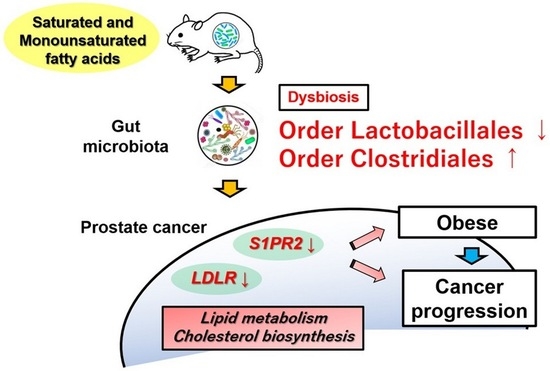
Specific Gut Microbial Environment in Lard Diet-Induced Prostate Cancer Development and Progression
Abstract
:1. Introduction
2. Results
2.1. SMFA Induces PCa Progression along with Weight Gain in Two Immunocompetent Mouse Models
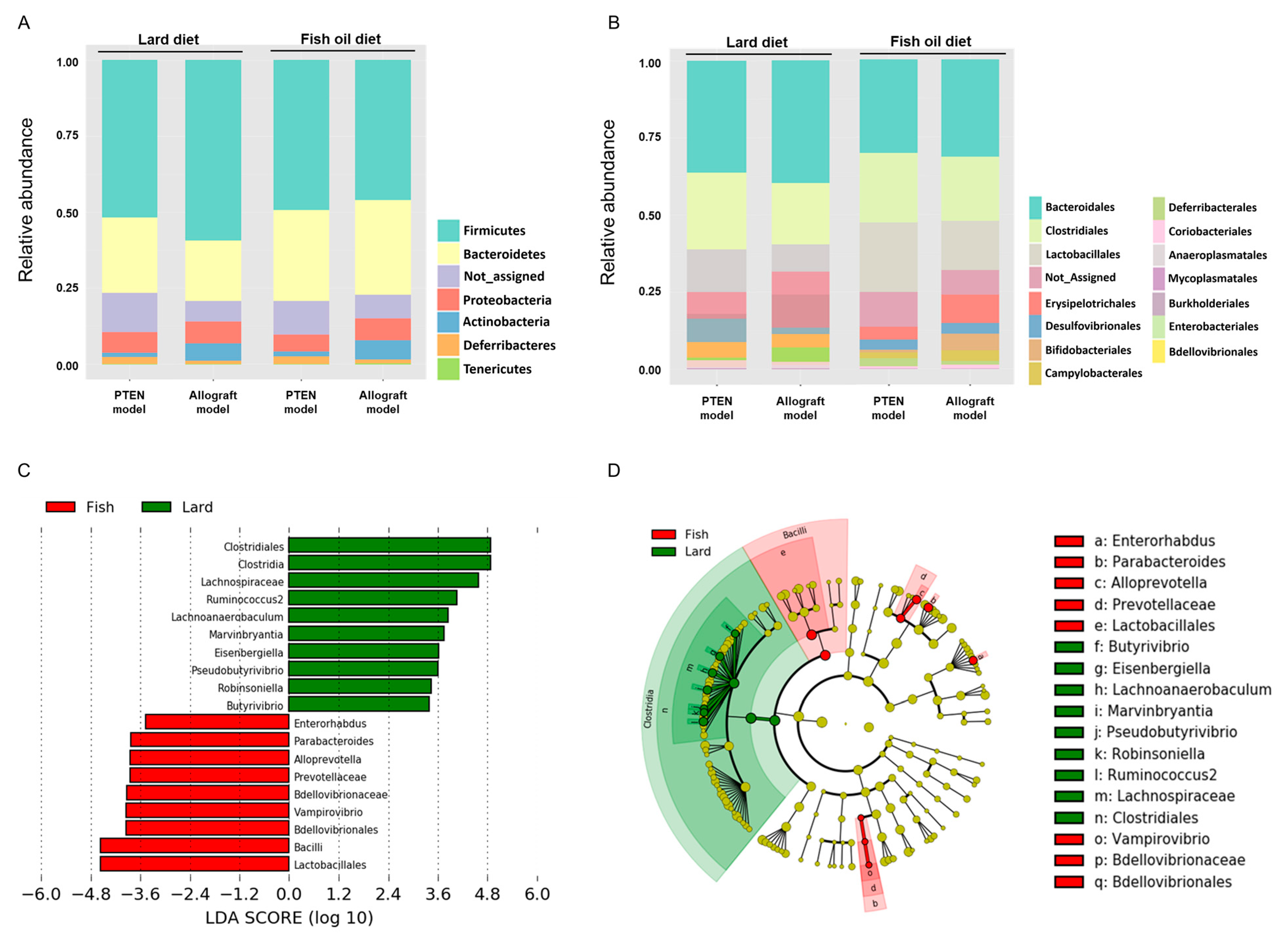
2.2. Gut Microbial Divergence in Mice Fed with LD and FOD
2.3. Taxonomic Differences of Gut Microbiota in the LD and FOD Groups
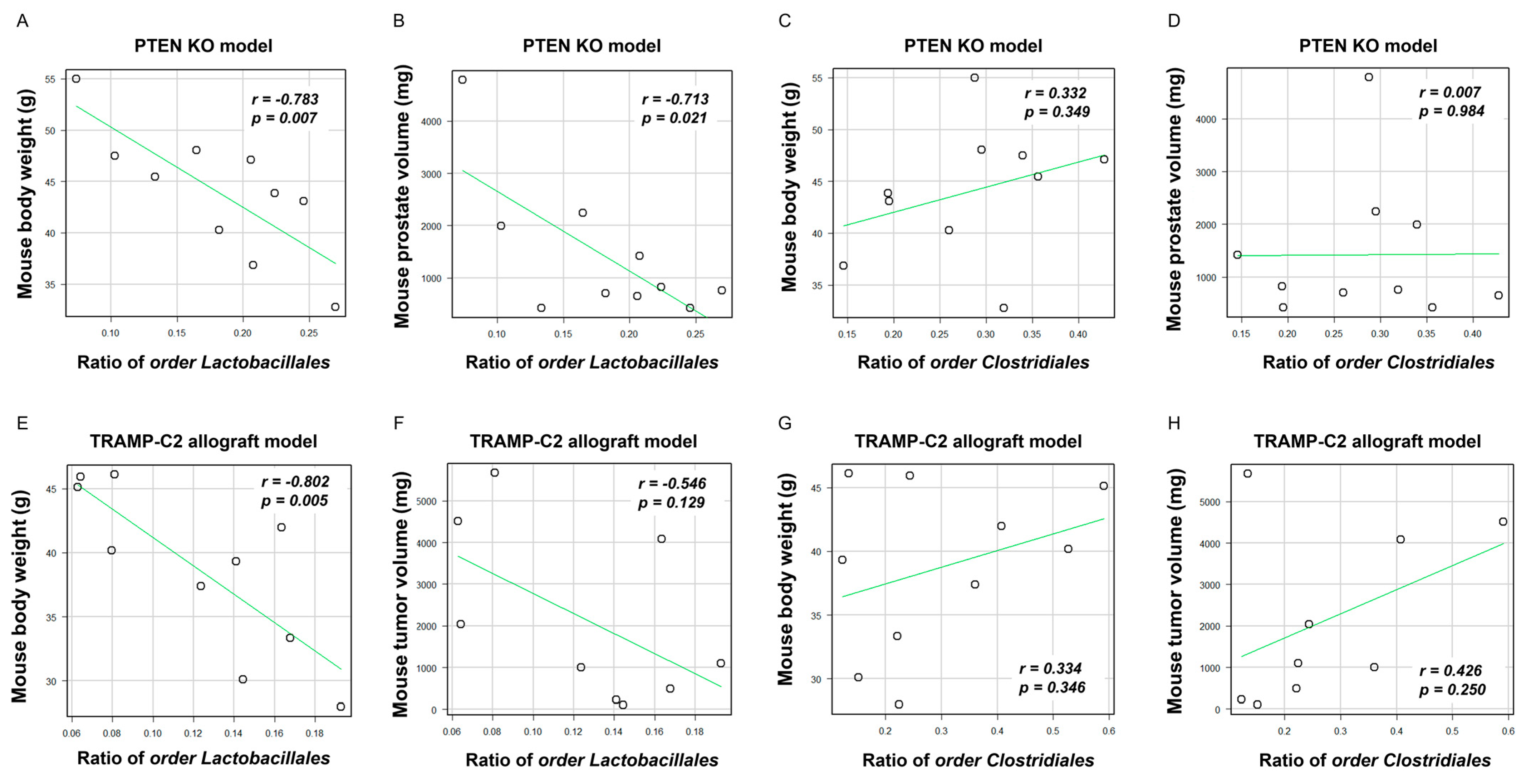
2.4. Specific Gut Microbial Species Correlated with SMFA-Induced Weight Gain and PCa Growth
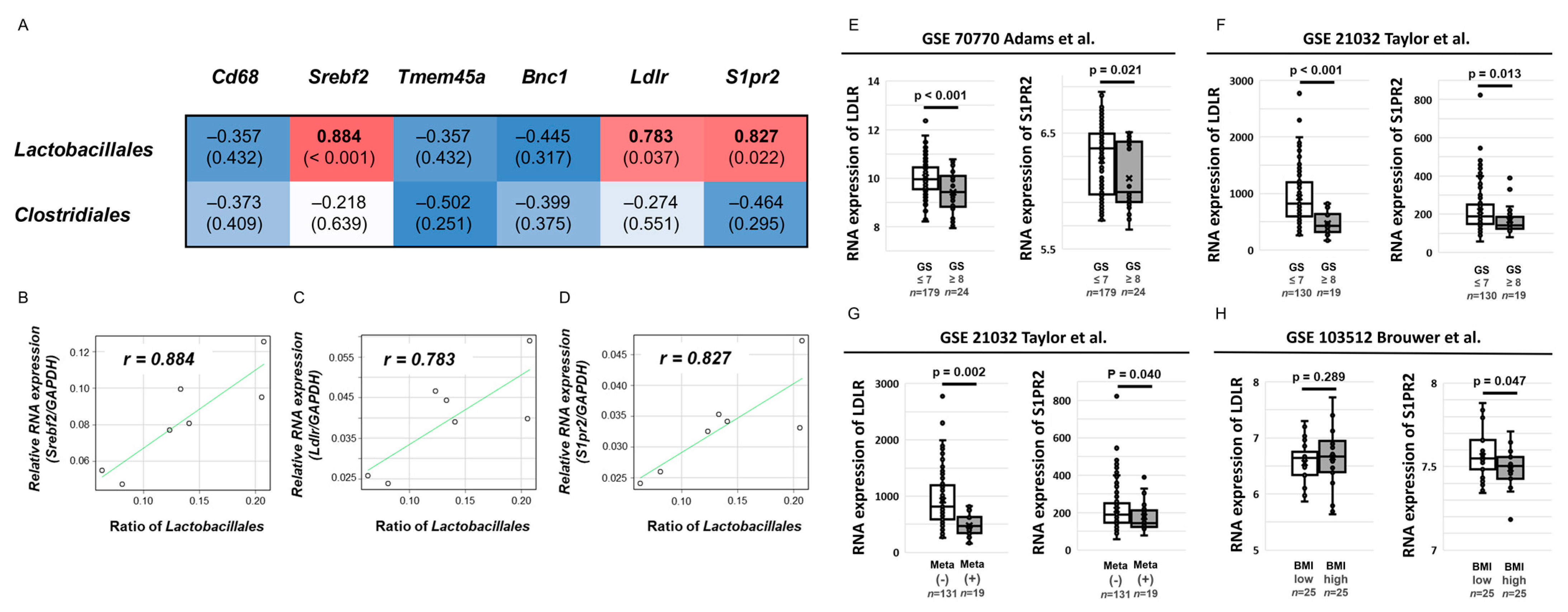
2.5. Relationship between Comprehensive Gene Expression in the Prostate and Allograft Tumors and Abundance of the Orders Lactobacillales and Clostridiales in the LD and FOD Groups
2.6. Clinical Effect of S1PR2 and LDLR Expression in Human PCa
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Animals
4.3. Diets
4.4. Design of Pten KO Mice Experiments
4.5. Design of TRAMP-C2 Allograft Mice Experiments
4.6. Fecal Collection, 16S Ribosomal RNA Sequencing, and Data Processing
4.7. RNA Extractions and Microarray Analysis
4.8. Bioinformatic Analysis in Human PCa Tissues
4.9. Statical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narita, S.; Nara, T.; Sato, H.; Koizumi, A.; Huang, M.; Inoue, T.; Habuchi, T. Research Evidence on High-Fat Diet-Induced Prostate Cancer Development and Progression. J. Clin. Med. 2019, 8, 597. [Google Scholar] [CrossRef] [Green Version]
- Allott, E.H.; Masko, E.M.; Freedland, S.J. Obesity and prostate cancer: Weighing the evidence. Eur. Urol. 2013, 63, 800–809. [Google Scholar] [CrossRef] [Green Version]
- Venkateswaran, V.; Klotz, L.H. Diet and prostate cancer: Mechanisms of action and implications for chemoprevention. Nat. Rev. Urol. 2010, 7, 442–453. [Google Scholar] [CrossRef]
- Kurahashi, N.; Inoue, M.; Iwasaki, M.; Sasazuki, S.; Tsugane, S. Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men. Cancer Epidemiol. Biomark. Prev. 2008, 17, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Pelser, C.; Mondul, A.M.; Hollenbeck, A.R.; Park, Y. Dietary fat, fatty acids, and risk of prostate cancer in the NIH-AARP diet and health study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, A.; Narita, S.; Nakanishi, H.; Ishikawa, M.; Eguchi, S.; Kimura, H.; Takasuga, S.; Huang, M.; Inoue, T.; Sasaki, J.; et al. Increased fatty acyl saturation of phosphatidylinositol phosphates in prostate cancer progression. Sci. Rep. 2019, 9, 13257. [Google Scholar] [CrossRef] [Green Version]
- Narita, S.; Tsuchiya, N.; Saito, M.; Inoue, T.; Kumazawa, T.; Yuasa, T.; Nakamura, A.; Habuchi, T. Candidate genes involved in enhanced growth of human prostate cancer under high fat feeding identified by microarray analysis. Prostate. 2008, 68, 321–335. [Google Scholar] [CrossRef]
- Huang, M.; Koizumi, A.; Narita, S.; Inoue, T.; Tsuchiya, N.; Nakanishi, H.; Numakura, K.; Tsuruta, H.; Saito, M.; Satoh, S.; et al. Diet-induced alteration of fatty acid synthase in prostate cancer progression. Oncogenesis. 2016, 5, e195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Fujita, K.; Nojima, S.; Hayashi, Y.; Nakano, K.; Shizuya, Y.; Wang, C.; Yamamoto, Y.; Kinouchi, T.; Matsuzaki, K.; et al. High-Fat Diet-Induced Inflammation Accelerates Prostate Cancer Growth via IL6 Signaling. Clin. Cancer Res. 2018, 24, 4309–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schimer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Horst, R.T.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell. 2016, 167, 125–1136. [Google Scholar] [CrossRef] [Green Version]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and fecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomized controlled-feeding trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ushiroda, C.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Higashimura, Y.; Yasukawa, Z.; Okubo, T.; Inoue, R.; Honda, A.; et al. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J. Clin. Biochem. Nutr. 2019, 65, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Liss, M.A.; White, J.R.; Goros, M.; Gelfond, J.; Leach, R.; Johnson-Pais, T.; Lai, Z.; Rourke, E.; Basler, J.; Ankerst, D.; et al. Metabolic Biosynthesis Pathways Identified from Fecal Microbiome Associated with Prostate Cancer. Eur. Urol. 2018, 74, 575–582. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Markowski, M.C.; Peiffer, L.B.; Ernst, S.E.; White, J.R.; Pienta, K.J.; Antonarakis, E.S.; Ross, A.E. Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis. 2018, 21, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernigoni, N.; Zagato, E.; Calcinotto, A.; Troiani, M.; Mestre, R.P.; Calì, C.; Attanasio, G.; Troisi, J.; Minini, M.; Mosole, S.; et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science 2021, 74, 216–224. [Google Scholar] [CrossRef]
- Matsushita, M.; Fujita, K.; Hayashi, T.; Kayama, H.; Motooka, D.; Hase, H.; Jingushi, K.; Yamamichi, G.; Yumiba, S.; Tomiyama, E.; et al. Gut Microbiota-Drived Short-Chain Fatty Acids Promote Prostate Cancer Growth via IGF1 Signaling. Cancer Res. 2021, 81, 4014–4026. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Madison, B.B. Srebp2: A master regulator of sterol and fatty acid synthesis. J. Lipid Res. 2016, 57, 333–335. [Google Scholar] [CrossRef] [Green Version]
- Schmit, K.; Michiels, C. TMEM Proteins in Cancer: A Review. Front. Pharmacol. 2018, 9, 1345. [Google Scholar] [CrossRef] [Green Version]
- Price, M.P.; Lewin, G.R.; McIlwrath, S.L.; Cheng, C.; Xie, J.; Heppenstall, P.A.; Stucky, C.L.; Mannsfeldt, A.G.; Brennan, T.J.; Drummond, H.A.; et al. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 2000, 407, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Go, G.W.; Mani, A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J. Biol. Med. 2012, 85, 19–28. [Google Scholar]
- Adada, M.; Canals, D.; Hannun, Y.A.; Obeid, L.M. Sphingosine-1-phosphate receptor 2. FEBS J. 2013, 280, 6354–6366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vourakis, M.; Mayer, G.; Rousseau, G. The Role of Gut Microbiota on Cholesterol Metabolism in Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 8074. [Google Scholar] [CrossRef] [PubMed]
- Kwong, E.K.; Zhou, H. Sphingosine-1-phoshate signaling and the gut-liver axis in liver diseases. Liver Res. 2019, 3, 19–24. [Google Scholar] [CrossRef]
- Ngo, T.H.; Barnard, R.J.; Cohen, P.; Freedland, S.; Tran, C.; de Gregorio, F.; Elshimali, Y.I.; Heber, D.; Aronson, W.J. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin. Cancer Res. 2003, 9, 2734–2743. [Google Scholar]
- Buettner, R.; Scholmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Llaverias, G.; Danilo, C.; Wang, Y.; Witkiewicz, A.K.; Daumer, K.; Lisanti, M.P.; Frank, P.G. A Western-type diet accelerates tumor progression in an autochthonous mouse model of prostate cancer. Am. J. Pathol. 2010, 177, 3180–3191. [Google Scholar] [CrossRef]
- Lloyd, J.C.; Antonelli, J.A.; Phillips, T.E.; Masko, E.M.; Thomas, J.A.; Poulton, S.H.; Pollak, M.; Freedland, S.J. Effect of isocaloric low fat diet on prostate cancer xenograft progression in a hormone deprivation model. J. Urol. 2010, 183, 1619–1624. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Chen, C.Y.; Wen, H.C.; Huang, C.Y.; Hung, M.S.; Lu, H.C.; Chen, W.L.; Chang, C.H. Caveolin-1 Secreted from Adipose Tissues and Adipocytes Functions as an Adipogenesis Enhancer. Obesity 2017, 25, 1932–1940. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Zhang, J.; Sampieri, K.; Clohessy, J.G.; Mendez, L.; Gonzalez-Billalabeitia, E.; Liu, X.S.; Lee, Y.R.; Fung, J.; Katon, J.M.; et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat. Genet. 2018, 50, 206–218. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Corr, J.G.; Tao, Y.; Fair, W.R.; Heston, W.D. Decreased growth of established human prostate LNCap tumors in nude mice fed a low-fat diet. J. Natl. Cancer Inst. 1995, 87, 1456–1462. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef] [Green Version]
- Liotti, A.; Cosimato, V.; Mirra, P.; Cali, G.; Conza, D.; Secondo, A.; Luongo, G.; Terracciano, D.; Forosamp, P.; Beguinot, F.; et al. Oleic acid promotes prostate cancer malignant phenotype via the G protein-coupled receptor FFA1/GPR40. Jwth of established human prostate LNCap tumors in nude mice fed a low-fat diet. J. Cell Physiol. 2018, 233, 7367–7378. [Google Scholar] [CrossRef]
- Caesar, R.; Tremarolo, V.; Kovatchva-Datchary, P.; Cani, P.D.; Backhed, F. Crosstalk Between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.S.; Fruge, A.D.; Pol, W.; Caston, N.E.; Morrow, C.D.; Demark-Wahnefried, W.; Carson, T.L. Gut microbial differences in breast and prostate cancer cases from two randomized controlled trials compared to matched cancer-free controls. Benef. Microbes. 2021, 12, 39–248. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.; Zhang, A.; Jiang, H. Gut Microbiota Dysbiosis Accelerate Prostate Cancer Progression through Increased LPCAT1 Expression and Enhanced DNA Repair Pathways. Front. Oncol. 2021, 11, 679712. [Google Scholar] [CrossRef]
- Lucki, N.C.; Sewer, M.B. Multiple roles for sphingolipids in steroid hormone biosynthesis. Subcell Biochem. 2008, 49, 387–412. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, X.; Jiang, H. High dietary fat intake lower serum equol concentration and promotes prostate carcinogenesis in a transgenic mouse prostate model. Nutr. Metab. 2019, 16, 24. [Google Scholar] [CrossRef]
- Kang, Y.; Kang, X.; Yang, H.; Liu, H.; Yang, X.; Liu, Q.; Tian, H.; Xue, Y.; Ren, P.; Kuang, X.; et al. Lactobacillus acidophilus ameliorates obesity in mice through modulation of gut microbiota dysbiosis and intestinal permeability. Phamacol. Res. 2022, 175, 106020. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Visitchanakun, P.; Saisorn, W.; Kanacharoen, S.; Wongsaroj, L.; Kullapanich, C.; Ngamwongsatit, N.; Settachaimongkon, S.; Somboonna, N.; et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci. Rep. 2021, 11, 6367. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, H.U. Effects of potential symbiotic interaction between Lactobacillus rhamnosus GG and salicylic acid on human colon and prostate cancer cells. Arch. Microbiol. 2021, 203, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Sen, P.; Alves, M.A.; Ribeiro, H.C.; Raunioniemi, P.; Hyotylainen, T.; Oresic, M. Linking Gut Microbiome and Lipid Metabolism; Moving beyond Associations. Metabolites 2021, 11, 55. [Google Scholar] [CrossRef]
- Takuwa, N.; Du, W.; Okamoto, Y.; Yoshioka, K.; Takuwa, Y. Tumor-suppressive sphingosine-1-phosphate receptor-2 counteracting tumor-promoting sphingosine-1-phosphate receptor-1 and sphingosine kinase1-Jekyll Hidden behind Hyde. Am. J. Cancer Res. 2011, 1, 460–481. [Google Scholar]
- Ren, S.; Shao, Y.; Zhao, X.; Hong, C.S.; Wang, F.; Lu, X.; Li, J.; Ye, G.; Yan, M.; Zhuang, Z.; et al. Integration of Metabolomics and Transcriptomics Reveals Major Metabolic Pathways and Potential Biomarker Involved in Prostate Cancer. Mol. Cell. Proteomics 2016, 15, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, B.O.; Backhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef]
- Foster, B.A.; Gingrich, J.R.; Kwon, E.D.; Greenberg, N.M. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997, 57, 3325–3330. [Google Scholar] [PubMed]
- Wu, X.; Huang, J.; Powell, W.C.; Zhang, J.; Matusik, R.J.; Sangiorgi, F.O.; Maxson, R.E.; Sucov, H.M.; Roy-Burman, P. Generation of a prostate epithelial cell-specific gene ablation. Mech. Dev. 2001, 102, 61–69. [Google Scholar] [CrossRef]
- Trotman, L.C.; Niki, M.; Dotan, Z.A.; Koutcher, J.A.; Cristofano, A.D.; Xiao, A.; Khoo, A.S.; Roy-Burman, P.; Greenberg, N.M.; Dyke, T.V.; et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003, 1, e59. [Google Scholar] [CrossRef]
- Mueller, S.; Saunier, K.; Hanisch, C.; Norin, E.; Alm, L.; Midtvedt, T.; Cresci, A.; Silvi, S.; Orpianesi, C.; Cristina, M.; et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: A cross-sectional study. Appl. Environ. Microbiol. 2006, 72, 1027–1103. [Google Scholar] [CrossRef] [Green Version]


| Gene Symbol | Gene Description | PTEN KO Model | TRAMP-C2 Allograft Model | ||
|---|---|---|---|---|---|
| Abundance Ratio(Lard/Fish) | p-Value | Abundance Ratio (Lard/Fish) | p-Value | ||
| 2010005H15Rik | RIKEN cDNA 2010005H15 gene | 1.468 | 0.05 | 1.399 | 0.008 |
| Gm22043 | predicted gene, 22043 | 1.351 | 0.012 | 1.252 | 0.02 |
| D130007C19Rik | RIKEN cDNA D130007C19 gene | 1.309 | 0.016 | 1.323 | 0.019 |
| n-R5s220 | nuclear encoded rRNA 5S 220 | 0.736 | 0.007 | 0.711 | 0.029 |
| Cd68 | CD68 antigen | 0.679 | 0.042 | 0.779 | <0.001 |
| Srebf2 | sterol regulatory element binding factor 2 | 0.754 | 0.002 | 0.655 | 0.022 |
| Tmem45a | transmembrane protein 45a | 0.662 | 0.025 | 0.742 | 0.007 |
| Bnc1 | basonuclin 1 | 0.668 | 0.048 | 0.64 | 0.024 |
| Ldlr | low density lipoprotein receptor | 0.661 | 0.014 | 0.604 | 0.011 |
| S1pr2 | sphingosine-1-phosphate receptor 2 | 0.746 | 0.005 | 0.759 | 0.026 |
| Wiki Pathway | Changed Genes | Total Genes | Z Score | p-Value |
|---|---|---|---|---|
| Cholesterol Bio synthesis | 5 | 15 | 9.62 | 1.27 × 10−5 |
| Cholesterol metabolism (includes both Bloch and Kandutsch-Russell pathways) | 8 | 48 | 8.16 | 2.53 × 10−6 |
| Matrix Metalloproteinases | 5 | 28 | 6.72 | 1.64 × 10−4 |
| SREBF and miR33 in cholesterol and lipid homeostasis | 3 | 11 | 6.66 | 1.32 × 10−3 |
| Endochondral Ossification | 7 | 62 | 5.95 | 1.10 × 10−4 |
| Lung fibrosis | 6 | 61 | 5.01 | 6.86 × 10−4 |
| Prostaglandin Synthesis and Regulation | 4 | 31 | 4.91 | 2.31 × 10−3 |
| Eicosanoid Synthesis | 2 | 18 | 3.15 | 4.10 × 10−2 |
| Small Ligand GPCRs | 2 | 18 | 3.15 | 4.10 × 10−2 |
| Statin Pathway | 2 | 19 | 3.03 | 4.49 × 10−2 |
| Retinol metabolism | 3 | 39 | 2.96 | 3.01 × 10−2 |
| Splicing factor NOVA regulated synaptic proteins | 3 | 42 | 2.79 | 3.60 × 10−2 |
| Adipogenesis genes | 6 | 133 | 2.58 | 2.47 × 10−2 |
| GO Term | Changed Genes | Total Genes | Z Score | p-Value |
|---|---|---|---|---|
| lipid metabolic process | 15 | 469 | 5.97 | 4.31 × 10−6 |
| aging | 7 | 165 | 5.07 | 3.67 × 10−4 |
| membrane organization | 2 | 32 | 3.52 | 2.82 × 10−2 |
| cell death | 2 | 35 | 3.32 | 3.30 × 10−2 |
| cell adhesion | 10 | 507 | 3.06 | 6.55 × 10−3 |
| embryo development | 3 | 80 | 3.03 | 2.63 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, H.; Narita, S.; Ishida, M.; Takahashi, Y.; Mingguo, H.; Kashima, S.; Yamamoto, R.; Koizumi, A.; Nara, T.; Numakura, K.; et al. Specific Gut Microbial Environment in Lard Diet-Induced Prostate Cancer Development and Progression. Int. J. Mol. Sci. 2022, 23, 2214. https://doi.org/10.3390/ijms23042214
Sato H, Narita S, Ishida M, Takahashi Y, Mingguo H, Kashima S, Yamamoto R, Koizumi A, Nara T, Numakura K, et al. Specific Gut Microbial Environment in Lard Diet-Induced Prostate Cancer Development and Progression. International Journal of Molecular Sciences. 2022; 23(4):2214. https://doi.org/10.3390/ijms23042214
Chicago/Turabian StyleSato, Hiromi, Shintaro Narita, Masanori Ishida, Yoshiko Takahashi, Huang Mingguo, Soki Kashima, Ryohei Yamamoto, Atsushi Koizumi, Taketoshi Nara, Kazuyuki Numakura, and et al. 2022. "Specific Gut Microbial Environment in Lard Diet-Induced Prostate Cancer Development and Progression" International Journal of Molecular Sciences 23, no. 4: 2214. https://doi.org/10.3390/ijms23042214