Blood–Brain Barrier Disruption Mediated by FFA1 Receptor—Evidence Using Miniscope
Abstract
:1. Introduction
2. Results
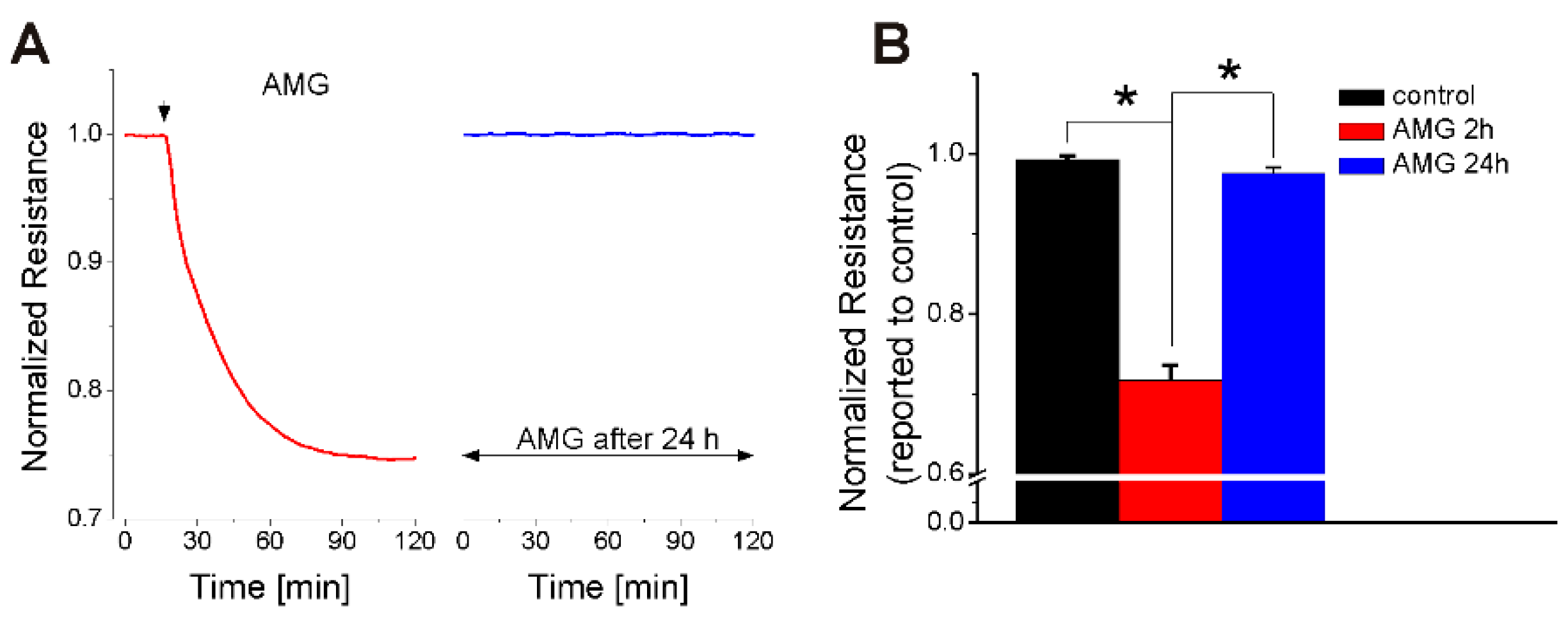
2.1. FFA1 Activation Produces a Transient Decrease in RBMVEC Monolayer Resistance
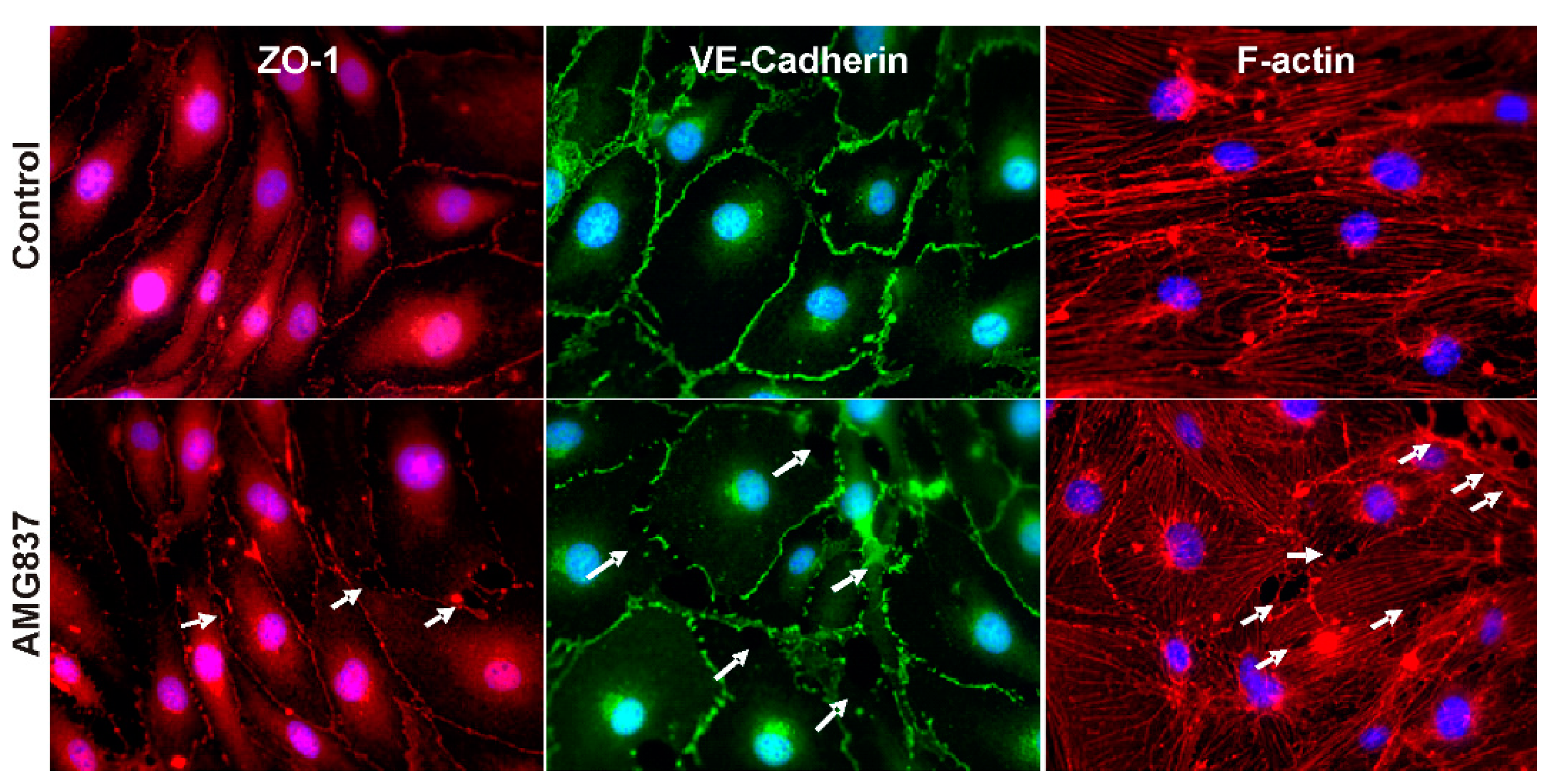
2.2. AMG837 Alters RBMVEC Tight and Adherens Junctions and Cytoskeleton
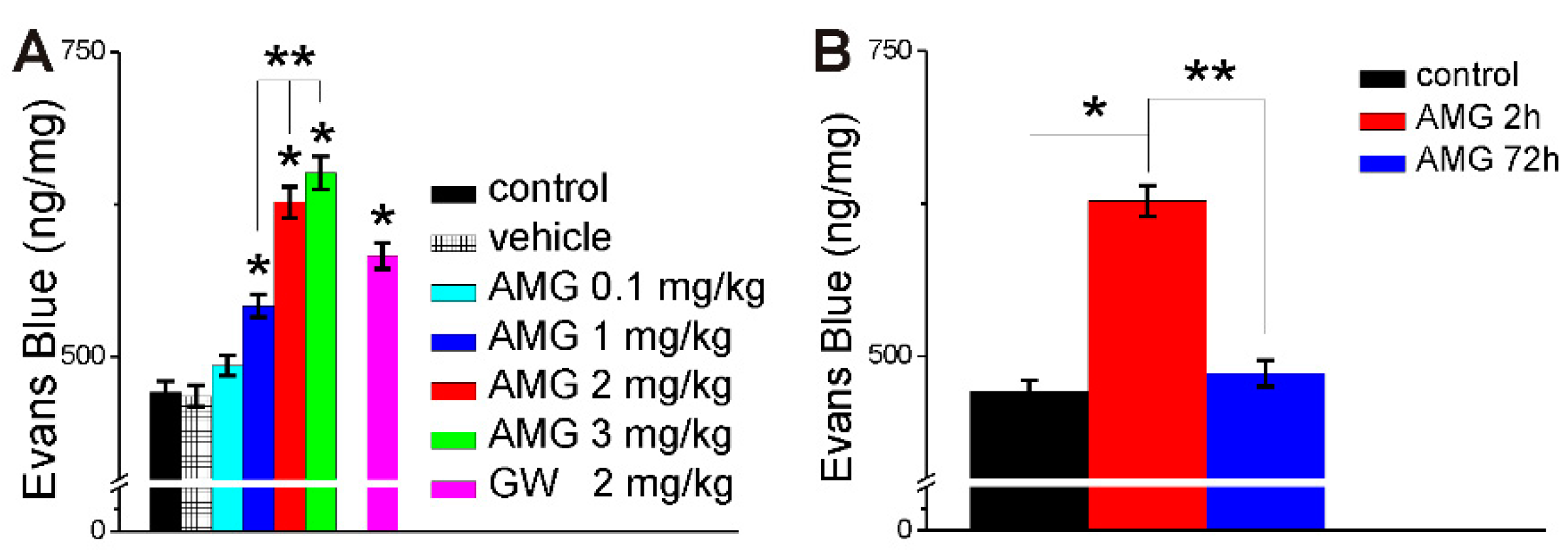
2.3. AMG837 Increases Brain Evans Blue Extravasation
2.4. AMG837 Increases Sodium Fluorescein Extravasation Visualized with Miniscope
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Cell Culture
4.4. Impedance Measurements
4.5. Immunofluorescence
4.6. Evans Blue Extravasation Method
4.7. Assessment of In Vivo BBB Permeability Using Miniaturized Fluorescence Microscopy (Miniscope)
4.8. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl. Health Stat. Rep. 2015, 79, 1–16. [Google Scholar]
- Black, L.I.; Clarke, T.C.; Barnes, P.M.; Stussman, B.J.; Nahin, R.L. Use of complementary health approaches among children aged 4-17 years in the United States: National Health Interview Survey, 2007-2012. Natl. Health Stat. Rep. 2015, 78, 1–19. [Google Scholar]
- Van Gelder, B.M.; Tijhuis, M.; Kalmijn, S.; Kromhout, D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: The Zutphen Elderly Study. Am. J. Clin. Nutr. 2007, 85, 1142–1147. [Google Scholar] [CrossRef]
- Nilsson, A.; Radeborg, K.; Salo, I.; Bjorck, I. Effects of supplementation with n-3 polyunsaturated fatty acids on cognitive performance and cardiometabolic risk markers in healthy 51 to 72 years old subjects: A randomized controlled cross-over study. Nutr. J. 2012, 11, 99. [Google Scholar] [CrossRef] [Green Version]
- D’Ascoli, T.A.; Mursu, J.; Voutilainen, S.; Kauhanen, J.; Tuomainen, T.P.; Virtanen, J.K. Association between serum long-chain omega-3 polyunsaturated fatty acids and cognitive performance in elderly men and women: The Kuopio Ischaemic Heart Disease Risk Factor Study. Eur. J. Clin. Nutr. 2016, 70, 970–975. [Google Scholar] [CrossRef]
- Ozawa, H.; Miyazawa, T.; Miyazawa, T. Effects of Dietary Food Components on Cognitive Functions in Older Adults. Nutrients 2021, 13, 2804. [Google Scholar] [CrossRef]
- Van der Burg, K.P.; Cribb, L.; Firth, J.; Karmacoska, D.; Mischoulon, D.; Byrne, G.J.; Bousman, C.; Stough, C.; Murphy, J.; Oliver, G.; et al. EPA and DHA as markers of nutraceutical treatment response in major depressive disorder. Eur. J. Nutr. 2019, 59, 2439–2447. [Google Scholar] [CrossRef]
- Van Dael, P. Role of n-3 long-chain polyunsaturated fatty acids in human nutrition and health: Review of recent studies and recommendations. Nutr. Res. Pract. 2021, 15, 137–159. [Google Scholar] [CrossRef]
- Barnes, S.; Chowdhury, S.; Gatto, N.M.; Fraser, G.E.; Lee, G.J. Omega-3 fatty acids are associated with blood-brain barrier integrity in a healthy aging population. Brain Behav. 2021, 11, e2273. [Google Scholar] [CrossRef]
- Kuo, Y.T.; So, P.W.; Parkinson, J.R.; Yu, W.S.; Hankir, M.; Herlihy, A.H.; Goldstone, A.P.; Frost, G.S.; Wasserfall, C.; Bell, J.D. The combined effects on neuronal activation and blood-brain barrier permeability of time and n-3 polyunsaturated fatty acids in mice, as measured in vivo using MEMRI. Neuroimage 2010, 50, 1384–1391. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Mu, H.; Zhu, W.; Jiang, X.; Hu, X.; Shi, Y.; Leak, R.K.; Dong, Q.; Chen, J.; et al. Omega-3 polyunsaturated fatty acids mitigate blood-brain barrier disruption after hypoxic-ischemic brain injury. Neurobiol. Dis. 2016, 91, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.Y.; Pan, H.C.; Yen, Y.J.; Wang, C.C.; Chuang, Y.H.; Chen, S.Y.; Lin, S.Y.; Liao, S.L.; Raung, S.L.; Wu, C.W.; et al. Detrimental effects of post-treatment with fatty acids on brain injury in ischemic rats. Neurotoxicology 2007, 28, 1220–1229. [Google Scholar] [CrossRef]
- Chang, C.Y.; Kuan, Y.H.; Li, J.R.; Chen, W.Y.; Ou, Y.C.; Pan, H.C.; Liao, S.L.; Raung, S.L.; Chang, C.J.; Chen, C.J. Docosahexaenoic acid reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. J. Nutr. Biochem. 2013, 24, 2127–2137. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Zhang, H.; Leak, R.K.; Shi, Y.; Hu, X.; Gao, Y.; Chen, J. Dietary supplementation with omega-3 polyunsaturated fatty acids robustly promotes neurovascular restorative dynamics and improves neurological functions after stroke. Exp. Neurol. 2015, 272, 170–180. [Google Scholar] [CrossRef] [Green Version]
- Briscoe, C.P.; Tadayyon, M.; Andrews, J.L.; Benson, W.G.; Chambers, J.K.; Eilert, M.M.; Ellis, C.; Elshourbagy, N.A.; Goetz, A.S.; Minnick, D.T.; et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 2003, 278, 11303–11311. [Google Scholar] [CrossRef] [Green Version]
- Sawzdargo, M.; George, S.R.; Nguyen, T.; Xu, S.; Kolakowski, L.F.; O’Dowd, B.F. A cluster of four novel human G protein-coupled receptor genes occurring in close proximity to CD22 gene on chromosome 19q13.1. Biochem. Biophys. Res. Commun. 1997, 239, 543–547. [Google Scholar] [CrossRef]
- Mancini, A.D.; Poitout, V. The fatty acid receptor FFA1/GPR40 a decade later: How much do we know? Trends Endocrinol. Metab. 2013, 24, 398–407. [Google Scholar] [CrossRef]
- Mohammad, S. GPR40 agonists for the treatment of type 2 diabetes mellitus: Benefits and challenges. Curr. Drug Targets 2016, 17, 1292–1300. [Google Scholar] [CrossRef]
- Sharma, N.; Bhagat, S.; Chundawat, T.S. Recent advances in development of GPR40 modulators (FFA1/FFAR1): An emerging target for type 2 diabetes. Mini Rev. Med. Chem. 2017, 17, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; He, L. The role of polyunsaturated fatty acids and GPR40 receptor in brain. Neuropharmacology 2017, 113, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K.; Aizawa, F.; Miyagi, K.; Yamashita, T.; Mankura, M.; Koyama, Y.; Kasuya, F.; Hirasawa, A.; Kurihara, T.; Miyata, A.; et al. Dysfunctional GPR40/FFAR1 signaling exacerbates pain behavior in mice. PLoS ONE 2017, 12, e0180610. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.-F.; Wu, H.-Y.; Tang, X.-Q.; Ali, U.; Liu, H.; Wang, Y.-X. Activation of GPR40 produces mechanical antiallodynia via the spinal glial interleukin-10/β-endorphin pathway. J. Neuroinflamm. 2019, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Falomir-Lockhart, L.J.; Cavazzutti, G.F.; Gimenez, E.; Toscani, A.M. Fatty Acid Signaling Mechanisms in Neural Cells: Fatty Acid Receptors. Front. Cell. Neurosci. 2019, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.L.; Lindenau, K.L.; Brailoiu, E.; Brailoiu, G.C. Direct evidence of bradycardic effect of omega-3 fatty acids acting on nucleus ambiguus. Neurosci. Lett. 2020, 735, 135196. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.C.; Guo, Q.; Luo, J.; Zhang, J.; Nguyen, K.; Chen, M.; Tran, T.; Dransfield, P.J.; Brown, S.P.; Houze, J.; et al. Identification and pharmacological characterization of multiple allosteric binding sites on the free fatty acid 1 receptor. Mol. Pharmacol. 2012, 82, 843–859. [Google Scholar] [CrossRef] [Green Version]
- Briscoe, C.P.; Peat, A.J.; McKeown, S.C.; Corbett, D.F.; Goetz, A.S.; Littleton, T.R.; McCoy, D.C.; Kenakin, T.P.; Andrews, J.L.; Ammala, C.; et al. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: Identification of agonist and antagonist small molecules. Br. J. Pharmacol. 2006, 148, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Stoddart, L.A.; Brown, A.J.; Milligan, G. Uncovering the pharmacology of the G protein-coupled receptor GPR40: High apparent constitutive activity in guanosine 5’-O-(3-[35S]thio)triphosphate binding studies reflects binding of an endogenous agonist. Mol. Pharmacol. 2007, 71, 994–1005. [Google Scholar] [CrossRef] [Green Version]
- Barr, J.L.; Brailoiu, G.C.; Abood, M.E.; Rawls, S.M.; Unterwald, E.M.; Brailoiu, E. Acute cocaine administration alters permeability of blood-brain barrier in freely-moving rats- Evidence using miniaturized fluorescence microscopy. Drug Alcohol Depend. 2020, 206, 107637. [Google Scholar] [CrossRef]
- Barr, J.L.; Brailoiu, G.C.; Unterwald, E.M.; Brailoiu, E. Assessment of Blood-Brain Barrier Permeability Using Miniaturized Fluorescence Microscopy in Freely Moving Rats. Methods Mol. Biol. 2021, 2367, 123–135. [Google Scholar] [CrossRef]
- Honore, J.C.; Kooli, A.; Hamel, D.; Alquier, T.; Rivera, J.C.; Quiniou, C.; Hou, X.; Kermorvant-Duchemin, E.; Hardy, P.; Poitout, V.; et al. Fatty acid receptor Gpr40 mediates neuromicrovascular degeneration induced by transarachidonic acids in rodents. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 954–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacombe, R.J.S.; Chouinard-Watkins, R.; Bazinet, R.P. Brain docosahexaenoic acid uptake and metabolism. Mol. Asp. Med. 2018, 64, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Zamarbide, M.; Etayo-Labiano, I.; Ricobaraza, A.; Martínez-Pinilla, E.; Aymerich, M.S.; Luis Lanciego, J.; Pérez-Mediavilla, A.; Franco, R. GPR40 activation leads to CREB and ERK phosphorylation in primary cultures of neurons from the mouse CNS and in human neuroblastoma cells. Hippocampus 2014, 24, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Nishinaka, T.; Yamashita, T.; Nakamoto, K.; Kasuya, F.; Tokuyama, S. Involvement of the long-chain fatty acid receptor GPR40 in depression-related behavior. J. Pharmacol. Sci. 2014, 125, 112–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aizawa, F.; Nishinaka, T.; Yamashita, T.; Nakamoto, K.; Kurihara, T.; Hirasawa, A.; Kasuya, F.; Miyata, A.; Tokuyama, S. GPR40/FFAR1 deficient mice increase noradrenaline levels in the brain and exhibit abnormal behavior. J. Pharmacol. Sci. 2016, 132, 249–254. [Google Scholar] [CrossRef]
- Khan, M.Z.; Zhuang, X.; He, L. GPR40 receptor activation leads to CREB phosphorylation and improves cognitive performance in an Alzheimer’s disease mouse model. Neurobiol. Learn. Mem. 2016, 131, 46–55. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, J.; Jin, Y.; Wang, C.; Zheng, M.; He, L. GW9508 ameliorates cognitive impairment via the cAMP-CREB and JNK pathways in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Neuropharmacology 2020, 164, 107899. [Google Scholar] [CrossRef]
- Gong, Y.; Li, Y.; Liu, X.; He, L. GW9508 ameliorates cognitive dysfunction via the external treatment of encephalopathy in Abeta1-42 induced mouse model of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 909, 174362. [Google Scholar] [CrossRef]
- Xie, Y.; Yan, L.; Zeng, H.; Chen, W.; Lu, J.H.; Wan, J.B.; Su, H.; Yao, X. Fish oil protects the blood-brain barrier integrity in a mouse model of Alzheimer’s disease. Chin. Med. 2020, 15, 29. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.H.; Chen, N.Y.; Tu, P.H.; Wu, C.T.; Chiu, S.C.; Huang, Y.C.; Lim, S.N.; Yip, P.K. DHA Attenuates Cerebral Edema Following Traumatic Brain Injury via the Reduction in Blood-Brain Barrier Permeability. Int. J. Mol. Sci. 2020, 21, 6291. [Google Scholar] [CrossRef] [PubMed]
- Giaever, I.; Keese, C.R. Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc. Natl. Acad. Sci. USA 1984, 81, 3761–3764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolwijk, J.A.; Matrougui, K.; Renken, C.W.; Trebak, M. Impedance analysis of GPCR-mediated changes in endothelial barrier function: Overview and fundamental considerations for stable and reproducible measurements. Pflug. Arch. Eur. J. Physiol. 2015, 467, 2193–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolwijk, J.A.; Zhang, X.; Gueguinou, M.; Zhang, W.; Matrougui, K.; Renken, C.; Trebak, M. Calcium Signaling Is Dispensable for Receptor Regulation of Endothelial Barrier Function. J. Biol. Chem. 2016, 291, 22894–22912. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Lin, H.; Zhang, Y.; Xu, T.; Wang, T.; Xue, X.; Zhang, W.; Liu, H. Activation of GPR40 Suppresses AGE-Induced Reduction of Type II Collagen and Aggrecan in Human SW1353 Chondrocytes. Drug Des. Devel Ther. 2020, 14, 2371–2379. [Google Scholar] [CrossRef]
- Sun, C.; Li, Y.; Li, X.; Sun, J. Agonism of Gpr40 Protects the Capacities of Epidermal Stem Cells (ESCs) Against Ultraviolet-B (UV-B). Drug Des. Devel Ther. 2020, 14, 5143–5153. [Google Scholar] [CrossRef]
- Milligan, G.; Shimpukade, B.; Ulven, T.; Hudson, B.D. Complex Pharmacology of Free Fatty Acid Receptors. Chem. Rev. 2017, 117, 67–110. [Google Scholar] [CrossRef]
- Roig-Pérez, S.; Guardiola, F.; Moretó, M.; Ferrer, R. Lipid peroxidation induced by DHA enrichment modifies paracellular permeability in Caco-2 cells: Protective role of taurine. J. Lipid Res. 2004, 45, 1418–1428. [Google Scholar] [CrossRef] [Green Version]
- Usami, M.; Komurasaki, T.; Hanada, A.; Kinoshita, K.; Ohata, A. Effect of γ-linolenic acid or docosahexaenoic acid on tight junction permeability in intestinal monolayer cells and their mechanism by protein kinase C activation and/or eicosanoid formation. Nutrition 2003, 19, 150–156. [Google Scholar] [CrossRef]
- Hossain, Z.; Kurihara, H.; Hosokawa, M.; Takahashi, K. Docosahexaenoic acid and eicosapentaenoic acid-enriched phosphatidylcholine liposomes enhance the permeability, transportation and uptake of phospholipids in Caco-2 cells. Mol. Cell. Biochem. 2006, 285, 155–163. [Google Scholar] [CrossRef]
- Liu, L.B.; Xue, Y.X.; Liu, Y.H.; Wang, Y.B. Bradykinin increases blood-tumor barrier permeability by down-regulating the expression levels of ZO-1, occludin, and claudin-5 and rearranging actin cytoskeleton. J. Neurosci. Res. 2008, 86, 1153–1168. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Shipsky, M.M.; Yan, G.; Abood, M.E.; Brailoiu, G.C. Mechanisms of modulation of brain microvascular endothelial cells function by thrombin. Brain Res. 2017, 1657, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, K.K.; Burns, L.D.; Cocker, E.D.; Nimmerjahn, A.; Ziv, Y.; Gamal, A.E.; Schnitzer, M.J. Miniaturized integration of a fluorescence microscope. Nat. Methods 2011, 8, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Drzazga, A.; Kristinsson, H.; Salaga, M.; Zatorski, H.; Koziolkiewicz, M.; Gendaszewska-Darmach, E.; Bergsten, P. Lysophosphatidylcholine and its phosphorothioate analogues potentiate insulin secretion via GPR40 (FFAR1), GPR55 and GPR119 receptors in a different manner. Mol. Cell. Endocrinol. 2018, 472, 117–125. [Google Scholar] [CrossRef]
- Drzazga, A.; Okulus, M.; Rychlicka, M.; Biegala, L.; Gliszczynska, A.; Gendaszewska-Darmach, E. Lysophosphatidylcholine Containing Anisic Acid Is Able to Stimulate Insulin Secretion Targeting G Protein Coupled Receptors. Nutrients 2020, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Drzazga, A.; Cichonska, E.; Koziolkiewicz, M.; Gendaszewska-Darmach, E. Formation of betaTC3 and MIN6 Pseudoislets Changes the Expression Pattern of Gpr40, Gpr55, and Gpr119 Receptors and Improves Lysophosphatidylcholines-Potentiated Glucose-Stimulated Insulin Secretion. Cells 2020, 9, 2062. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, C.; Yang, J.; Zhou, J.; Ye, Z.; Feng, D.; Yue, N.; Tong, J.; Huang, W.; Qian, H. Design, synthesis and biological evaluation of novel FFA1/GPR40 agonists: New breakthrough in an old scaffold. Eur. J. Med. Chem. 2019, 179, 608–622. [Google Scholar] [CrossRef]
- Grundmann, M.; Bender, E.; Schamberger, J.; Eitner, F. Pharmacology of Free Fatty Acid Receptors and Their Allosteric Modulators. Int. J. Mol. Sci. 2021, 22, 1763. [Google Scholar] [CrossRef]
- Rani, L.; Grewal, A.S.; Sharma, N.; Singh, S. Recent Updates on Free Fatty Acid Receptor 1 (GPR-40) Agonists for the Treatment of Type 2 Diabetes Mellitus. Mini Rev. Med. Chem. 2021, 21, 426–470. [Google Scholar] [CrossRef]
- Han, L. Modulation of the Blood-Brain Barrier for Drug Delivery to Brain. Pharmaceutics 2021, 13, 2024. [Google Scholar] [CrossRef]
- Han, L.; Jiang, C. Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm. Sin. B 2021, 11, 2306–2325. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Barlow, C.L.; Ramirez, S.H.; Abood, M.E.; Brailoiu, G.C. Effects of Platelet-Activating Factor on Brain Microvascular Endothelial Cells. Neuroscience 2018, 377, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Leo, L.M.; Familusi, B.; Hoang, M.; Smith, R.; Lindenau, K.; Sporici, K.T.; Brailoiu, E.; Abood, M.E.; Brailoiu, G.C. GPR55-mediated effects on brain microvascular endothelial cells and the blood-brain barrier. Neuroscience 2019, 414, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain In Stereotaxic Coordinates, 7th ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindenau, K.L.; Barr, J.L.; Higgins, C.R.; Sporici, K.T.; Brailoiu, E.; Brailoiu, G.C. Blood–Brain Barrier Disruption Mediated by FFA1 Receptor—Evidence Using Miniscope. Int. J. Mol. Sci. 2022, 23, 2258. https://doi.org/10.3390/ijms23042258
Lindenau KL, Barr JL, Higgins CR, Sporici KT, Brailoiu E, Brailoiu GC. Blood–Brain Barrier Disruption Mediated by FFA1 Receptor—Evidence Using Miniscope. International Journal of Molecular Sciences. 2022; 23(4):2258. https://doi.org/10.3390/ijms23042258
Chicago/Turabian StyleLindenau, Kristen L., Jeffrey L. Barr, Christopher R. Higgins, Kevin T. Sporici, Eugen Brailoiu, and Gabriela C. Brailoiu. 2022. "Blood–Brain Barrier Disruption Mediated by FFA1 Receptor—Evidence Using Miniscope" International Journal of Molecular Sciences 23, no. 4: 2258. https://doi.org/10.3390/ijms23042258
APA StyleLindenau, K. L., Barr, J. L., Higgins, C. R., Sporici, K. T., Brailoiu, E., & Brailoiu, G. C. (2022). Blood–Brain Barrier Disruption Mediated by FFA1 Receptor—Evidence Using Miniscope. International Journal of Molecular Sciences, 23(4), 2258. https://doi.org/10.3390/ijms23042258





