostic Test for Individuals with Developmental Disabilities or Congenital Anomalies. Am. J. Hum. Genet. 2010, 86, 749. [Google Scholar] [CrossRef] [PubMed]
- Haraksingh, R.R.; Abyzov, A.; Urban, A.E. Comprehensive Performance Comparison of High-Resolution Array Platforms for Genome-Wide Copy Number Variation (CNV) Analysis in Humans. BMC Genom. 2017, 18, 321. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Darvishi, K.; Shi, X.; Rajan, D.; Rigler, D.; Fitzgerald, T.; Lionel, A.C.; Thiruvahindrapuram, B.; MacDonald, J.R.; Mills, R.; et al. Comprehensive Assessment of Array-Based Platforms and Calling Algorithms for Detection of Copy Number Variants. Nat. Biotechnol. 2011, 29, 512–520. [Google Scholar] [CrossRef]
- Veltman, J.A.; Fridlyand, J.; Pejavar, S.; Olshen, A.B.; Korkola, J.E.; DeVries, S.; Carroll, P.; Kuo, W.-L.; Pinkel, D.; Albertson, D.; et al. Array-Based Comparative Genomic Hybridization for Genome-Wide Screening of DNA Copy Number in Bladder Tumors. Cancer Res. 2003, 63, 2872–2880. [Google Scholar] [PubMed]
- Array-Based Comparative Genomic Hybridization for the Genomewide Detection of Submicroscopic Chromosomal Abnormalities. Am. J. Hum. Genet. 2003, 73, 1261–1270. [CrossRef] [PubMed]
- Comparative Genomic Hybridization–Array Analysis Enhances the Detection of Aneuploidies and Submicroscopic Imbalances in Spontaneous Miscarriages. Am. J. Hum. Genet. 2004, 74, 1168–1174. [CrossRef] [PubMed]
- Shaw-Smith, C.; Redon, R.; Rickman, L.; Rio, M.; Willatt, L.; Fiegler, H.; Firth, H.; Sanlaville, D.; Winter, R.; Colleaux, L.; et al. Microarray Based Comparative Genomic Hybridisation (array-CGH) Detects Submicroscopic Chromosomal Deletions and Duplications in Patients with Learning Disability/mental Retardation and Dysmorphic Features. J. Med. Genet. 2004, 41, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Schwaenen, C.; Nessling, M.; Wessendorf, S.; Salvi, T.; Wrobel, G.; Radlwimmer, B.; Kestler, H.A.; Haslinger, C.; Stilgenbauer, S.; Döhner, H.; et al. Automated Array-Based Genomic Profiling in Chronic Lymphocytic Leukemia: Development of a Clinical Tool and Discovery of Recurrent Genomic Alterations. Proc. Natl. Acad. Sci. USA 2004, 101, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- :Olshen, A.B.; Venkatraman, E.S.; Lucito, R.; Wigler, M. Circular Binary Segmentation for the Analysis of Array-Based DNA Copy Number Data. Biostatistics 2004, 5, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Jong, K.; Marchiori, E.; van der Vaart, A.; Ylstra, B.; Weiss, M.; Meijer, G. Chromosomal Breakpoint Detection in Human Cancer. In Proceedings of the Applications of Evolutionary Computing, Essex, UK, 14–16 April 2003; Springer: Berlin/Heidelberg, Germany, 2003; pp. 54–65. [Google Scholar]
- Hupé, P.; Stransky, N.; Thiery, J.P.; Radvanyi, F.; Barillot, E. Analysis of Array CGH Data: From Signal Ratio to Gain and Loss of DNA Regions. Bioinformatics 2004, 20, 3413–3422. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R.; Wang, P. Spatial Smoothing and Hot Spot Detection for CGH Data Using the Fused Lasso. Biostatistics 2008, 9, 18–29. [Google Scholar] [CrossRef]
- Jeng, X.J.; Cai, T.T.; Li, H. Optimal Sparse Segment Identification with Application in Copy Number Variation Analysis. J. Am. Stat. Assoc. 2010, 105, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.S.; Zhang, H. The screening and ranking algorithm to detect dna copy number variations. Ann. Appl. Stat. 2012, 6, 1306–1326. [Google Scholar] [CrossRef]
- de Vries, B.B.A.; Pfundt, R.; Leisink, M.; Koolen, D.A.; Vissers, L.E.L.; Janssen, I.M.; van Reijmersdal, S.; Nillesen, W.M.; Huys, E.H.L.P.; de Leeuw, N.; et al. Diagnostic Genome Profiling in Mental Retardation. Am. J. Hum. Genet. 2005, 77, 606. [Google Scholar] [CrossRef]
- Zhao, X.; Li, C.; Guillermo Paez, J.; Chin, K.; Jänne, P.A.; Chen, T.-H.; Girard, L.; Minna, J.; Christiani, D.; Leo, C.; et al. An Integrated View of Copy Number and Allelic Alterations in the Cancer Genome Using Single Nucleotide Polymorphism Arrays. Cancer Res. 2004, 64, 3060–3071. [Google Scholar] [CrossRef]
- Picard, F.; Robin, S.; Lavielle, M.; Vaisse, C.; Daudin, J.-J. A Statistical Approach for Array CGH Data Analysis. BMC Bioinform. 2005, 6, 27. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hadley, D.; Liu, R.; Glessner, J.; Grant, S.F.A.; Hakonarson, H.; Bucan, M. PennCNV: An Integrated Hidden Markov Model Designed for High-Resolution Copy Number Variation Detection in Whole-Genome SNP Genotyping Data. Genome Res. 2007, 17, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, A.E.; Saw, S.M.; Goh, L.K.; Seielstad, M.; Young, T.L.; Li, Y.J. Comparative Analyses of Seven Algorithms for Copy Number Variant Identification from Single Nucleotide Polymorphism Arrays. Nucleic Acids Res. 2010, 38, e105. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Motsinger, R.A. Evaluation of Calling Algorithms for Array-CGH. Front. Genet. 2013, 4, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Winchester, L.; Yau, C.; Ragoussis, J. Comparing CNV Detection Methods for SNP Arrays. Brief. Funct. Genom. 2009, 8, 353–366. [Google Scholar] [CrossRef]
- Korn, J.M.; Kuruvilla, F.G.; McCarroll, S.A.; Wysoker, A.; Nemesh, J.; Cawley, S.; Hubbell, E.; Veitch, J.; Collins, P.J.; Darvishi, K.; et al. Integrated Genotype Calling and Association Analysis of SNPs, Common Copy Number Polymorphisms and Rare CNVs. Nat. Genet. 2008, 40, 1253–1260. [Google Scholar] [CrossRef]
- Sun, W.; Wright, F.A.; Tang, Z.; Nordgard, S.H.; Van Loo, P.; Yu, T.; Kristensen, V.N.; Perou, C.M. Integrated Study of Copy Number States and Genotype Calls Using High-Density SNP Arrays. Nucleic Acids Res. 2009, 37, 5365–5377. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, K. Application of Nexus Copy Number Software for CNV Detection and Analysis. Curr. Protoc. Hum. Genet. 2010, 65, 4–14. [Google Scholar] [CrossRef]
- Colella, S.; Yau, C.; Taylor, J.M.; Mirza, G.; Butler, H.; Clouston, P.; Bassett, A.S.; Seller, A.; Holmes, C.C.; Ragoussis, J. QuantiSNP: An Objective Bayes Hidden-Markov Model to Detect and Accurately Map Copy Number Variation Using SNP Genotyping Data. Nucleic Acids Res. 2007, 35, 2013–2025. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Q.; Wang, Q.; Jia, P.; Zhao, Z. Computational Tools for Copy Number Variation (CNV) Detection Using next-Generation Sequencing Data: Features and Perspectives. BMC Bioinform. 2013, 14, S1. [Google Scholar] [CrossRef]
- Korbel, J.O.; Urban, A.E.; Affourtit, J.P.; Godwin, B.; Grubert, F.; Simons, J.F.; Kim, P.M.; Palejev, D.; Carriero, N.J.; Du, L.; et al. Paired-End Mapping Reveals Extensive Structural Variation in the Human Genome. Science 2007, 318, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wallis, J.W.; McLellan, M.D.; Larson, D.E.; Kalicki, J.M.; Pohl, C.S.; McGrath, S.D.; Wendl, M.C.; Zhang, Q.; Locke, D.P.; et al. BreakDancer: An Algorithm for High-Resolution Mapping of Genomic Structural Variation. Nat. Methods 2009, 6, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Korbel, J.O.; Abyzov, A.; Mu, X.J.; Carriero, N.; Cayting, P.; Zhang, Z.; Snyder, M.; Gerstein, M.B. PEMer: A Computational Framework with Simulation-Based Error Models for Inferring Genomic Structural Variants from Massive Paired-End Sequencing Data. Genome Biol. 2009, 10, R23. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hormozdiari, F.; Alkan, C.; Brudno, M. MoDIL: Detecting Small Indels from Clone-End Sequencing with Mixtures of Distributions. Nat. Methods 2009, 6, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Pyon, Y.S.; Li, J. A Model-Based Clustering Method for Genomic Structural Variant Prediction and Genotyping Using Paired-End Sequencing Data. PLoS ONE 2012, 7, e52881. [Google Scholar] [CrossRef]
- Marschall, T.; Costa, I.G.; Canzar, S.; Bauer, M.; Klau, G.W.; Schliep, A.; Schönhuth, A. CLEVER: Clique-Enumerating Variant Finder. Bioinformatics 2012, 28, 2875–2882. [Google Scholar] [CrossRef] [PubMed]
- Trappe, K.; Emde, A.-K.; Ehrlich, H.-C.; Reinert, K. Gustaf: Detecting and Correctly Classifying SVs in the NGS Twilight Zone. Bioinformatics 2014, 30, 3484–3490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ye, K.; Schulz, M.H.; Long, Q.; Apweiler, R.; Ning, Z. Pindel: A Pattern Growth Approach to Detect Break Points of Large Deletions and Medium Sized Insertions from Paired-End Short Reads. Bioinformatics 2009, 25, 2865–2871. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Xuan, Z.; Makarov, V.; Ye, K.; Sebat, J. Sensitive and Accurate Detection of Copy Number Variants Using Read Depth of Coverage. Genome Res. 2009, 19, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Tammi, M.T. CNV-Seq, a New Method to Detect Copy Number Variation Using High-Throughput Sequencing. BMC Bioinform. 2009, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Gusnanto, A.; Taylor, C.C.; Nafisah, I.; Wood, H.M.; Rabbitts, P.; Berri, S. Estimating Optimal Window Size for Analysis of Low-Coverage next-Generation Sequence Data. Bioinformatics 2014, 30, 1823–1829. [Google Scholar] [CrossRef]
- Benjamini, Y.; Speed, T.P. Summarizing and Correcting the GC Content Bias in High-Throughput Sequencing. Nucleic Acids Res. 2012, 40, e72. [Google Scholar] [CrossRef]
- Talevich, E.; Hunter Shain, A.; Botton, T.; Bastian, B.C. CNVkit: Genome-Wide Copy Number Detection and Visualization from Targeted DNA Sequencing. PLoS Comput. Biol. 2016, 12, e1004873. [Google Scholar] [CrossRef]
- Abyzov, A.; Urban, A.E.; Snyder, M.; Gerstein, M. CNVnator: An Approach to Discover, Genotype, and Characterize Typical and Atypical CNVs from Family and Population Genome Sequencing. Genome Res. 2011, 21, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Dharanipragada, P.; Vogeti, S.; Parekh, N. iCopyDAV: Integrated Platform for Copy Number variations—Detection, Annotation and Visualization. PLoS ONE 2018, 13, e0195334. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Sun, W.; Crowley, J.J.; Szatkiewicz, J.P. Allele-Specific Copy-Number Discovery from Whole-Genome and Whole-Exome Sequencing. Nucleic Acids Res. 2015, 43, e90. [Google Scholar] [CrossRef]
- Xi, R.; Lee, S.; Xia, Y.; Kim, T.-M.; Park, P.J. Copy Number Analysis of Whole-Genome Data Using BIC-seq2 and Its Application to Detection of Cancer Susceptibility Variants. Nucleic Acids Res. 2016, 44, 6274–6286. [Google Scholar] [CrossRef]
- Boeva, V.; Zinovyev, A.; Bleakley, K.; Vert, J.P.; Janoueix-Lerosey, I.; Delattre, O.; Barillot, E. Control-Free Calling of Copy Number Alterations in Deep-Sequencing Data Using GC-Content Normalization. Bioinformatics 2011, 27, 268–269. [Google Scholar] [CrossRef]
- Miller, C.A.; Hampton, O.; Coarfa, C.; Milosavljevic, A. ReadDepth: A Parallel R Package for Detecting Copy Number Alterations from Short Sequencing Reads. PLoS ONE 2011, 6, e16327. [Google Scholar] [CrossRef]
- Gordeeva, V.; Sharova, E.; Babalyan, K.; Sultanov, R.; Govorun, V.M.; Arapidi, G. Benchmarking Germline CNV Calling Tools from Exome Sequencing Data. Sci. Rep. 2021, 11, 14416. [Google Scholar] [CrossRef]
- Fromer, M.; Moran, J.L.; Chambert, K.; Banks, E.; Bergen, S.E.; Ruderfer, D.M.; Handsaker, R.E.; McCarroll, S.A.; O’Donovan, M.C.; Owen, M.J.; et al. Discovery and Statistical Genotyping of Copy-Number Variation from Whole-Exome Sequencing Depth. Am. J. Hum. Genet. 2012, 91, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Oldridge, D.A.; Diskin, S.J.; Zhang, N.R. CODEX: A Normalization and Copy Number Variation Detection Method for Whole Exome Sequencing. Nucleic Acids Res. 2015, 43, e39. [Google Scholar] [CrossRef]
- Plagnol, V.; Curtis, J.; Epstein, M.; Mok, K.Y.; Stebbings, E.; Grigoriadou, S.; Wood, N.W.; Hambleton, S.; Burns, S.O.; Thrasher, A.J.; et al. A Robust Model for Read Count Data in Exome Sequencing Experiments and Implications for Copy Number Variant Calling. Bioinformatics 2012, 28, 2747–2754. [Google Scholar] [CrossRef]
- Love, M.I.; Myšičková, A.; Sun, R.; Kalscheuer, V.; Vingron, M.; Haas, S.A. Modeling Read Counts for CNV Detection in Exome Sequencing Data. Stat. Appl. Genet. Mol. Biol. 2011, 10. [Google Scholar] [CrossRef]
- D’Aurizio, R.; Pippucci, T.; Tattini, L.; Giusti, B.; Pellegrini, M.; Magi, A. Enhanced Copy Number Variants Detection from Whole-Exome Sequencing Data Using EXCAVATOR2. Nucleic Acids Res. 2016, 44, e154. [Google Scholar] [CrossRef] [PubMed]
- Kuśmirek, W.; Szmurło, A.; Wiewiórka, M.; Nowak, R.; Gambin, T. Comparison of kNN and K-Means Optimization Methods of Reference Set Selection for Improved CNV Callers Performance. BMC Bioinform. 2019, 20, 266. [Google Scholar] [CrossRef]
- Klambauer, G.; Schwarzbauer, K.; Mayr, A.; Clevert, D.-A.; Mitterecker, A.; Bodenhofer, U.; Hochreiter, S. cn.MOPS: Mixture of Poissons for Discovering Copy Number Variations in next-Generation Sequencing Data with a Low False Discovery Rate. Nucleic Acids Res. 2012, 40, e69. [Google Scholar] [CrossRef] [PubMed]
- Krumm, N.; Sudmant, P.H.; Ko, A.; O’Roak, B.J.; Malig, M.; Coe, B.P.; Quinlan, A.R.; Nickerson, D.A.; Eichler, E.E. Copy Number Variation Detection and Genotyping from Exome Sequence Data. Genome Res. 2012, 22, 1525–1532. [Google Scholar] [CrossRef]
- Johansson, L.F.; van Dijk, F.; de Boer, E.N.; van Dijk-Bos, K.K.; Jongbloed, J.D.; van der Hout, A.H.; Westers, H.; Sinke, R.J.; Swertz, M.A.; Sijmons, R.H.; et al. CoNVaDING: Single Exon Variation Detection in Targeted NGS Data. Hum. Mutat. 2016, 37, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.; Mahamdallie, S.; Ruark, E.; Seal, S.; Ramsay, E.; Clarke, M.; Uddin, I.; Wylie, H.; Strydom, A.; Lunter, G.; et al. Accurate Clinical Detection of Exon Copy Number Variants in a Targeted NGS Panel Using DECoN. Wellcome Open Res. 2016, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Pevzner, P.A.; Tang, H.; Waterman, M.S. An Eulerian Path Approach to DNA Fragment Assembly. Proc. Natl. Acad. Sci. USA 2001, 98, 9748–9753. [Google Scholar] [CrossRef] [PubMed]
- Nijkamp, J.F.; van den Broek, M.A.; Geertman, J.-M.A.; Reinders, M.J.T.; Daran, J.-M.G.; de Ridder, D. De Novo Detection of Copy Number Variation by Co-Assembly. Bioinformatics 2012, 28, 3195–3202. [Google Scholar] [CrossRef]
- Iqbal, Z.; Caccamo, M.; Turner, I.; Flicek, P.; McVean, G. De Novo Assembly and Genotyping of Variants Using Colored de Bruijn Graphs. Nat. Genet. 2012, 44, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.L.; Schröder, J.; Penington, J.S.; Do, H.; Molania, R.; Dobrovic, A.; Speed, T.P.; Papenfuss, A.T. GRIDSS: Sensitive and Specific Genomic Rearrangement Detection Using Positional de Bruijn Graph Assembly. Genome Res. 2017, 27, 2050–2060. [Google Scholar] [CrossRef]
- Wang, J.; Mullighan, C.G.; Easton, J.; Roberts, S.; Heatley, S.L.; Ma, J.; Rusch, M.C.; Chen, K.; Harris, C.C.; Ding, L.; et al. CREST Maps Somatic Structural Variation in Cancer Genomes with Base-Pair Resolution. Nat. Methods 2011, 8, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Using ERDS to Infer Copy-Number Variants in High-Coverage Genomes. Am. J. Hum. Genet. 2012, 91, 408–421. [CrossRef] [PubMed]
- Layer, R.M.; Chiang, C.; Quinlan, A.R.; Hall, I.M. LUMPY: A Probabilistic Framework for Structural Variant Discovery. Genome Biol. 2014, 15, e1004572. [Google Scholar] [CrossRef]
- Chen, X.; Schulz-Trieglaff, O.; Shaw, R.; Barnes, B.; Schlesinger, F.; Källberg, M.; Cox, A.J.; Kruglyak, S.; Saunders, C.T. Manta: Rapid Detection of Structural Variants and Indels for Germline and Cancer Sequencing Applications. Bioinformatics 2015, 32, 1220–1222. [Google Scholar] [CrossRef] [PubMed]
- Handsaker, R.E.; Korn, J.M.; Nemesh, J.; McCarroll, S.A. Discovery and Genotyping of Genome Structural Polymorphism by Sequencing on a Population Scale. Nat. Genet. 2011, 43, 269–276. [Google Scholar] [CrossRef]
- Rausch, T.; Zichner, T.; Schlattl, A.; Stütz, A.M.; Benes, V.; Korbel, J.O. DELLY: Structural Variant Discovery by Integrated Paired-End and Split-Read Analysis. Bioinformatics 2012, 28, i333–i339. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Clark, R.A.; Sokolova, S.; Leibowitz, M.L.; Zhang, Y.; Hurles, M.E.; Mell, J.C.; Hall, I.M. Genome-Wide Mapping and Assembly of Structural Variant Breakpoints in the Mouse Genome. Genome Res. 2010, 20, 623–635. [Google Scholar] [CrossRef]
- Mohiyuddin, M.; Mu, J.C.; Li, J.; Bani Asadi, N.; Gerstein, M.B.; Abyzov, A.; Wong, W.H.; Lam, H.Y.K. MetaSV: An Accurate and Integrative Structural-Variant Caller for next Generation Sequencing. Bioinformatics 2015, 31, 2741–2744. [Google Scholar] [CrossRef]
- Michaelson, J.J.; Sebat, J. forestSV: Structural Variant Discovery through Statistical Learning. Nat. Methods 2012, 9, 819–821. [Google Scholar] [CrossRef]
- Parikh, H.; Mohiyuddin, M.; Lam, H.Y.K.; Iyer, H.; Chen, D.; Pratt, M.; Bartha, G.; Spies, N.; Losert, W.; Zook, J.M.; et al. Svclassify: A Method to Establish Benchmark Structural Variant Calls. BMC Genom. 2016, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wu, Y.; Gao, J. DeepSV: Accurate Calling of Genomic Deletions from High-Throughput Sequencing Data Using Deep Convolutional Neural Network. BMC Bioinform. 2019, 20, 665. [Google Scholar] [CrossRef]
- Mills, R.E.; Walter, K.; Stewart, C.; Handsaker, R.E.; Chen, K.; Alkan, C.; Abyzov, A.; Yoon, S.C.; Ye, K.; Cheetham, R.K.; et al. Mapping Copy Number Variation by Population-Scale Genome Sequencing. Nature 2011, 470, 59–65. [Google Scholar] [CrossRef]
- Sudmant, P.H.; Rausch, T.; Gardner, E.J.; Handsaker, R.E.; Abyzov, A.; Huddleston, J.; Zhang, Y.; Ye, K.; Jun, G.; Fritz, M.H.-Y.; et al. An Integrated Map of Structural Variation in 2504 Human Genomes. Nature 2015, 526, 75–81. [Google Scholar] [CrossRef]
- Collins, R.L.; Brand, H.; Karczewski, K.J.; Zhao, X.; Alföldi, J.; Francioli, L.C.; Khera, A.V.; Lowther, C.; Gauthier, L.D.; Wang, H.; et al. A Structural Variation Reference for Medical and Population Genetics. Nature 2020, 581, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Cretu Stancu, M.; van Roosmalen, M.J.; Renkens, I.; Nieboer, M.M.; Middelkamp, S.; de Ligt, J.; Pregno, G.; Giachino, D.; Mandrile, G.; Espejo Valle-Inclan, J.; et al. Mapping and Phasing of Structural Variation in Patient Genomes Using Nanopore Sequencing. Nat. Commun. 2017, 8, 1326. [Google Scholar] [CrossRef] [PubMed]
- English, A.C.; Salerno, W.J.; Reid, J.G. PBHoney: Identifying Genomic Variants via Long-Read Discordance and Interrupted Mapping. BMC Bioinform. 2014, 15, 180. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, S.; Cao, S.; Liu, Y.; Cui, Z.; Wang, Y.; Guo, H. Long-Read Sequencing Settings for Efficient Structural Variation Detection Based on Comprehensive Evaluation. BMC Bioinform. 2021, 22, 552. [Google Scholar] [CrossRef] [PubMed]
- Spies, N.; Weng, Z.; Bishara, A.; McDaniel, J.; Catoe, D.; Zook, J.M.; Salit, M.; West, R.B.; Batzoglou, S.; Sidow, A. Genome-Wide Reconstruction of Complex Structural Variants Using Read Clouds. Nat. Methods 2017, 14, 915–920. [Google Scholar] [CrossRef]
- Elyanow, R.; Wu, H.T.; Raphael, B.J. Identifying Structural Variants Using Linked-Read Sequencing Data. Bioinformatics 2018, 34, 353–360. [Google Scholar] [CrossRef]
- Hills, M.; O’Neill, K.; Falconer, E.; Brinkman, R.; Lansdorp, P.M. BAIT: Organizing Genomes and Mapping Rearrangements in Single Cells. Genome Med. 2013, 5, 82. [Google Scholar] [CrossRef]
- Wang, S.; Lee, S.; Chu, C.; Jain, D.; Kerpedjiev, P.; Nelson, G.M.; Walsh, J.M.; Alver, B.H.; Park, P.J. HiNT: A Computational Method for Detecting Copy Number Variations and Translocations from Hi-C Data. Genome Biol. 2020, 21, 73. [Google Scholar] [CrossRef]
- Schwartz, D.C.; Li, X.; Hernandez, L.I.; Ramnarain, S.P.; Huff, E.J.; Wang, Y.K. Ordered Restriction Maps of Saccharomyces Cerevisiae Chromosomes Constructed by Optical Mapping. Science 1993, 262, 110–114. [Google Scholar] [CrossRef]
- Latreille, P.; Norton, S.; Goldman, B.S.; Henkhaus, J.; Miller, N.; Barbazuk, B.; Bode, H.B.; Darby, C.; Du, Z.; Forst, S.; et al. Optical Mapping as a Routine Tool for Bacterial Genome Sequence Finishing. BMC Genom. 2007, 8, 321. [Google Scholar] [CrossRef]
- Pendleton, M.; Sebra, R.; Pang, A.W.C.; Ummat, A.; Franzen, O.; Rausch, T.; Stütz, A.M.; Stedman, W.; Anantharaman, T.; Hastie, A.; et al. Assembly and Diploid Architecture of an Individual Human Genome via Single-Molecule Technologies. Nat. Methods 2015, 12, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Optical Genome Mapping Enables Constitutional Chromosomal Aberration Detection. Am. J. Hum. Genet. 2021, 108, 1409–1422. [CrossRef]
- Li, L.; Leung, A.K.-Y.; Kwok, T.-P.; Lai, Y.Y.Y.; Pang, I.K.; Chung, G.T.-Y.; Mak, A.C.Y.; Poon, A.; Chu, C.; Li, M.; et al. OMSV Enables Accurate and Comprehensive Identification of Large Structural Variations from Nanochannel-Based Single-Molecule Optical Maps. Genome Biol. 2017, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, H.; Hong, X.; Di Narzo, A.F.; Franzen, O.; Peng, S.; Ruusalepp, A.; Kovacic, J.C.; Bjorkegren, J.L.M.; Wang, X.; et al. EnsembleCNV: An Ensemble Machine Learning Algorithm to Identify and Genotype Copy Number Variation Using SNP Array Data. Nucleic Acids Res. 2019, 47, e39. [Google Scholar] [CrossRef] [PubMed]
- Pounraja, V.K.; Jayakar, G.; Jensen, M.; Kelkar, N.; Girirajan, S. A Machine-Learning Approach for Accurate Detection of Copy-Number Variants from Exome Sequencing. Genome Res. 2019, 29, 1134–1143. [Google Scholar] [CrossRef]
- Akbarinejad, S.; Hadadian Nejad Yousefi, M.; Goudarzi, M. SVNN: An Efficient PacBio-Specific Pipeline for Structural Variations Calling Using Neural Networks. BMC Bioinform. 2021, 22, 335. [Google Scholar] [CrossRef]
- Kosugi, S.; Momozawa, Y.; Liu, X.; Terao, C.; Kubo, M.; Kamatani, Y. Comprehensive Evaluation of Structural Variation Detection Algorithms for Whole Genome Sequencing. Genome Biol. 2019, 20, 1–18. [Google Scholar] [CrossRef]
- Zook, J.M.; Hansen, N.F.; Olson, N.D.; Chapman, L.; Mullikin, J.C.; Xiao, C.; Sherry, S.; Koren, S.; Phillippy, A.M.; Boutros, P.C.; et al. A Robust Benchmark for Detection of Germline Large Deletions and Insertions. Nat. Biotechnol. 2020, 38, 1347–1355. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).