Investigation of the Antiremodeling Effects of Losartan, Mirabegron and Their Combination on the Development of Doxorubicin-Induced Chronic Cardiotoxicity in a Rat Model
Abstract
:1. Introduction
2. Results
2.1. Early Echocardiographic Signs of Systolic Dysfunction Developed in the DOXO Groups before Starting the Treatments at Week 4
2.2. DOXO-Treated Groups Presented Lower Body Weight and Higher Serum Cholesterol Levels Irrespective of the Treatments with Losartan, Mirabegron, and their Combination at Week 9
2.3. Echocardiographic Signs of the DOXO-Induced Chronic Cardiotoxicity Were Alleviated by Mirabegron and the Combination Treatment but Not by Losartan at Week 8
2.4. DOXO-Indued Heart Weight Loss Was Alleviated by Mirabegron at Week 9
2.5. DOXO-Indued Cardiac Fibrosis Was Significantly Reduced Only by Mirabegron at Week 9
2.6. Overexpression of Smad2 and Smad3 Were Ameliorated by Treatments with Losartan, Mirabegron and their Combination in DOXO-Indued Chronic Cardiotoxicity
2.7. DOXO-Indued Repression of SERCA2a Was Ameliorated by Losartan at Week 9
2.8. Left Ventricular β3AR Protein Levels were Decreased in all DOXO-Treated Groups Irrespective of Treatments with Losartan, Mirabegron, and their Combination
2.9. Left Ventricular Expression of the Inducible Nitric Oxide Synthase was Increased in All DOXO-Treated Groups Irrespective of Treatments with Losartan, Mirabegron, and their Combination
2.10. Left Ventricular Overexpression of Inflammatory Markers Il1, Il6 and Tnf Were Ameliorated by Losartan and Mirabegron in DOXO-Induced Chronic Cardiotoxicity at Week 9
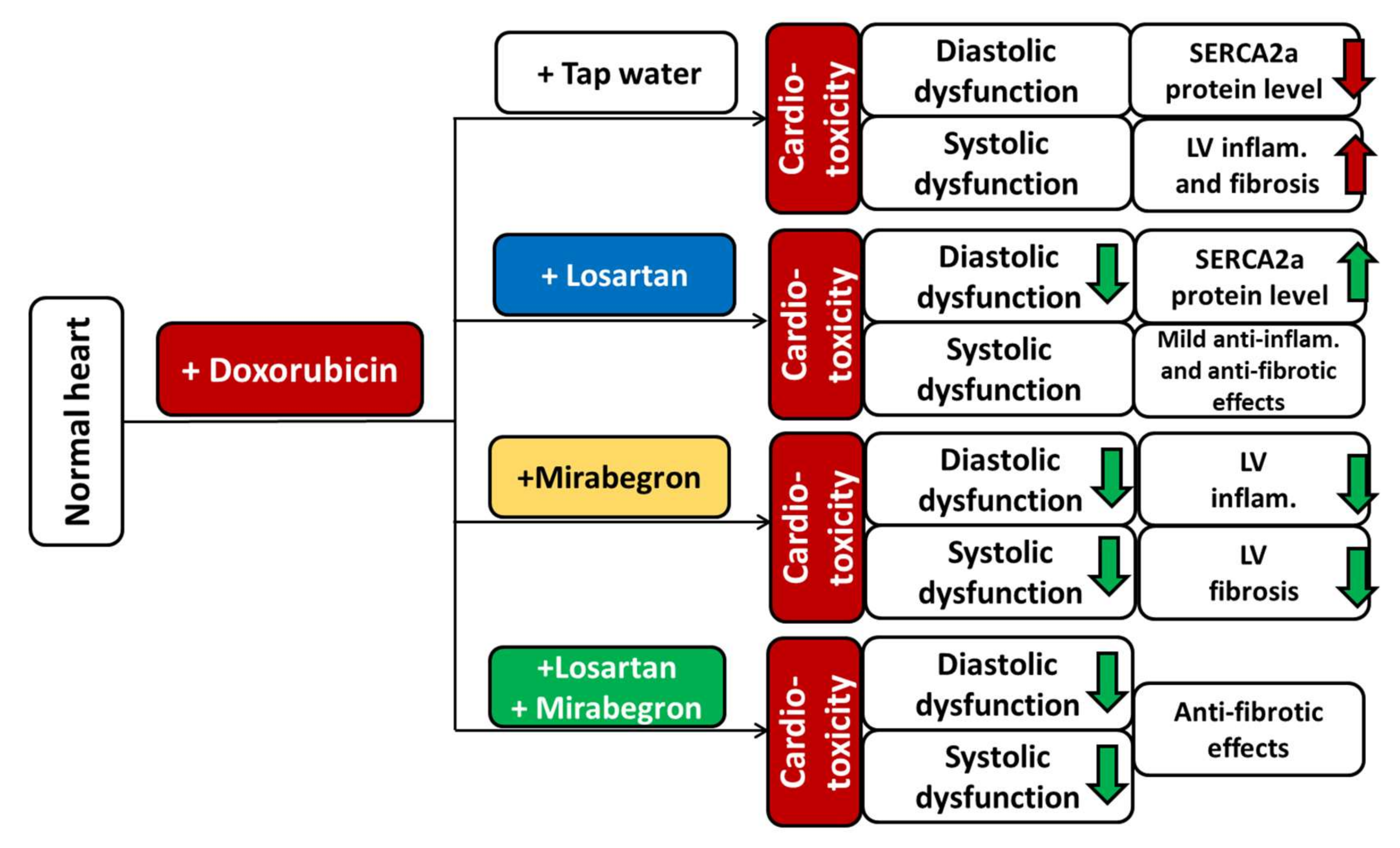
3. Discussion
4. Materials and Methods
4.1. Ethics Approval
4.2. Animals
4.3. Experimental Setup
4.4. Transthoracic Echocardiography
4.5. Blood Pressure Measurement
4.6. Blood Serum Parameters
4.7. Tissue Harvesting
4.8. Hematoxylin-Eosin and Picrosirius Red and Fast Green Stainings
4.9. mRNA Expression Profiling by qRT-PCR
4.10. Western Blot
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 19 January 2022).
- WHO. WHO Cancer 2020. Available online: https://www.who.int/health-topics/cancer (accessed on 19 January 2022).
- Lenneman, C.G.; Sawyer, D.B. Cardio-Oncology: An Update on Cardiotoxicity of Cancer-Related Treatment. Circ. Res. 2016, 118, 1008–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Sárközy, M.; Varga, Z.; Gáspár, R.; Szűcs, G.; Kovács, M.G.; Kovács, Z.Z.A.; Dux, L.; Kahán, Z.; Csont, T. Pathomechanisms and therapeutic opportunities in radiation-induced heart disease: From bench to bedside. Clin. Res. Cardiol. 2021, 110, 507–531. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Abilash, V.G.; Tirupathi Pichiah, P.B.; Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy—An update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Geisberg, C.A.; Sawyer, D.B. Mechanisms of anthracycline cardiotoxicity and strategies to decrease cardiac damage. Curr. Hypertens. Rep. 2010, 12, 404–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipshultz, S.E.; Adams, M.J.; Colan, S.D.; Constine, L.S.; Herman, E.H.; Hsu, D.T.; Hudson, M.M.; Kremer, L.C.; Landy, D.C.; Miller, T.L.; et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation 2013, 128, 1927–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; de Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinherz, L.J.; Steinherz, P.G.; Tan, C.T.; Heller, G.; Murphy, M.L. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 1991, 266, 1672–1677. [Google Scholar] [CrossRef]
- Chang, H.M.; Moudgil, R.; Scarabelli, T.; Okwuosa, T.M.; Yeh, E.T. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 1. J. Am. Coll. Cardiol. 2017, 70, 2536–2551. [Google Scholar] [CrossRef]
- Vallakati, A.; Konda, B.; Lenihan, D.J.; Baliga, R.R. Management of Cancer Therapeutics–Related Cardiac Dysfunction. Heart Fail. Clin. 2018, 14, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B.; Sardão, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Ferrario, C.M.; Mullick, A.E. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacol. Res. 2017, 125, 57–71. [Google Scholar] [CrossRef]
- Metra, M.; Teerlink, J.R. Heart failure. Lancet 2017, 390, 1981–1995. [Google Scholar] [CrossRef]
- Matouk, A.I.; Taye, A.; Heeba, G.H.; El-Moselhy, M.A. Quercetin augments the protective effect of losartan against chronic doxorubicin cardiotoxicity in rats. Environ. Toxicol. Pharmacol. 2013, 36, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Feridooni, T.; Mac Donald, C.; Shao, D.; Yeung, P.; Agu, R.U. Cytoprotective potential of anti-ischemic drugs against chemotherapy-induced cardiotoxicity in H9c2 myoblast cell line. Acta Pharm. 2013, 63, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Michel, L.Y.M.; Balligand, J.-L. New and Emerging Therapies and Targets: Beta-3 Agonists. Handb. Exp. Pharmacol. 2017, 243, 205–223. [Google Scholar] [CrossRef]
- García-Prieto, J.; García-Ruiz, J.M.; Sanz-Rosa, D.; Pun, A.; García-Alvarez, A.; Davidson, S.M.; Fernández-Friera, L.; Nuno-Ayala, M.; Fernández-Jiménez, R.; Bernal, J.A.; et al. β3 adrenergic receptor selective stimulation during ischemia/reperfusion improves cardiac function in translational models through inhibition of mPTP opening in cardiomyocytes. Basic Res. Cardiol. 2014, 109, 422. [Google Scholar] [CrossRef]
- Niu, X.; Watts, V.L.; Cingolani, O.H.; Sivakumaran, V.; Leyton-Mange, J.S.; Ellis, C.L.; Miller, K.L.; Vandegaer, K.; Bedja, D.; Gabrielson, K.L.; et al. Cardioprotective effect of beta-3 adrenergic receptor agonism: Role of neuronal nitric oxide synthase. J. Am. Coll. Cardiol. 2012, 59, 1979–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehvari, N.; da Silva Junior, E.D.; Bengtsson, T.; Hutchinson, D.S. Mirabegron: Potential off target effects and uses beyond the bladder. Br. J. Pharmacol. 2018, 175, 4072–4082. [Google Scholar] [CrossRef] [PubMed]
- Belge, C.; Hammond, J.; Dubois-Deruy, E.; Manoury, B.; Hamelet, J.; Beauloye, C.; Markl, A.; Pouleur, A.-C.; Bertrand, L.; Esfahani, H.; et al. Enhanced expression of β3-adrenoceptors in cardiac myocytes attenuates neurohormone-induced hypertrophic remodeling through nitric oxide synthase. Circulation 2014, 129, 451–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.-Y.; Li, Y.-F.; Zhi-Li, J.; Guo, Y.-Q. Effects of β(3)-adrenoceptor activation on expression of pancreatic adrenoceptors and angiotensin II receptors in ApoE(−/−) mice. Eur. J. Pharmacol. 2015, 764, 134–139. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Y.-F.; Song, J.-Y.; Guo, Y.-Q. Effects of beta3-adrenoceptor activation on the interaction between adrenoceptors and angiotensin II receptors in apolipoprotein E knockout mouse lung. Eur. J. Pharmacol. 2014, 742, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Leblais, V.; Kobzik, L.; Trochu, J.N.; Khandoudi, N.; Bril, A.; Balligand, J.L.; Le Marec, H. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J. Clin. Investig. 1998, 102, 1377–1384. [Google Scholar] [CrossRef]
- Kovács, Z.Z.A.; Szűcs, G.; Freiwan, M.; Kovács, M.G.; Márványkövi, F.M.; Dinh, H.; Siska, A.; Farkas, K.; Kovács, F.; Kriston, A.; et al. Comparison of the antiremodeling effects of losartan and mirabegron in a rat model of uremic cardiomyopathy. Sci. Rep. 2021, 11, 17495. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Axelsson, A.; Hartvig Thomsen, J.; Sørgaard, M.; Kofoed, K.F.; Hasselbalch, R.; Fry, N.A.S.; Valeur, N.; Boesgaard, S.; Gustafsson, F.; et al. The first-in-man randomized trial of a beta3 adrenoceptor agonist in chronic heart failure: The BEAT-HF trial. Eur. J. Heart Fail. 2017, 19, 566–575. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Janssen, P.M.L. Molecular basis of diastolic dysfunction. Heart Fail. Clin. 2008, 4, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Carnicer, R.; Crabtree, M.J.; Sivakumaran, V.; Casadei, B.; Kass, D.A. Nitric oxide synthases in heart failure. Antioxid. Redox Signal. 2013, 18, 1078–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungvári, Z.; Gupte, S.A.; Recchia, F.A.; Bátkai, S.; Pacher, P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr. Vasc. Pharmacol. 2005, 3, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Aroor, A.R.; Hill, M.A.; Sowers, J.R. Role of Renin-Angiotensin-Aldosterone System Activation in Promoting Cardiovascular Fibrosis and Stiffness. Hypertension 2018, 72, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Lódi, M.; Priksz, D.; Fülöp, G.Á.; Bódi, B.; Gyöngyösi, A.; Nagy, L.; Kovács, Á.; Kertész, A.B.; Kocsis, J.; Édes, I.; et al. Advantages of prophylactic versus conventionally scheduled heart failure therapy in an experimental model of doxorubicin-induced cardiomyopathy. J. Transl. Med. 2019, 17, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babaei, H.; Razmaraii, N.; Assadnassab, G.; Mohajjel Nayebi, A.; Azarmi, Y.; Mohammadnejad, D.; Azami, A. Ultrastructural and Echocardiographic Assessment of Chronic Doxorubicin-Induced Cardiotoxicity in Rats. Arch. Razi Inst. 2020, 75, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Baniahmad, B.; Safaeian, L.; Vaseghi, G.; Rabbani, M.; Mohammadi, B. Cardioprotective effect of vanillic acid against doxorubicin-induced cardiotoxicity in rat. Res. Pharm. Sci. 2020, 15, 87–96. [Google Scholar] [CrossRef]
- Eisvand, F.; Imenshahidi, M.; Ghasemzadeh Rahbardar, M.; Tabatabaei Yazdi, S.A.; Rameshrad, M.; Razavi, B.M.; Hosseinzadeh, H. Cardioprotective effects of alpha-mangostin on doxorubicin-induced cardiotoxicity in rats. Phytother. Res. 2022, 36, 506–524. [Google Scholar] [CrossRef]
- Wu, R.; Yao, P.-A.; Wang, H.-L.; Gao, Y.; Yu, H.-L.; Wang, L.; Cui, X.-H.; Xu, X.; Gao, J.-P. Effect of fermented Cordyceps sinensis on doxorubicin-induced cardiotoxicity in rats. Mol. Med. Rep. 2018, 18, 3229–3241. [Google Scholar] [CrossRef] [Green Version]
- Ikewuchi, J.C.; Ikewuchi, C.C.; Ifeanacho, M.O.; Jaja, V.S.; Okezue, E.C.; Jamabo, C.N.; Adeku, K.A. Attenuation of doxorubicin-induced cardiotoxicity in Wistar rats by aqueous leaf-extracts of Chromolaena odorata and Tridax procumbens. J. Ethnopharmacol. 2021, 274, 114004. [Google Scholar] [CrossRef]
- Haybar, H.; Goudarzi, M.; Mehrzadi, S.; Aminzadeh, A.; Khodayar, M.J.; Kalantar, M.; Fatemi, I. Effect of gemfibrozil on cardiotoxicity induced by doxorubicin in male experimental rats. Biomed. Pharmacother. 2019, 109, 530–535. [Google Scholar] [CrossRef]
- Podyacheva, E.Y.; Kushnareva, E.A.; Karpov, A.A.; Toropova, Y.G. Analysis of Models of Doxorubicin-Induced Cardiomyopathy in Rats and Mice. A Modern View From the Perspective of the Pathophysiologist and the Clinician. Front. Pharmacol. 2021, 12, 670479. [Google Scholar] [CrossRef]
- Hanna, A.D.; Lam, A.; Tham, S.; Dulhunty, A.F.; Beard, N.A. Adverse effects of doxorubicin and its metabolic product on cardiac RyR2 and SERCA2A. Mol. Pharmacol. 2014, 86, 438–449. [Google Scholar] [CrossRef] [Green Version]
- Mattila, M.; Koskenvuo, J.; Söderström, M.; Eerola, K.; Savontaus, M. Intramyocardial injection of SERCA2a-expressing lentivirus improves myocardial function in doxorubicin-induced heart failure. J. Gene Med. 2016, 18, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, K.-P.; Liu, X.-C.; Wang, W.; Li, C.; Li, M.; Qiu, C.-G. Valsartan regulates TGF-β/Smads and TGF-β/p38 pathways through lncRNA CHRF to improve doxorubicin-induced heart failure. Arch. Pharm. Res. 2018, 41, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Ivanová, M.; Dovinová, I.; Okruhlicová, L.; Tribulová, N.; Simončíková, P.; Barteková, M.; Vlkovičová, J.; Barančík, M. Chronic cardiotoxicity of doxorubicin involves activation of myocardial and circulating matrix metalloproteinases in rats. Acta Pharmacol. Sin. 2012, 33, 459–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sárközy, M.; Kovács, Z.Z.A.; Kovács, M.G.; Gáspár, R.; Szűcs, G.; Dux, L. Mechanisms and Modulation of Oxidative/Nitrative Stress in Type 4 Cardio-Renal Syndrome and Renal Sarcopenia. Front. Physiol. 2018, 9, 1648. [Google Scholar] [CrossRef]
- Kovács, M.G.; Kovács, Z.Z.A.; Varga, Z.; Szűcs, G.; Freiwan, M.; Farkas, K.; Kővári, B.; Cserni, G.; Kriston, A.; Kovács, F.; et al. Investigation of the Antihypertrophic and Antifibrotic Effects of Losartan in a Rat Model of Radiation-Induced Heart Disease. Int. J. Mol. Sci. 2021, 22, 12963. [Google Scholar] [CrossRef]
- Zong, W.; Yang, X.; Chen, X.; Huang, H.; Zheng, H.; Qin, X.; Yong, Y.; Cao, K.; Huang, J.; Lu, X. Regulation of angiotensin-(1-7) and angiotensin II type 1 receptor by telmisartan and losartan in adriamycin-induced rat heart failure. Acta Pharmacol. Sin. 2011, 32, 1345–1350. [Google Scholar] [CrossRef]
- Luu, A.Z.; Chowdhury, B.; Al-Omran, M.; Teoh, H.; Hess, D.A.; Verma, S. Role of Endothelium in Doxorubicin-Induced Cardiomyopathy. JACC Basic Transl. Sci. 2018, 3, 861–870. [Google Scholar] [CrossRef]
- Klein, I.; Danzi, S. Thyroid disease and the heart. Circulation 2007, 116, 1725–1735. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Kanda, T.; Takahashi, T.; Saegusa, S.; Moriya, J.; Kurabayashi, M. Interleukin-6-induced reciprocal expression of SERCA and natriuretic peptides mRNA in cultured rat ventricular myocytes. J. Int. Med. Res. 2004, 32, 57–61. [Google Scholar] [CrossRef]
- Moreo, A.; Ambrosio, G.; de Chiara, B.; Pu, M.; Tran, T.; Mauri, F.; Raman, S.V. Influence of myocardial fibrosis on left ventricular diastolic function: Noninvasive assessment by cardiac magnetic resonance and echo. Circ. Cardiovasc. Imaging 2009, 2, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Cannavo, A.; Koch, W.J. Targeting β3-Adrenergic Receptors in the Heart: Selective Agonism and β-Blockade. J. Cardiovasc. Pharmacol. 2017, 69, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moniotte, S.; Kobzik, L.; Feron, O.; Trochu, J.N.; Gauthier, C.; Balligand, J.L. Upregulation of beta(3)-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation 2001, 103, 1649–1655. [Google Scholar] [CrossRef] [Green Version]
- Myagmar, B.-E.; Flynn, J.M.; Cowley, P.M.; Swigart, P.M.; Montgomery, M.D.; Thai, K.; Nair, D.; Gupta, R.; Deng, D.X.; Hosoda, C.; et al. Adrenergic Receptors in Individual Ventricular Myocytes: The Beta-1 and Alpha-1B Are in All Cells, the Alpha-1A Is in a Subpopulation, and the Beta-2 and Beta-3 Are Mostly Absent. Circ. Res. 2017, 120, 1103–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germack, R.; Dickenson, J.M. Induction of beta3-adrenergic receptor functional expression following chronic stimulation with noradrenaline in neonatal rat cardiomyocytes. J. Pharmacol. Exp. Ther. 2006, 316, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Balligand, J.-L.; Michel, L.Y.M. Letter by Balligand and Michel Regarding Article, “Adrenergic Receptors in Individual Ventricular Myocytes: The Beta-1 and Alpha-1B Are in All Cells, the Alpha-1A Is in a Subpopulation, and the Beta-2 and Beta-3 Are Mostly Absent”. Circ. Res. 2017, 120, e54–e55. [Google Scholar] [CrossRef]
- Balligand, J.-L. Cardiac salvage by tweaking with beta-3-adrenergic receptors. Cardiovasc. Res. 2016, 111, 128–133. [Google Scholar] [CrossRef]
- Hermida, N.; Michel, L.; Esfahani, H.; Dubois-Deruy, E.; Hammond, J.; Bouzin, C.; Markl, A.; Colin, H.; van Steenbergen, A.; de Meester, C.; et al. Cardiac myocyte β3-adrenergic receptors prevent myocardial fibrosis by modulating oxidant stress-dependent paracrine signaling. Eur. Heart J. 2018, 39, 888–898. [Google Scholar] [CrossRef]
- Hadi, T.; Douhard, R.; Dias, A.M.M.; Wendremaire, M.; Pezzè, M.; Bardou, M.; Sagot, P.; Garrido, C.; Lirussi, F. Beta3 adrenergic receptor stimulation in human macrophages inhibits NADPHoxidase activity and induces catalase expression via PPARγ activation. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1769–1784. [Google Scholar] [CrossRef]
- Lirussi, F.; Rakotoniaina, Z.; Madani, S.; Goirand, F.; Breuiller-Fouché, M.; Leroy, M.-J.; Sagot, P.; Morrison, J.J.; Dumas, M.; Bardou, M. ADRB3 adrenergic receptor is a key regulator of human myometrial apoptosis and inflammation during chorioamnionitis. Biol. Reprod. 2008, 78, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Dhindsa, S.; Ghanim, H.; Chaudhuri, A. Angiotensin II and inflammation: The effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J. Hum. Hypertens. 2007, 21, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Lipsitz, S.R.; Mone, S.M.; Goorin, A.M.; Sallan, S.E.; Sanders, S.P.; Orav, E.J.; Gelber, R.D.; Colan, S.D. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N. Engl. J. Med. 1995, 332, 1738–1743. [Google Scholar] [CrossRef]
- Ashour, A.E.; Sayed-Ahmed, M.M.; Abd-Allah, A.R.; Korashy, H.M.; Maayah, Z.H.; Alkhalidi, H.; Mubarak, M.; Alhaider, A. Metformin rescues the myocardium from doxorubicin-induced energy starvation and mitochondrial damage in rats. Oxid. Med. Cell. Longev. 2012, 2012, 434195. [Google Scholar] [CrossRef] [PubMed]
- Lončar-Turukalo, T.; Vasić, M.; Tasić, T.; Mijatović, G.; Glumac, S.; Bajić, D.; Japunžić-Žigon, N. Heart rate dynamics in doxorubicin-induced cardiomyopathy. Physiol. Meas. 2015, 36, 727–739. [Google Scholar] [CrossRef]
- Merlet, N.; Piriou, N.; Rozec, B.; Grabherr, A.; Lauzier, B.; Trochu, J.-N.; Gauthier, C. Increased beta2-adrenoceptors in doxorubicin-induced cardiomyopathy in rat. PLoS ONE 2013, 8, e64711. [Google Scholar] [CrossRef] [Green Version]
- Medeiros-Lima, D.J.M.; Carvalho, J.J.; Tibirica, E.; Borges, J.P.; Matsuura, C. Time course of cardiomyopathy induced by doxorubicin in rats. Pharmacol. Rep. 2019, 71, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Hydock, D.S.; Lien, C.-Y.; Jensen, B.T.; Parry, T.L.; Schneider, C.M.; Hayward, R. Rehabilitative exercise in a rat model of doxorubicin cardiotoxicity. Exp. Biol. Med. 2012, 237, 1483–1492. [Google Scholar] [CrossRef]
- Amadori, D.; Frassineti, G.L.; Zoli, W.; Milandri, C.; Serra, P.; Tienghi, A.; Ravaioli, A.; Gentile, A.; Salzano, E. Doxorubicin and paclitaxel (sequential combination) in the treatment of advanced breast cancer. Oncology 1997, 11, 30–33. [Google Scholar]
- Armenian, S.; Bhatia, S. Predicting and Preventing Anthracycline-Related Cardiotoxicity. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 3–12. [Google Scholar] [CrossRef]
- Feijen, E.A.M.; Leisenring, W.M.; Stratton, K.L.; Ness, K.K.; van der Pal, H.J.H.; van Dalen, E.C.; Armstrong, G.T.; Aune, G.J.; Green, D.M.; Hudson, M.M.; et al. Derivation of Anthracycline and Anthraquinone Equivalence Ratios to Doxorubicin for Late-Onset Cardiotoxicity. JAMA Oncol. 2019, 5, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Escher, S.E.; Batke, M.; Hoffmann-Doerr, S.; Messinger, H.; Mangelsdorf, I. Interspecies extrapolation based on the RepDose database--a probabilistic approach. Toxicol. Lett. 2013, 218, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; McNeill, J.H. To scale or not to scale: The principles of dose extrapolation. Br. J. Pharmacol. 2009, 157, 907–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sárközy, M.; Márványkövi, F.M.; Szűcs, G.; Kovács, Z.Z.A.; Szabó, M.R.; Gáspár, R.; Siska, A.; Kővári, B.; Cserni, G.; Földesi, I.; et al. Ischemic preconditioning protects the heart against ischemia-reperfusion injury in chronic kidney disease in both males and females. Biol. Sex Differ. 2021, 12, 49. [Google Scholar] [CrossRef]
- Sárközy, M.; Gáspár, R.; Zvara, Á.; Siska, A.; Kővári, B.; Szűcs, G.; Márványkövi, F.; Kovács, M.G.; Diószegi, P.; Bodai, L.; et al. Chronic kidney disease induces left ventricular overexpression of the pro-hypertrophic microRNA-212. Sci. Rep. 2019, 9, 1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sárközy, M.; Gáspár, R.; Zvara, Á.; Kiscsatári, L.; Varga, Z.; Kővári, B.; Kovács, M.G.; Szűcs, G.; Fábián, G.; Diószegi, P.; et al. Selective Heart Irradiation Induces Cardiac Overexpression of the Pro-hypertrophic miR-212. Front. Oncol. 2019, 9, 598. [Google Scholar] [CrossRef] [Green Version]
- Kiscsatári, L.; Sárközy, M.; Kővári, B.; Varga, Z.; Gömöri, K.; Morvay, N.; Leprán, I.; Hegyesi, H.; Fábián, G.; Cserni, B.; et al. High-dose Radiation Induced Heart Damage in a Rat Model. Vivo 2016, 30, 623–631. [Google Scholar]


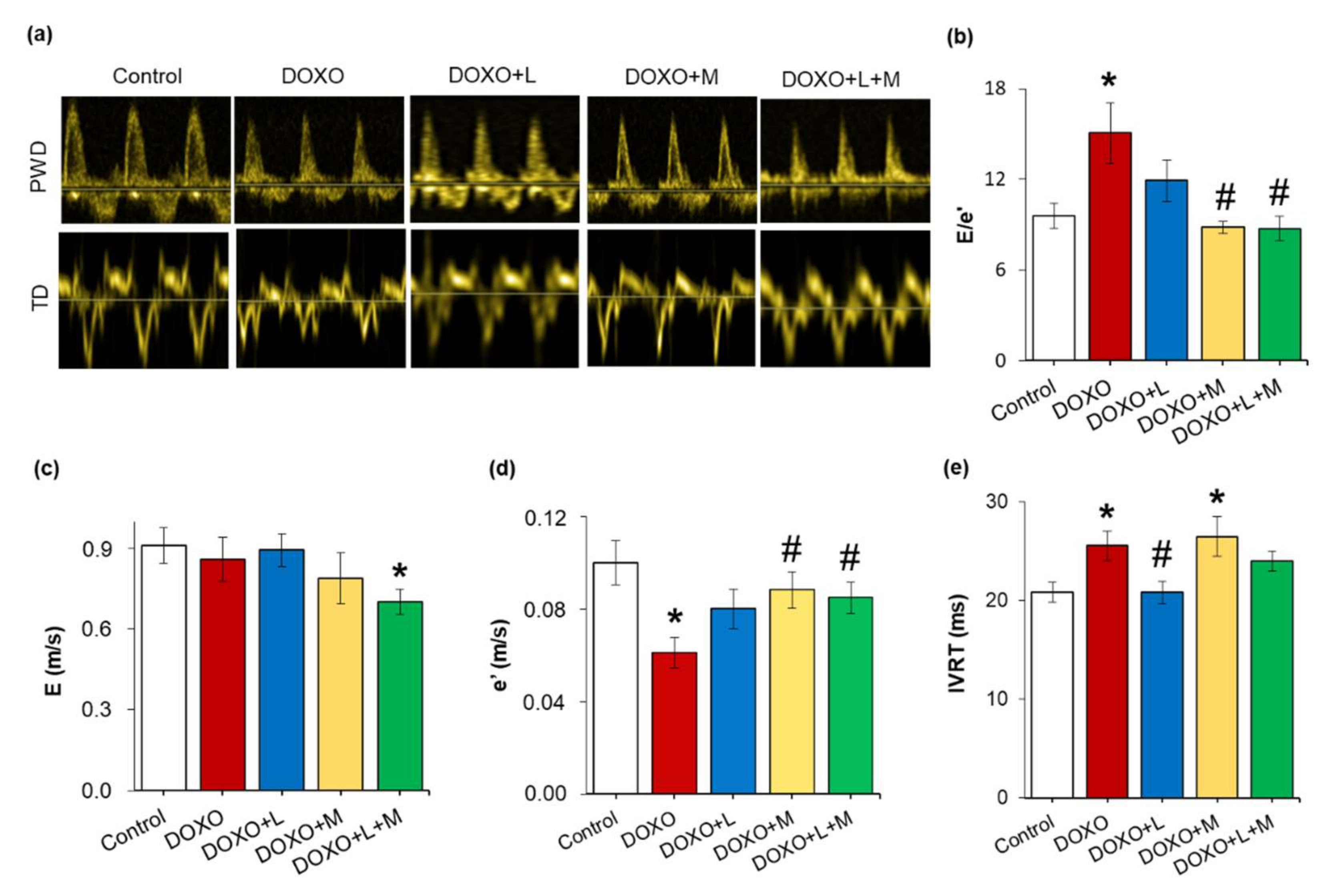

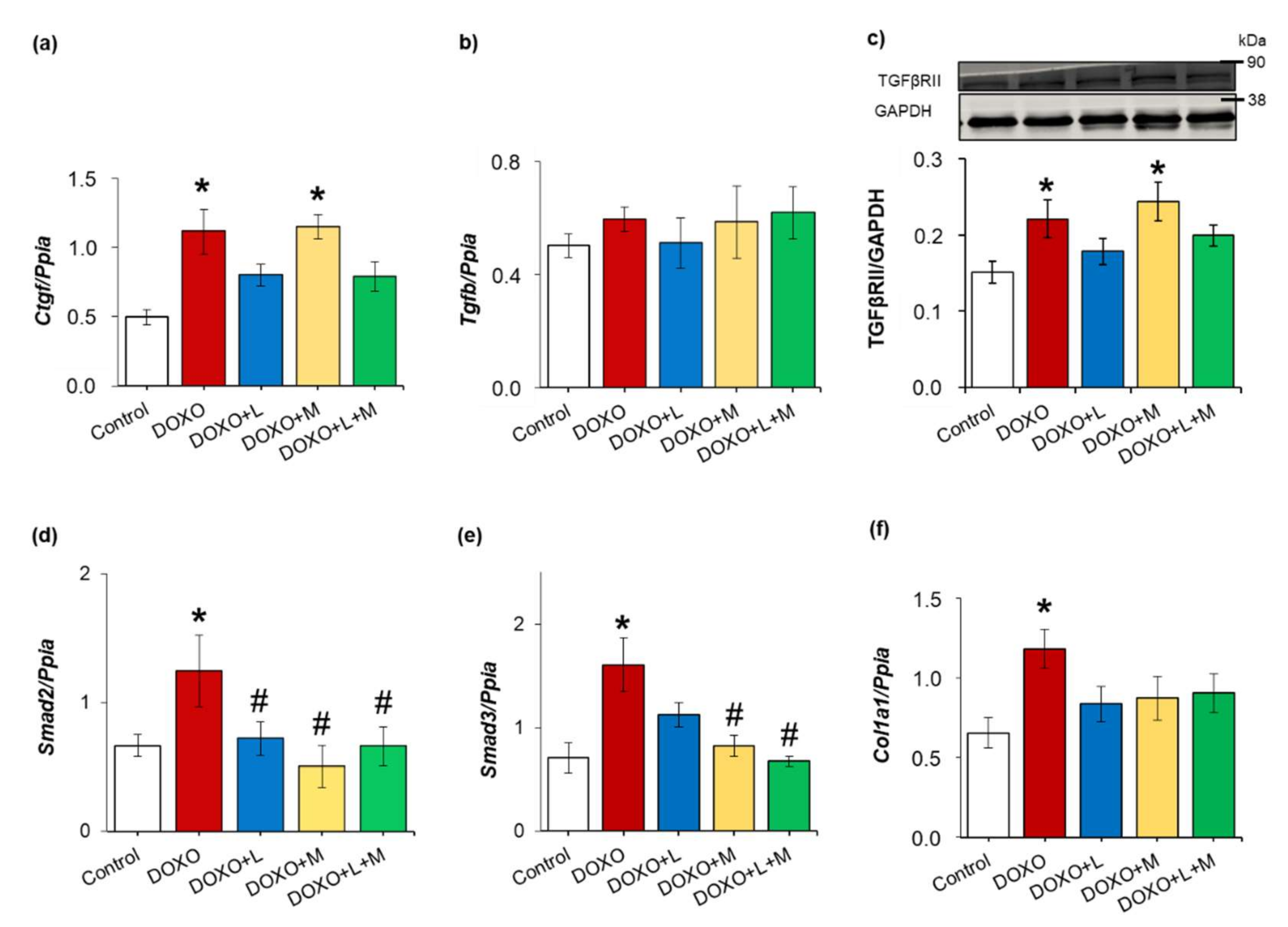


| Parameter (Unit) | Groups | ||||
|---|---|---|---|---|---|
| Control | DOXO | DOXO + L | DOXO + M | DOXO + L + M | |
| SWTs (mm) | 3.57 ± 0.06 | 3.43 ± 0.09 | 3.35 ± 0.19 | 3.53 ± 0.15 | 3.34 ± 0.16 |
| SWTd (mm) | 2.17 ± 0.04 | 1.92 ± 0.09 | 1.90 ± 0.09 | 1.99 ± 0.09 | 1.98 ± 0.14 |
| PWTs (mm) | 3.1 ± 0.11 | 2.82 ± 0.10 | 2.97 ± 0.15 | 3.16 ± 0.12 | 3.09 ± 0.10 |
| PWTd (mm) | 1.76 ± 0.05 | 1.73 ± 0.09 | 1.86 ± 0.07 | 1.91 ± 0.05 | 1.83 ± 0.02 |
| AWTs (mm) | 3.34 ± 0.11 | 3.05 ± 0.13 | 3.09 ± 0.15 | 3.21 ± 0.16 | 3.04 ± 0.12 |
| AWTd (mm) | 2.01 ± 0.08 | 1.84 ± 0.08 | 1.84 ± 0.09 | 1.88 ± 0.07 | 1.82 ± 0.09 |
| IWTs (mm) | 3.09 ± 0.1 | 2.88 ± 0.17 | 2.93 ± 0.11 | 3.01 ± 0.23 | 2.97 ± 0.07 |
| IWTd (mm) | 1.81 ± 0.07 | 1.85 ± 0.15 | 1.81 ± 0.1 | 1.94 ± 0.13 | 1.86 ± 0.08 |
| LVEDD (mm) | 7.02 ± 0.26 | 7.07 ± 0.21 | 7.10 ± 0.19 | 6.72 ± 0.24 | 6.86 ± 0.31 |
| LVESD (mm) | 3.04 ± 0.17 | 3.55 ± 0.23 * | 3.62 ± 0.25 * | 3.41 ± 0.08 * | 3.43 ± 0.31 * |
| FS (%) | 57 ± 1 | 50 ± 2 | 49 ± 3 | 53 ± 2 | 51 ± 3 |
| EF (%) | 90 ± 1 | 85 ± 1 | 84 ± 3 | 88 ± 1 | 86 ± 2 |
| E (m/s) | 0.90 ± 0.04 | 0.92 ± 0.06 | 0.93 ± 0.02 | 0.91 ± 0.08 | 0.92 ± 0.05 |
| e’ (m/s) | 0.089 ± 0.009 | 0.076 ± 0.008 | 0.066 ± 0.007 | 0.064 ± 0.009 | 0.085 ± 0.014 |
| E/e’ | 10 ± 2 | 13 ± 1 | 15 ± 2 | 15 ± 3 | 13 ± 3 |
| IVRT (ms) | 17 ± 0.64 | 18 ± 0.81 | 16 ± 0.65 | 16 ± 0.95 | 17 ± 0.7 |
| IVCT (ms) | 16 ± 0.67 | 15 ± 0.67 | 15 ± 0.47 | 16 ± 0.99 | 15 ± 0.78 |
| HR (1/min) | 365 ± 9 | 368 ± 9 | 363 ± 11 | 382 ± 15 | 381 ± 7 |
| Parameter (Unit) | Groups | ||||
|---|---|---|---|---|---|
| Control | DOXO | DOXO + L | DOXO + M | DOXO + L + M | |
| Body weight before DOXO (g) | 362 ± 10 | 357 ± 8 | 360 ± 11 | 364 ± 9 | 358 ± 9 |
| Body weight after DOXO at week 0 (g) | 394 ± 8 | 353 ± 11 * | 355 ± 9 * | 367 ± 9 * | 363 ± 9 * |
| Body weight at week 9 (g) | 451 ± 7 | 372 ± 19 * | 360 ± 13 * | 351 ± 18 * | 379 ± 11 * |
| Serum carbamide (mmol/L) | 7.76 ± 0.53 | 9.07 ± 0.80 | 7.93 ± 0.74 | 9.66 ± 1.82 | 9.23 ± 1.03 |
| Serum creatinine (μmol/L) | 36 ± 1.91 | 36 ± 3.59 | 35 ± 2.17 | 32 ± 3.44 | 27 ± 1.96 |
| Serum cholesterol (mmol/L) | 1.75 ± 0.07 | 8.91 ± 1.08 * | 6.14 ± 0.93 * | 6.01 ± 1.07 * | 6.96 ± 1.20 * |
| Serum triglyceride (mmol/L) | 0.69 ± 0.06 | 2.94 ± 0.30 * | 2.71 ± 0.50 * | 1.19 ± 0.30 # | 1.79 ± 0.24 * |
| SBP (mmHg) | 148 ± 5 | 146 ± 8 | 119 ± 5 *# | 153 ± 8 | 128 ± 6 |
| DBP (mmHg) | 108 ± 4 | 111 ± 5 | 79 ± 5 *# | 116 ± 8 | 92 ± 4 |
| MBP (mmHg) | 122 ± 4 | 123 ± 7 | 98 ± 4 *# | 131 ± 8 | 106 ± 5 |
| Parameter (Unit) | Groups | ||||
|---|---|---|---|---|---|
| Control | DOXO | DOXO + L | DOXO + M | DOXO + L + M | |
| SWTd (mm) | 2.10 ± 0.04 | 1.69 ± 0.09 * | 1.76 ± 0.1 | 2.32 ± 0.25 # | 1.83 ± 0.1 |
| PWTs (mm) | 3.10 ± 0.09 | 2.52 ± 0.13 * | 2.79 ± 0.18 | 3.01 ± 0.22 | 2.72 ± 0.18 |
| PWTd (mm) | 1.79 ± 0.12 | 1.69 ± 0.11 | 1.90 ± 0.10 | 1.92 ± 0.12 | 1.67 ± 0.13 |
| AWTs (mm) | 3.04 ± 0.10 | 2.57 ± 0.12 * | 2.61 ± 0.19 * | 3.21 ± 0.17 # | 3.31 ± 0.22 # |
| AWTd (mm) | 1.71 ± 0.07 | 1.60 ± 0.06 | 1.63 ± 0.16 | 2.03 ± 0.16 # | 2.02 ± 0.14 # |
| IWTd (mm) | 1.75 ± 0.07 | 1.57 ± 0.13 | 1.65 ± 0.1 | 1.84 ± 0.16 | 1.64 ± 0.1 |
| IVCT (ms) | 16 ± 2 | 21 ± 1 * | 16 ± 1 # | 18 ± 2 | 20 ± 1 |
| HR (1/min) | 371 ± 11 | 347 ± 12 | 349 ± 15 | 343 ± 12 | 335 ± 10 # |
| Parameter (Unit) | Groups | ||||
|---|---|---|---|---|---|
| Control | DOXO | DOXO + L | DOXO + M | DOXO + L + M | |
| Tibia length (cm) | 4.13 ± 0.02 | 4.06 ± 0.03 | 4.04 ± 0.04 | 4.07 ± 0.05 | 4.09 ± 0.03 |
| Heart weight (mg) | 1167 ± 20 | 972 ± 43 * | 989 ± 52 * | 1076 ± 75 | 1011 ± 27 * |
| LV weight (mg) | 838 ± 15 | 693 ± 31 * | 690 ± 36 * | 768 ± 55 | 698 ± 22 * |
| RV weight (mg) | 218 ± 12 | 178 ± 12 * | 190 ± 17 | 181 ± 14 | 184 ± 4 * |
| Lung weight (mg) | 1581 ± 29 | 1497 ± 44 | 1615 ± 60 | 1665 ± 106 | 1684 ± 52 |
| Relative Gene Expression | Groups | ||||
|---|---|---|---|---|---|
| Control | DOXO | DOXO + L | DOXO + M | DOXO + L + M | |
| Mmp2/Ppia | 1.04 ± 0.07 | 1.71 ± 0.22 * | 1.19 ± 0.15 | 1.29 ± 0.18 | 1.12 ± 0.28 |
| Mmp9/Ppia | 0.75 ± 0.06 | 1.5 ± 0.17 * | 1.55 ± 0.28 * | 1.27 ± 0.22 | 0.92 ± 0.08 # |
| Nppa/Ppia | 0.26 ± 0.05 | 1.49 ± 0.28 * | 0.78 ± 0.15 # | 1.59 ± 0.27 * | 0.82 ± 0.16 |
| Nppb/Ppia | 0.75 ± 0.12 | 1.14 ± 0.12 * | 0. 82 ± 0.09 | 1.43 ± 0.12 * | 0.73 ± 0.13 # |
| Bax/Ppia | 0.89 ± 0.19 | 1.69 ± 0.34 | 0.97 ± 0.29 | 0.71 ± 0.08 | 0.68 ± 0.12 |
| Bcl2/Ppia | 0.91 ± 0.08 | 1.01 ± 0.14 | 0.83 ± 0.1 | 0.80 ± 0.05 | 0.80 ± 0.19 |
| Bax/Bcl2 | 0.96 ± 0.18 | 1.63 ± 0.13 * | 1.26 ± 0.26 | 0.87 ± 0.15 # | 1.21 ± 0.34 |
| Relative Gene Expression | Groups | ||||
|---|---|---|---|---|---|
| Control | DOXO | DOXO + L | DOXO + M | DOXO + L + M | |
| Nos1/Ppia | 0.80 ± 0.17 | 0.68 ± 0.07 | 0.7 ± 0.16 | 0.64 ± 0.22 | 0.65 ± 0.05 |
| Nos2/Ppia | 0.48 ± 0.04 | 1.00 ± 0.17 * | 0.75 ± 0.10 * | 0.98 ± 0.2 * | 0.64 ± 0.06 * |
| Nox4/Ppia | 0.96 ± 0.15 | 1.24 ± 0.21 | 1.20 ± 0.39 | 0.24 ± 0.10 * # | 0.38 ± 0.11 # |
| Sod1/Ppia | 1.05 ± 0.05 | 0.97 ± 0.06 | 0.96 ± 0.13 | 0.78 ± 0.15 | 0.62 ± 0.09 * |
| Sod2/Ppia | 0.94 ± 0.05 | 0.88 ± 0.06 | 0.80 ± 0.09 | 0.82 ± 0.09 | 0.51 ± 0.07 * # |
| Sod3/Ppia | 1.21 ± 0.15 | 1.09 ± 0.2 | 0.96 ± 0.21 | 0.54 ± 0.17 * | 0.41 ± 0.09 * # |
| Cat/Ppia | 0.46 ± 0.04 | 0.43 ± 0.03 | 0.48 ± 0.08 | 0.37 ± 0.04 | 0.24 ± 0.04 * # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freiwan, M.; Kovács, M.G.; Kovács, Z.Z.A.; Szűcs, G.; Dinh, H.; Losonczi, R.; Siska, A.; Kriston, A.; Kovács, F.; Horváth, P.; et al. Investigation of the Antiremodeling Effects of Losartan, Mirabegron and Their Combination on the Development of Doxorubicin-Induced Chronic Cardiotoxicity in a Rat Model. Int. J. Mol. Sci. 2022, 23, 2201. https://doi.org/10.3390/ijms23042201
Freiwan M, Kovács MG, Kovács ZZA, Szűcs G, Dinh H, Losonczi R, Siska A, Kriston A, Kovács F, Horváth P, et al. Investigation of the Antiremodeling Effects of Losartan, Mirabegron and Their Combination on the Development of Doxorubicin-Induced Chronic Cardiotoxicity in a Rat Model. International Journal of Molecular Sciences. 2022; 23(4):2201. https://doi.org/10.3390/ijms23042201
Chicago/Turabian StyleFreiwan, Marah, Mónika G. Kovács, Zsuzsanna Z. A. Kovács, Gergő Szűcs, Hoa Dinh, Réka Losonczi, Andrea Siska, András Kriston, Ferenc Kovács, Péter Horváth, and et al. 2022. "Investigation of the Antiremodeling Effects of Losartan, Mirabegron and Their Combination on the Development of Doxorubicin-Induced Chronic Cardiotoxicity in a Rat Model" International Journal of Molecular Sciences 23, no. 4: 2201. https://doi.org/10.3390/ijms23042201
APA StyleFreiwan, M., Kovács, M. G., Kovács, Z. Z. A., Szűcs, G., Dinh, H., Losonczi, R., Siska, A., Kriston, A., Kovács, F., Horváth, P., Földesi, I., Cserni, G., Dux, L., Csont, T., & Sárközy, M. (2022). Investigation of the Antiremodeling Effects of Losartan, Mirabegron and Their Combination on the Development of Doxorubicin-Induced Chronic Cardiotoxicity in a Rat Model. International Journal of Molecular Sciences, 23(4), 2201. https://doi.org/10.3390/ijms23042201








