Platelet-Released Factors: Their Role in Viral Disease and Applications for Extracellular Vesicle (EV) Therapy
Abstract
:1. Background
1.1. Platelet Activation
Virus Entry and Activation in Platelets
2. Platelet Effector Functions
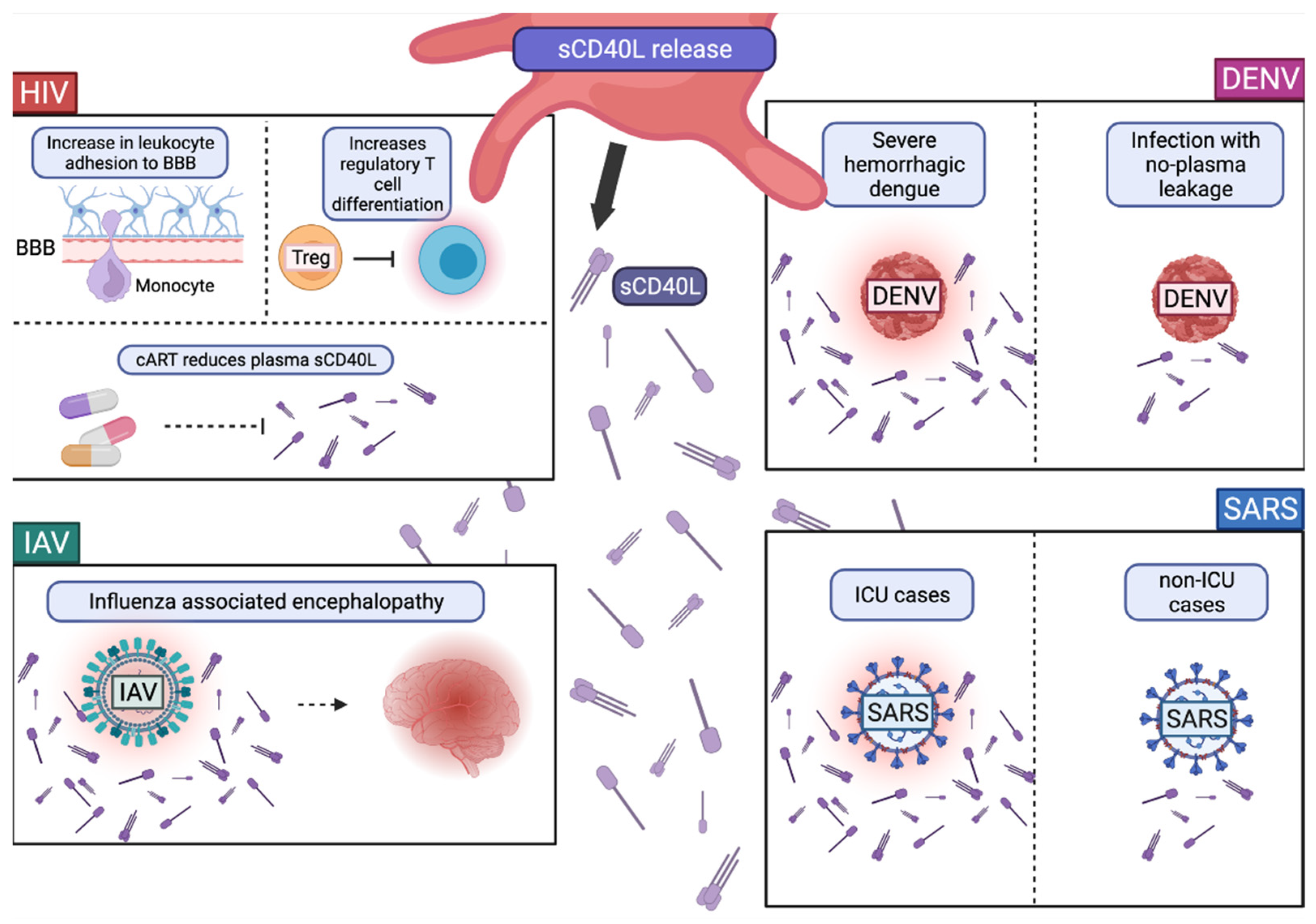
2.1. Expression and Release of CD40L
Role of CD40L and sCD40L in Viral Infections
2.2. Platelet Extracellular Vesicle Release
The Role of PMVs in Viral Infections
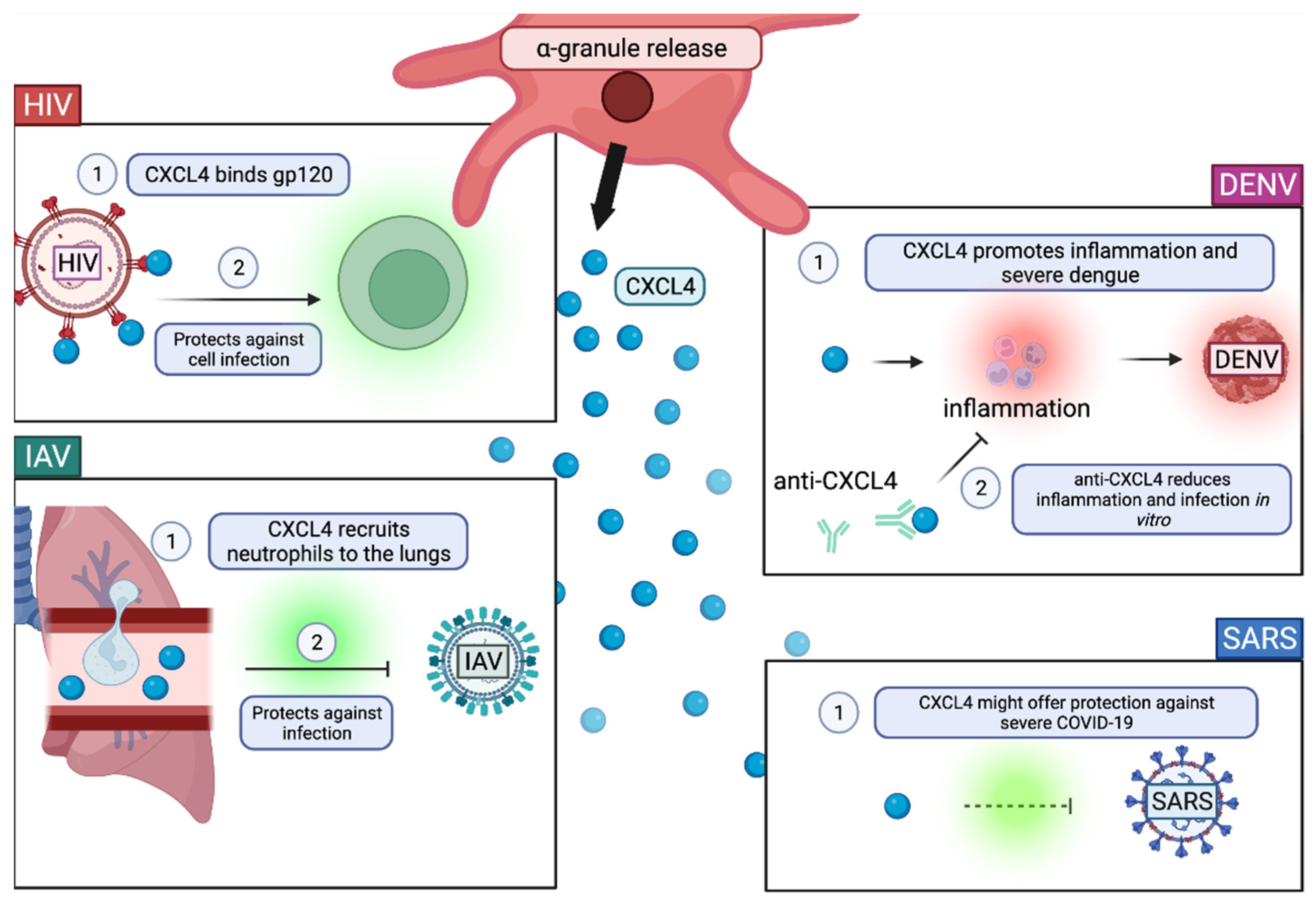
2.3. Degranulation
The Role of α-Granules in Viral Infection
3. Therapeutic Applications
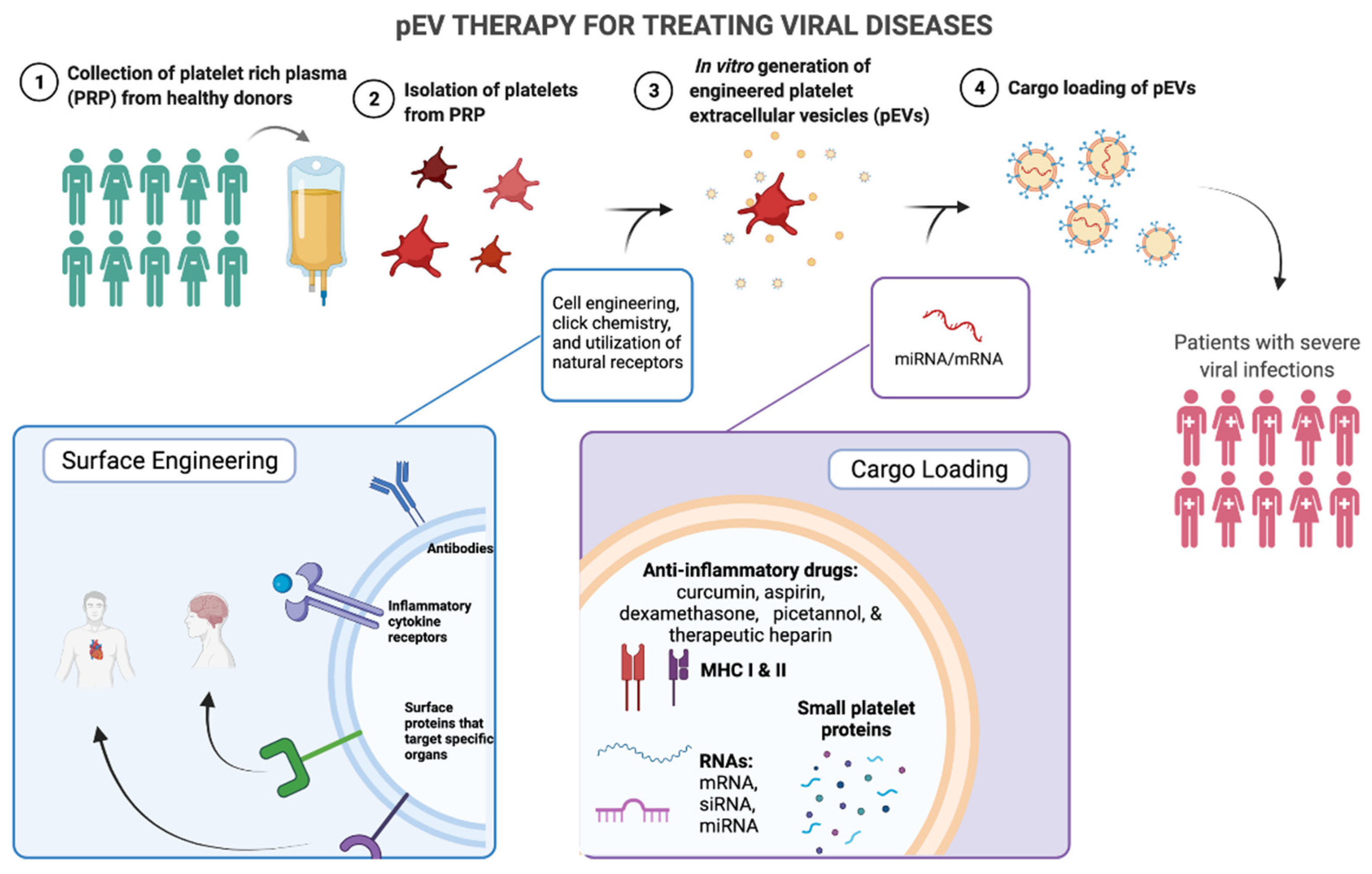
3.1. Advantages of pEV Therapy
3.2. Platelet Rich Plasma as a Source for pEV Therapy
3.3. In Vitro Engineering of EVs
3.3.1. Cargo-Loading of EVs
3.3.2. Surface Engineering of EVs
3.4. Targeting Viruses with pEV Engineering
3.4.1. Anti-Viral Mediators in the Cargo of pEVs
- MHC and peptides. The loading of MHC molecules with peptides could target specific T cells, inducing their effector functions against viruses.
- RNAs. EV RNAs have already been shown to exert effects on surrounding cells, influencing their production of molecules. Thus, using immunologically specific RNAs to alter immune cell phenotypes, is of great interest for viral disease.
- Anti-inflammatory mediators. Platelet-derived factors, such as sCD40L and components of α-granules (CXCL4), are of therapeutic interest due to their anti-inflammatory effects, specific targeting of immune cells, and role in viral disease progression.
3.4.2. Anti-Viral pEV Surface Markers
- Viral binding proteins. Engineered surface markers for viral binding (TLRs, CLEC-2, DC-SIGN, and ACE2) on the surface of pEVs is of potential therapeutic interest in order to prevent excessive platelet activation and thrombocytopenia. Viruses could be targeted to bind pEVs instead of platelets, just by the availability of engineered pEV surface molecules compared to platelets. In conjunction, these pEVs could be equipped with anti-viral molecules that release upon viral binding, preventing viral replication. In many severe viral diseases, thrombocytopenia is a biomarker of mortality; thus, by reducing platelet infection, perhaps platelets may more easily carry out their anti-viral effector functions.
- Sequestering inflammatory platelet mediators. Another application for engineering surface markers is to specifically target platelet-released factors such as sCD40L or inflammatory markers using monoclonal antibodies. This application would reduce an excess of inflammatory effects, which is often characteristic of severe disease. Severe viral infections often have elevated sCD40L and inflammatory cytokines. By sequestering these factors through antibody binding on pEVs, inflammatory positive feedback loops could be reduced.
- Homing to damaged tissues. PMV-like nanovesicles were engineered with the surface receptors CPIIb/IIIa and P-selectin in order to target the site of clot formation [190]. In viral diseases such as Dengue where the coagulation cascade can be disrupted, it might be beneficial to utilize receptors that home cells to the site of capillary leakage.
3.5. Considerations for pEV Therapy
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bizzozero, G. Sur Un Nouvel Èlèment Morphologique Du Sang Chez Les Mammiferes et Son Importance Dans La Thrombose et Dans La Coagulation. Arch. Ital. Biol. 1882, 1, 1–5. [Google Scholar]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in Inflammation and Atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieswandt, B.; Varga-Szabo, D.; Elvers, M. Integrins in Platelet Activation. J. Thromb. Haemost. 2009, 7, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Radley, J.; Haller, C. The Demarcation Membrane System of the Megakaryocyte: A Misnomer? Blood 1982, 60, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cognasse, F.; Hamzeh, H.; Chavarin, P.; Acquart, S.; Genin, C.; Garraud, O. Evidence of Toll-like Receptor Molecules on Human Platelets. Immunol. Cell Biol. 2005, 83, 196–198. [Google Scholar] [CrossRef]
- Andonegui, G.; Kerfoot, S.M.; McNagny, K.; Ebbert, K.V.J.; Patel, K.D.; Kubes, P. Platelets Express Functional Toll-like Receptor-4. Blood 2005, 106, 2417–2423. [Google Scholar] [CrossRef]
- Blair, P.; Rex, S.; Vitseva, O.; Beaulieu, L.; Tanriverdi, K.; Chakrabarti, S.; Hayashi, C.; Genco, C.A.; Iafrati, M.; Freedman, J.E. Stimulation of Toll-Like Receptor 2 in Human Platelets Induces a Thromboinflammatory Response Through Activation of Phosphoinositide 3-Kinase. Circ. Res. 2009, 104, 346–354. [Google Scholar] [CrossRef] [Green Version]
- Cognasse, F.; Nguyen, K.A.; Damien, P.; McNicol, A.; Pozzetto, B.; Hamzeh-Cognasse, H.; Garraud, O. The Inflammatory Role of Platelets via Their TLRs and Siglec Receptors. Front. Immunol. 2015, 6, 83. [Google Scholar] [CrossRef] [Green Version]
- Chapman, L.M.; Aggrey, A.A.; Field, D.J.; Srivastava, K.; Ture, S.; Yui, K.; Topham, D.J.; Baldwin, W.M.; Morrell, C.N. Platelets Present Antigen in the Context of MHC Class I. J. Immunol. 2012, 189, 916–923. [Google Scholar] [CrossRef] [Green Version]
- Cognasse, F.; Hamzeh-Cognasse, H.; Lafarge, S.; Chavarin, P.; Cogné, M.; Richard, Y.; Garraud, O. Human Platelets Can Activate Peripheral Blood B Cells and Increase Production of Immunoglobulins. Exp. Hematol. 2007, 35, 1376–1387. [Google Scholar] [CrossRef]
- Nishat, S.; Wuescher, L.M.; Worth, R.G. Platelets Enhance Dendritic Cell Responses against Staphylococcus Aureus through CD40-CD40L. Infect. Immun. 2018, 86, e00186-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schattner, M. Platelet TLR4 at the Crossroads of Thrombosis and the Innate Immune Response. J. Leukoc. Biol. 2019, 105, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Suzuki-Inoue, K.; Inoue, O. Platelet Receptors Activated via Mulitmerization: Glycoprotein VI, GPIb-IX-V, and CLEC-2. J. Thromb. Haemost. 2013, 11, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Stegner, D.; Nieswandt, B. Platelet Receptor Signaling in Thrombus Formation. J. Mol. Med. 2011, 89, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Ossovskaya, V.S.; Bunnett, N.W. Protease-Activated Receptors: Contribution to Physiology and Disease. Physiol. Rev. 2004, 84, 579–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlin, S.R. Protease-Activated Receptors in Hemostasis, Thrombosis and Vascular Biology. J. Thromb. Haemost. 2005, 3, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Nandi, D.; Batra, H.; Singhal, R.; Annarapu, G.K.; Bhattacharyya, S.; Seth, T.; Dar, L.; Medigeshi, G.R.; Vrati, S.; et al. Platelet Activation Determines the Severity of Thrombocytopenia in Dengue Infection. Sci. Rep. 2017, 7, 41697. [Google Scholar] [CrossRef]
- Koupenova, M.; Vitseva, O.; MacKay, C.R.; Beaulieu, L.M.; Benjamin, E.J.; Mick, E.; Kurt-Jones, E.A.; Ravid, K.; Freedman, J.E. Platelet-TLR7 Mediates Host Survival and Platelet Count during Viral Infection in the Absence of Platelet-Dependent Thrombosis. Blood 2014, 124, 791–802. [Google Scholar] [CrossRef] [Green Version]
- Nieswandt, B.; Watson, S.P. Platelet-Collagen Interaction: Is GPVI the Central Receptor? Blood 2003, 102, 449–461. [Google Scholar] [CrossRef]
- Watson, S.P.; Herbert, J.M.J.; Pollitt, A.Y. GPVI and CLEC-2 in Hemostasis and Vascular Integrity. J. Thromb. Haemost. 2010, 8, 1456–1467. [Google Scholar] [CrossRef]
- Zuchtriegel, G.; Uhl, B.; Puhr-Westerheide, D.; Pörnbacher, M.; Lauber, K.; Krombach, F.; Reichel, C.A. Platelets Guide Leukocytes to Their Sites of Extravasation. PLoS Biol. 2016, 14, e1002459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diacovo, T.G.; Puri, K.D.; Warnock, R.A.; Springer, T.A.; von Andrian, U.H. Platelet-Mediated Lymphocyte Delivery to High Endothelial Venules. Science 1996, 273, 252–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 Binds Platelet ACE2 to Enhance Thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Chabert, A.; Hamzeh-Cognasse, H.; Pozzetto, B.; Cognasse, F.; Schattner, M.; Gomez, R.M.; Garraud, O. Human Platelets and Their Capacity of Binding Viruses: Meaning and Challenges? BMC Immunol. 2015, 16, 26. [Google Scholar] [CrossRef] [Green Version]
- Pretorius, E.; Smit, E.; Oberholzer, H.M.; Steyn, E.; Briedenhann, S.; Franz, R.C. Investigating the Ultrastructure of Platelets of HIV Patients Treated with the Immuno-Regulator, Canova: A Qualitative Scanning Electron Microscopy Study. Histol. Histopathol. 2009, 24, 399–405. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Nardi, M.A.; Li, Z. HIV-1 Tat-Induced Platelet Activation and Release of CD154 Contribute to HIV-1-Associated Autoimmune Thrombocytopenia. J. Thromb. Haemost. 2011, 9, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Simpson, S.R.; Singh, M.V.; Dewhurst, S.; Schifitto, G.; Maggirwar, S.B. Platelets Function as an Acute Viral Reservoir during HIV-1 Infection by Harboring Virus and T-Cell Complex Formation. Blood Adv. 2020, 4, 4512–4521. [Google Scholar] [CrossRef]
- Simon, A.Y.; Sutherland, M.R.; Pryzdial, E.L.G. Dengue Virus Binding and Replication by Platelets. Blood 2015, 126, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Sung, P.-S.; Huang, T.-F.; Hsieh, S.-L. Extracellular Vesicles from CLEC2-Activated Platelets Enhance Dengue Virus-Induced Lethality via CLEC5A/TLR2. Nat. Commun. 2019, 10, 2402. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.-H.; Wu, W.-C.; Lai, Y.; Tsai, P.; Perng, G.; Lin, Y.-S.; Yeh, T. Dengue Virus Nonstructural Protein 1 Activates Platelets via Toll-like Receptor 4, Leading to Thrombocytopenia and Hemorrhage. PLoS Pathog. 2019, 15, e1007625. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.C.Q.; Rozini, S.V.; Lima, G.B.; Coelho, D.R.; Carneiro, P.H.; Borges, R.M.; Bozza, P.T.; Hottz, E.D. Inflammatory Signaling in Dengue-Infected Platelets Requires Translation and Secretion of Nonstructural Protein 1. Blood Adv. 2020, 4, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Trugilho, M.R.; Hottz, E.D.; Brunoro, G.V.F.; Teixeira-Ferreira, A.; Carvalho, P.C.; Salazar, G.A.; Zimmerman, G.A.; Bozza, F.A.; Bozza, P.T.; Perales, J. Platelet Proteome Reveals Novel Pathways of Platelet Activation and Platelet-Mediated Immunoregulation in Dengue. PLoS Pathog. 2017, 13, e1006385. [Google Scholar]
- Alonzo, M.T.G.; Lacuesta, T.L.V.; Dimaano, E.M.; Kurosu, T.; Suarez, L.-A.C.; Mapua, C.A.; Akeda, Y.; Matias, R.R.; Kuter, D.J.; Nagata, S.; et al. Platelet Apoptosis and Apoptotic Platelet Clearance by Macrophages in Secondary Dengue Virus Infections. J. Infect. Dis. 2012, 205, 1321–1329. [Google Scholar] [CrossRef]
- Koupenova, M.; Corkrey, H.A.; Vitseva, O.; Manni, G.; Pang, C.J.; Clancy, L.; Yao, C.; Rade, J.; Levy, D.; Wang, J.P.; et al. The Role of Platelets in Mediating a Response to Human Influenza Infection. Nat. Commun. 2019, 10, 1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondina, M.T.; Brewster, B.; Grissom, C.K.; Zimmerman, G.A.; Kastendieck, D.H.; Harris, E.S.; Weyrich, A.S. In Vivo Platelet Activation in Critically Ill Patients With Primary 2009 Influenza A(H1N1). Chest 2012, 141, 1490–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroczek, R.A.; Graf, D.; Giliani, D.B.S.; Korthauer, U.; Senger, A.U.G.; Mages, H.W.; Villa, A.; Notarangelo, L.D. Defective Expression of CD40 Ligand on T Cells Causes “X-Linked Immunodeficiency with Hyper-IgM (HIGMl)”. Immunol. Rev. 1994, 138, 39–59. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Nannizzi-Alaimo, L.; Prasad, S.K.; Phillips, D.R. Platelet-Derived CD40L. Circulation 2002, 106, 896–899. [Google Scholar] [CrossRef] [Green Version]
- Henn, V.; Slupsky, J.R.; Gräfe, M.; Anagnostopoulos, I.; Förster, R.; Müller-Berghaus, G.; Kroczek, R.A. CD40 Ligand on Activated Platelets Triggers an Inflammatory Reaction of Endothelial Cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef]
- Aukrust, P.; Müller, F.; Ueland, T.; Berget, T.; Aaser, E.; Brunsvig, A.; Solum, N.O.; Forfang, K.; Frøland, S.S.; Gullestad, L. Enhanced Levels of Soluble and Membrane-Bound CD40 Ligand in Patients With Unstable Angina. Circulation 1999, 100, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Henn, V.; Steinbach, S.; Büchner, K.; Presek, P.; Kroczek, R.A. The Inflammatory Action of CD40 Ligand (CD154) Expressed on Activated Human Platelets Is Temporally Limited by Coexpressed CD40. Blood 2001, 98, 1047–1054. [Google Scholar] [CrossRef]
- Hammwöhner, M.; Ittenson, A.; Dierkes, J.; Bukowska, A.; Klein, H.U.; Lendeckel, U.; Goette, A. Platelet Expression of CD40/CD40 Ligand and Its Relation to Inflammatory Markers and Adhesion Molecules in Patients with Atrial Fibrillation. Exp. Biol. Med. 2007, 232, 581–589. [Google Scholar]
- Anand, X.S.; Viles-Gonzalez, J.F.; Badimon, J.J.; Cavusoglu, E.; Marmur, J.D. Membrane-Associated CD40L and SCD40L in Atherothrombotic Disease. Thromb. Haemost. 2003, 90, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.V.; Davidson, D.C.; Jackson, J.W.; Singh, V.B.; Silva, J.; Ramirez, S.H.; Maggirwar, S.B. Characterization of Platelet-Monocyte Complexes in HIV-1-Infected Individuals: Possible Role in HIV-Associated Neuroinflammation. J. Immunol. 2014, 192, 4674–4684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, D.C.; Hirschman, M.P.; Sun, A.; Singh, M.V.; Kasischke, K.; Maggirwar, S.B. Excess Soluble CD40L Contributes to Blood Brain Barrier Permeability In Vivo: Implications for HIV-Associated Neurocognitive Disorders. PLoS ONE 2012, 7, e51793. [Google Scholar] [CrossRef] [Green Version]
- Sui, Z.; Sniderhan, L.F.; Schifitto, G.; Phipps, R.P.; Gelbard, H.A.; Dewhurst, S.; Maggirwar, S.B. Functional Synergy between CD40 Ligand and HIV-1 Tat Contributes to Inflammation: Implications in HIV Type 1 Dementia. J. Immunol. 2007, 178, 3226–3236. [Google Scholar] [CrossRef] [Green Version]
- Jenabian, M.-A.; Patel, M.; Kema, I.; Vyboh, K.; Kanagaratham, C.; Radzioch, D.; Thébault, P.; Lapointe, R.; Gilmore, N.; Ancuta, P.; et al. Soluble CD40-Ligand (SCD40L, SCD154) Plays an Immunosuppressive Role via Regulatory T Cell Expansion in HIV Infection. Clin. Exp. Immunol. 2014, 178, 102–111. [Google Scholar] [CrossRef]
- Steel, H.C.; Venter, W.D.F.; Theron, A.J.; Anderson, R.; Feldman, C.; Arulappan, N.; Rossouw, T.M. Differential Responsiveness of the Platelet Biomarkers, Systemic CD40 Ligand, CD62P, and Platelet-Derived Growth Factor-BB, to Virally-Suppressive Antiretroviral Therapy. Front. Immunol. 2021, 11, 3590. [Google Scholar] [CrossRef]
- Núñez-Avellaneda, D.; Mosso-Pani, M.A.; Sánchez-Torres, L.E.; Castro-Mussot, M.E.; Corona-de la Peña, N.A.; Salazar, M.I. Dengue Virus Induces the Release of SCD40L and Changes in Levels of Membranal CD42b and CD40L Molecules in Human Platelets. Viruses 2018, 10, 357. [Google Scholar] [CrossRef] [Green Version]
- Tomashek, K.M.; Lorenzi, O.D.; Andújar-Pérez, D.A.; Torres-Velásquez, B.C.; Hunsperger, E.A.; Munoz-Jordan, J.L.; Perez-Padilla, J.; Rivera, A.; Gonzalez-Zeno, G.E.; Sharp, T.M.; et al. Clinical and Epidemiologic Characteristics of Dengue and Other Etiologic Agents among Patients with Acute Febrile Illness, Puerto Rico, 2012–2015. PLoS Negl. Trop. Dis. 2017, 11, e0005859. [Google Scholar] [CrossRef]
- Tramontini Gomes de Sousa Cardozo, F.; Baimukanova, G.; Lanteri, M.C.; Keating, S.M.; Moraes Ferreira, F.; Heitman, J.; Pannuti, C.S.; Pati, S.; Romano, C.M.; Cerdeira Sabino, E. Serum from Dengue Virus-Infected Patients with and without Plasma Leakage Differentially Affects Endothelial Cells Barrier Function in Vitro. PLoS ONE 2017, 12, e0178820. [Google Scholar] [CrossRef]
- McElroy, A.K.; Erickson, B.R.; Flietstra, T.D.; Rollin, P.E.; Nichol, S.T.; Towner, J.S.; Spiropoulou, C.F. Ebola Hemorrhagic Fever: Novel Biomarker Correlates of Clinical Outcome. J. Infect. Dis. 2014, 210, 558–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Tamimi, A.O.; Yusuf, A.M.; Jayakumar, M.N.; Ansari, A.W.; Elhassan, M.; AbdulKarim, F.; Kannan, M.; Halwani, R.; Ahmad, F. Induction Of Soluble P-Selectin And CD40 Ligand And, FXIII Deficiency Promote Aberrant Coagulation And Thromboembolism In Severe COVID-19. Circ. Res. 2021, 129, AP357. [Google Scholar] [CrossRef]
- Campo, G.; Contoli, M.; Fogagnolo, A.; Vieceli Dalla Sega, F.; Zucchetti, O.; Ronzoni, L.; Verri, M.; Fortini, F.; Pavasini, R.; Morandi, L.; et al. Over Time Relationship between Platelet Reactivity, Myocardial Injury and Mortality in Patients with SARS-CoV-2-Associated Respiratory Failure. Platelets 2021, 32, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Ageno, W.; Sessa, F.; Salvini, M.; Caramazza, D.; Mora, B.; Rossi, A.; Rovelli, C.; Passamonti, F.; Grossi, P. Recurrence of Immune Thrombocytopenia at the Time of SARS-CoV-2 Infection. Ann. Hematol. 2020, 99, 1951–1952. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J.; Lee, A.H.; Xia, Y.; Lin, L.H.; Black, M.; Cotzia, P.; Hochman, J.; Berger, J.S. Platelet and Vascular Biomarkers Associate With Thrombosis and Death in Coronavirus Disease. Circ. Res. 2020, 127, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Di Ienno, L.; Zucchetti, O.; D’Aniello, E.; Verri, M.; Biscaglia, S.; Pavasini, R.; Contoli, M.; Savino, S.; Guardigli, G. The Relation between Platelet Activation, Myocardial Injury, and Mortality in Patients with SARS-CoV-2-Associated Respiratory Failure (ATTAC-Co). Eur. Heart J. Suppl. 2021, 23, C1–C48. [Google Scholar]
- Zhu, R.; Chen, C.; Wang, Q.; Zhang, X.; Lu, C.; Sun, Y. Routine Blood Parameters Are Helpful for Early Identification of Influenza Infection in Children. BMC Infect. Dis. 2020, 20, 864. [Google Scholar] [CrossRef]
- Obi, A.T.; Tignanelli, C.J.; Jacobs, B.N.; Arya, S.; Park, P.K.; Wakefield, T.W.; Henke, P.K.; Napolitano, L.M. Empirical Systemic Anticoagulation Is Associated with Decreased Venous Thromboembolism in Critically Ill Influenza A H1N1 Acute Respiratory Distress Syndrome Patients. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 317–324. [Google Scholar] [CrossRef]
- Ashar, H.K.; Mueller, N.C.; Rudd, J.M.; Snider, T.A.; Achanta, M.; Prasanthi, M.; Pulavendran, S.; Thomas, P.G.; Ramachandran, A.; Malayer, J.R.; et al. The Role of Extracellular Histones in Influenza Virus Pathogenesis. Am. J. Pathol. 2018, 188, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Lê, V.B.; Schneider, J.G.; Boergeling, Y.; Berri, F.; Ducatez, M.; Guerin, J.-L.; Adrian, I.; Errazuriz-Cerda, E.; Frasquilho, S.; Antunes, L.; et al. Platelet Activation and Aggregation Promote Lung Inflammation and Influenza Virus Pathogenesis. Am. J. Respir. Crit. Care Med. 2015, 191, 804–819. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, T.; Morishima, T.; Suenaga, N.; Kajimoto, M.; Matsubara, T.; Furukawa, S. Analysis of Serum Soluble CD40 Ligand in Patients with Influenza Virus-Associated Encephalopathy. J. Neurol. Sci. 2005, 239, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ståhl, A.; Johansson, K.; Mossberg, M.; Kahn, R.; Karpman, D. Exosomes and Microvesicles in Normal Physiology, Pathophysiology, and Renal Diseases. Pediatr. Nephrol. 2019, 34, 11–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding Microvesicles: Artefacts No More. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef]
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging Role of Extracellular Vesicles in Inflammatory Diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef]
- Horstman, L.L.; Ahn, Y.S. Platelet Microparticles: A Wide-Angle Perspective. Crit. Rev. Oncol. /Hematol. 1999, 30, 111–142. [Google Scholar] [CrossRef]
- Joop, K.; Berckmans, R.J.; Nieuwland, R.; Berkhout, J.; Romijn, F.P.H.T.M.; Hack, C.E.; Sturk, A. Microparticles from Patients with Multiple Organ Dysfunction Syndrome and Sepsis Support Coagulation through Multiple Mechanisms. Thromb. Haemost. 2001, 85, 810–820. [Google Scholar] [CrossRef]
- Berckmans, R.J.; Nieuwland, R.; Böing, A.N.; Romijn, F.P.H.T.M.; Hack, C.E.; Sturk, A. Cell-Derived Microparticles Circulate in Healthy Humans and Support Low Grade Thrombin Generation. Thromb. Haemost. 2001, 85, 639–649. [Google Scholar]
- Flaumenhaft, R.; Dilks, J.R.; Richardson, J.; Alden, E.; Patel-Hett, S.R.; Battinelli, E.; Klement, G.L.; Sola-Visner, M.; Italiano, J.E., Jr. Megakaryocyte-Derived Microparticles: Direct Visualization and Distinction from Platelet-Derived Microparticles. Blood 2009, 113, 1112–1121. [Google Scholar] [CrossRef] [Green Version]
- Tesse, A.; Martinez, M.C.; Meziani, F.; Hugel, B.; Panaro, M.A.; Mitolo, V.; Freyssinet, J.-M.; Andriantsitohaina, R. Origin and Biological Significance of Shed-Membrane Microparticles. Endocr. Metab. Immune Disord. Drug Targets (Former. Curr. Drug Targets Immune Endocr. Metab. Disord. ) 2006, 6, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pujol, S.; Marker, P.H.; Key, N.S. Platelet Microparticles Are Heterogeneous and Highly Dependent on the Activation Mechanism: Studies Using a New Digital Flow Cytometer. Cytometry Part A 2007, 71A, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.; Goldfinger, L.E. Platelet Microparticles and MiRNA Transfer in Cancer Progression: Many Targets, Modes of Action, and Effects Across Cancer Stages. Front. Cardiovasc. Med. 2018, 5, 13. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs Are Transported in Plasma and Delivered to Recipient Cells by High-Density Lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duchez, A.-C.; Boudreau, L.H.; Naika, G.S.; Bollinger, J.; Belleannée, C.; Cloutier, N.; Laffont, B.; Mendoza-Villarroel, R.E.; Lévesque, T.; Rollet-Labelle, E.; et al. Platelet Microparticles Are Internalized in Neutrophils via the Concerted Activity of 12-Lipoxygenase and Secreted Phospholipase A2-IIA. Proc. Natl. Acad. Sci. USA 2015, 112, E3564–E3573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melki, I.; Tessandier, N.; Zufferey, A.; Boilard, E. Platelet Microvesicles in Health and Disease. Platelets 2017, 28, 214–221. [Google Scholar] [CrossRef]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A Multifunctional Alarmin Driving Autoimmune and Inflammatory Disease. Nat. Rev. Rheumatol. 2012, 8, 195–202. [Google Scholar] [CrossRef]
- Lood, C.; Tydén, H.; Gullstrand, B.; Jönsen, A.; Källberg, E.; Mörgelin, M.; Kahn, R.; Gunnarsson, I.; Leanderson, T.; Ivars, F.; et al. Platelet-Derived S100A8/A9 and Cardiovascular Disease in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2016, 68, 1970–1980. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fang, C.; Gao, H.; Bilodeau, M.L.; Zhang, Z.; Croce, K.; Liu, S.; Morooka, T.; Sakuma, M.; Nakajima, K.; et al. Platelet-Derived S100 Family Member Myeloid-Related Protein-14 Regulates Thrombosis. J. Clin. Investig. 2014, 124, 2160–2171. [Google Scholar] [CrossRef] [Green Version]
- Melki, I.; Allaeys, I.; Tessandier, N.; Lévesque, T.; Cloutier, N.; Laroche, A.; Vernoux, N.; Becker, Y.; Benk-Fortin, H.; Zufferey, A.; et al. Platelets Release Mitochondrial Antigens in Systemic Lupus Erythematosus. Sci. Transl. Med. 2021, 13, eaav5928. [Google Scholar] [CrossRef] [PubMed]
- Tsiantoulas, D.; Perkmann, T.; Afonyushkin, T.; Mangold, A.; Prohaska, T.A.; Papac-Milicevic, N.; Millischer, V.; Bartel, C.; Hörkkö, S.; Boulanger, C.M.; et al. Circulating Microparticles Carry Oxidation-Specific Epitopes and Are Recognized by Natural IgM Antibodies1[S]. J. Lipid Res. 2015, 56, 440–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poveda, E.; Tabernilla, A.; Fitzgerald, W.; Salgado-Barreira, Á.; Grandal, M.; Pérez, A.; Mariño, A.; Álvarez, H.; Valcarce, N.; González-García, J.; et al. Massive Release of CD9+ Microvesicles in Human Immunodeficiency Virus Infection, Regardless of Virologic Control. J. Infect. Dis. 2020, jiaa375. [Google Scholar] [CrossRef] [PubMed]
- Falasca, K.; Lanuti, P.; Ucciferri, C.; Pieragostino, D.; Cufaro, M.C.; Bologna, G.; Federici, L.; Miscia, S.; Pontolillo, M.; Auricchio, A.; et al. Circulating Extracellular Vesicles as New Inflammation Marker in HIV Infection. AIDS 2021, 35, 595–604. [Google Scholar] [CrossRef]
- Snopkova, S.; Matyskova, M.; Havlickova, K.; Jarkovsky, J.; Svoboda, M.; Zavrelova, J.; Svacinka, R.; Penka, M.; Husa, P. Increasing Procoagulant Activity of Circulating Microparticles in Patients Living with HIV. Méd. Mal. Infect. 2020, 50, 555–561. [Google Scholar] [CrossRef]
- Bazié, W.W.; Boucher, J.; Vitry, J.; Goyer, B.; Routy, J.P.; Tremblay, C.; Trottier, S.; Jenabian, M.-A.; Provost, P.; Alary, M.; et al. Plasma Extracellular Vesicle Subtypes May Be Useful as Potential Biomarkers of Immune Activation in People With HIV. Pathog. Immun. 2021, 6, 1–28. [Google Scholar] [CrossRef]
- Hijmans, J.G.; Stockelman, K.A.; Garcia, V.; Levy, M.V.; Brewster, L.M.; Bammert, T.D.; Greiner, J.J.; Stauffer, B.L.; Connick, E.; DeSouza, C.A. Circulating Microparticles Are Elevated in Treated HIV-1 Infection and Are Deleterious to Endothelial Cell Function. J. Am. Heart Assoc. 2019, 8, e011134. [Google Scholar] [CrossRef]
- van der Heijden, W.A.; van de Wijer, L.; Jaeger, M.; Grintjes, K.; Netea, M.G.; Urbanus, R.T.; van Crevel, R.; van den Heuvel, L.P.; Brink, M.; Rodenburg, R.J.; et al. Long-Term Treated HIV Infection Is Associated with Platelet Mitochondrial Dysfunction. Sci. Rep. 2021, 11, 6246. [Google Scholar] [CrossRef]
- Rozmyslowicz, T.; Majka, M.; Kijowski, J.; Murphy, S.L.; Conover, D.O.; Poncz, M.; Ratajczak, J.; Gaulton, G.N.; Ratajczak, M.Z. Platelet- and Megakaryocyte-Derived Microparticles Transfer CXCR4 Receptor to CXCR4-Null Cells and Make Them Susceptible to Infection by X4-HIV. AIDS 2003, 17, 33–42. [Google Scholar] [CrossRef]
- Hamali, H.A.; Mobarki, A.A.; Akhter, M.S.; Saboor, M.; Madkhali, A.M.; Halawani, A.J.; Hakami, A.M.; Eisa, Z.M.; Dobie, G.; Hobani, Y. Elevated Levels of Procoagulant Microvesicles in Patients with Dengue Fever. Future Virol. 2020, 15, 701–706. [Google Scholar] [CrossRef]
- Patil, R.; Bajpai, S.; Ghosh, K.; Shetty, S. Microparticles as Prognostic Biomarkers in Dengue Virus Infection. Acta Trop. 2018, 181, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Paré, G.; Rousseau, M.; Cloutier, N.; Dubuc, I.; Lévesque, T.; Borgeat, P.; Flamand, L. Influenza Virus H1N1 Activates Platelets through FcγRIIA Signaling and Thrombin Generation. Blood 2014, 123, 2854–2863. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N.; Grover, S.P.; Antoniak, S. Tissue Factor Expression, Extracellular Vesicles, and Thrombosis after Infection with the Respiratory Viruses Influenza A Virus and Coronavirus. J. Thromb. Haemost. 2021, 19, 2652–2658. [Google Scholar] [CrossRef]
- Jansen, A.J.G.; Spaan, T.; Low, H.Z.; Di Iorio, D.; van den Brand, J.; Tieke, M.; Barendrecht, A.; Rohn, K.; van Amerongen, G.; Stittelaar, K.; et al. Influenza-Induced Thrombocytopenia Is Dependent on the Subtype and Sialoglycan Receptor and Increases with Virus Pathogenicity. Blood Adv. 2020, 4, 2967–2978. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, E.S.; Chen, Y.; Fitzgerald, K.A.; Jamieson, A.M. Lung Epithelial Cell Transcriptional Regulation as a Factor in COVID-19–Associated Coagulopathies. Am. J. Respir. Cell Mol. Biol. 2021, 64, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Zaid, Y.; Puhm, F.; Allaeys, I.; Naya, A.; Oudghiri, M.; Khalki, L.; Limami, Y.; Zaid, N.; Sadki, K.; Ben El Haj, R.; et al. Platelets Can Associate With SARS-CoV-2 RNA and Are Hyperactivated in COVID-19. Circ. Res. 2020, 127, 1404–1418. [Google Scholar] [CrossRef]
- Guervilly, C.; Bonifay, A.; Burtey, S.; Sabatier, F.; Cauchois, R.; Abdili, E.; Arnaud, L.; Lano, G.; Pietri, L.; Robert, T.; et al. Dissemination of Extreme Levels of Extracellular Vesicles: Tissue Factor Activity in Patients with Severe COVID-19. Blood Adv. 2021, 5, 628–634. [Google Scholar] [CrossRef]
- Cappellano, G.; Raineri, D.; Rolla, R.; Giordano, M.; Puricelli, C.; Vilardo, B.; Manfredi, M.; Cantaluppi, V.; Sainaghi, P.P.; Castello, L.; et al. Circulating Platelet-Derived Extracellular Vesicles Are a Hallmark of Sars-Cov-2 Infection. Cells 2021, 10, 85. [Google Scholar] [CrossRef]
- Theresa, L.; Whiteside. Procoagulant Activity of Extracellular Vesicles in Plasma of Patients with SARS-CoV-2 Infection. EBioMedicine 2021, 68, 103411. [Google Scholar] [CrossRef]
- Puhm, F.; Flamand, L.; Boilard, E. Platelet Extracellular Vesicles in COVID-19: Potential Markers and Makers. J. Leukoc. Biol. 2021, 111, 63–74. [Google Scholar] [CrossRef]
- Rausch, L.; Lutz, K.; Schifferer, M.; Winheim, E.; Gruber, R.; Rinke, L.; Hellmuth, J.C.; Scherer, C.; Muenchhoff, M.; Mandel, C.; et al. Binding of Phosphatidylserine-Positive Microparticles by PBMCs Classifies Disease Severity in COVID-19 Patients. J. Extracell. Vesicles 2021, 10, e12173. [Google Scholar] [CrossRef]
- Stenberg, P.E.; Shuman, M.A.; Levine, S.P.; Bainton, D.F. Redistribution of Alpha-Granules and Their Contents in Thrombin-Stimulated Platelets. J. Cell Biol. 1984, 98, 748–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escolar, G.; White, J.G. The Platelet Open Canalicular System: A Final Common Pathway. Blood Cells 1991, 17, 467–485; discussion 486–495. [Google Scholar] [PubMed]
- Singh, A.; Bisht, P.; Bhattacharya, S.; Guchhait, P. Role of Platelet Cytokines in Dengue Virus Infection. Front. Cell. Infect. Microbiol. 2020, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet α-Granules: Basic Biology and Clinical Correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frojmovic, M.M.; Milton, J.G. Human Platelet Size, Shape, and Related Functions in Health and Disease. Physiol. Rev. 1982, 62, 185–261. [Google Scholar] [CrossRef]
- Zufferey, A.; Schvartz, D.; Nolli, S.; Reny, J.-L.; Sanchez, J.-C.; Fontana, P. Characterization of the Platelet Granule Proteome: Evidence of the Presence of MHC1 in Alpha-Granules. J. Proteom. 2014, 101, 130–140. [Google Scholar] [CrossRef]
- Klockenbusch, C.; Walsh, G.M.; Brown, L.M.; Hoffman, M.D.; Ignatchenko, V.; Kislinger, T.; Kast, J. Global Proteome Analysis Identifies Active Immunoproteasome Subunits in Human Platelets. Mol. Cell. Proteom. 2014, 13, 3308–3319. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.S.; Smith, M.C.P.; Campbell, R.A.; Zimmerman, P.W.; Franks, Z.B.; Kraemer, B.F.; Machlus, K.R.; Ling, J.; Kamba, P.; Schwertz, H.; et al. Proteasome Function Is Required for Platelet Production. J. Clin. Investig. 2014, 124, 3757–3766. [Google Scholar] [CrossRef]
- Berger, G.; Massé, J.M.; Cramer, E.M. Alpha-Granule Membrane Mirrors the Platelet Plasma Membrane and Contains the Glycoproteins Ib, IX, and V. Blood 1996, 87, 1385–1395. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Murasaki, K.; Kodama, K.; Takayama, H. Intracellular Localization of Glycoprotein VI in Human Platelets and Its Surface Expression upon Activation. Br. J. Haematol. 2003, 121, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Coppinger, J.A.; Cagney, G.; Toomey, S.; Kislinger, T.; Belton, O.; McRedmond, J.P.; Cahill, D.J.; Emili, A.; Fitzgerald, D.J.; Maguire, P.B. Characterization of the Proteins Released from Activated Platelets Leads to Localization of Novel Platelet Proteins in Human Atherosclerotic Lesions. Blood 2004, 103, 2096–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rendu, F.; Brohard-Bohn, B. The Platelet Release Reaction: Granules’ Constituents, Secretion and Functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Novotny, W.F.; Girard, T.J.; Miletich, J.P.; Broze, G.J. Platelets Secrete a Coagulation Inhibitor Functionally and Antigenically Similar to the Lipoprotein Associated Coagulation Inhibitor. Blood 1988, 72, 2020–2025. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, H.P.; Heeb, M.J.; Wencel-Drake, J.D.; Griffin, J.H. Identification and Quantitation of Protein S in Human Platelets. Blood 1985, 66, 1452–1455. [Google Scholar] [CrossRef] [Green Version]
- Van Nostrand, W.E.; Schmaier, A.H.; Farrow, J.S.; Cunningham, D.D. Protease Nexin-II (Amyloid Beta-Protein Precursor): A Platelet Alpha-Granule Protein. Science 1990, 248, 745–748. [Google Scholar] [CrossRef]
- Brandt, E.; Petersen, F.; Ludwig, A.; Ehlert, J.E.; Bock, L.; Flad, H.D. The Beta-Thromboglobulins and Platelet Factor 4: Blood Platelet-Derived CXC Chemokines with Divergent Roles in Early Neutrophil Regulation. J. Leukoc. Biol. 2000, 67, 471–478. [Google Scholar] [CrossRef]
- Kowalska, M.A.; Rauova, L.; Poncz, M. Role of the Platelet Chemokine Platelet Factor 4 (PF4) in Hemostasis and Thrombosis. Thromb. Res. 2010, 125, 292–296. [Google Scholar] [CrossRef]
- Scheuerer, B.; Ernst, M.; Dürrbaum-Landmann, I.; Fleischer, J.; Grage-Griebenow, E.; Brandt, E.; Flad, H.-D.; Petersen, F. The CXC-Chemokine Platelet Factor 4 Promotes Monocyte Survival and Induces Monocyte Differentiation into Macrophages. Blood 2000, 95, 1158–1166. [Google Scholar] [CrossRef]
- Hartwig, H.; Drechsler, M.; Lievens, D.; Kramp, B.; von Hundelshausen, P.; Lutgens, E.; Weber, C.; Döring, Y.; Soehnlein, O. Platelet-Derived PF4 Reduces Neutrophil Apoptosis Following Arterial Occlusion. Thromb. Haemost. 2014, 112, 562–564. [Google Scholar]
- Liu, C.Y.; Battaglia, M.; Lee, S.H.; Sun, Q.-H.; Aster, R.H.; Visentin, G.P. Platelet Factor 4 Differentially Modulates CD4+CD25+ (Regulatory) versus CD4+CD25- (Nonregulatory) T Cells. J. Immunol. 2005, 174, 2680–2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fricke, I.; Mitchell, D.; Petersen, F.; Böhle, A.; Bulfone-Paus, S.; Brandau, S. Platelet Factor 4 in Conjunction with IL-4 Directs Differentiation of Human Monocytes into Specialized Antigen-Presenting Cells. FASEB J. 2004, 18, 1588–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, A.G. Platelet-Derived Chemokines in Atherogenesis: What’s New? Curr. Vasc. Pharmacol. 2012, 10, 563–569. [Google Scholar]
- Cole, A.M.; Ganz, T.; Liese, A.M.; Burdick, M.D.; Liu, L.; Strieter, R.M. Cutting Edge: IFN-Inducible ELR- CXC Chemokines Display Defensin-like Antimicrobial Activity. J. Immunol. 2001, 167, 623–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.-Q.; Yeaman, M.R.; Selsted, M.E. Antimicrobial Peptides from Human Platelets. Infect. Immun. 2002, 70, 6524–6533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaujac, C.; Boukour, S.; Cramer-Bordé, E. Platelets and Viruses: An Ambivalent Relationship. Cell. Mol. Life Sci. 2010, 67, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, D.J.; Lin, Y.; Miao, H.; Cimbro, R.; DiFiore, M.J.; Gianolini, M.E.; Furci, L.; Biswas, P.; Fauci, A.S.; Lusso, P. Identification of the Platelet-Derived Chemokine CXCL4/PF-4 as a Broad-Spectrum HIV-1 Inhibitor. Proc. Natl. Acad. Sci. USA 2012, 109, 9569–9574. [Google Scholar] [CrossRef] [Green Version]
- Parker, Z.F.; Rux, A.H.; Riblett, A.M.; Lee, F.-H.; Rauova, L.; Cines, D.B.; Poncz, M.; Sachais, B.S.; Doms, R.W. Platelet Factor 4 Inhibits and Enhances HIV-1 Infection in a Concentration-Dependent Manner by Modulating Viral Attachment. AIDS Res. Hum. Retrovir. 2016, 32, 705–717. [Google Scholar] [CrossRef] [Green Version]
- Solomon Tsegaye, T.; Gnirß, K.; Rahe-Meyer, N.; Kiene, M.; Krämer-Kühl, A.; Behrens, G.; Münch, J.; Pöhlmann, S. Platelet Activation Suppresses HIV-1 Infection of T Cells. Retrovirology 2013, 10, 48. [Google Scholar] [CrossRef] [Green Version]
- Poon, T.C.W.; Pang, R.T.K.; Chan, K.C.A.; Lee, N.L.S.; Chiu, R.W.K.; Tong, Y.-K.; Chim, S.S.C.; Ngai, S.M.; Sung, J.J.Y.; Lo, Y.M.D. Proteomic Analysis Reveals Platelet Factor 4 and Beta-Thromboglobulin as Prognostic Markers in Severe Acute Respiratory Syndrome. Electrophoresis 2012, 33, 1894–1900. [Google Scholar] [CrossRef]
- Guo, L.; Feng, K.; Wang, Y.C.; Mei, J.J.; Ning, R.T.; Zheng, H.W.; Wang, J.J.; Worthen, G.S.; Wang, X.; Song, J.; et al. Critical Role of CXCL4 in the Lung Pathogenesis of Influenza (H1N1) Respiratory Infection. Mucosal. Immunol. 2017, 10, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Bhasym, A.; Mukherjee, S.; Annarapu, G.K.; Bhakuni, T.; Akbar, I.; Seth, T.; Vikram, N.K.; Vrati, S.; Basu, A.; et al. Platelet Factor 4 Promotes Rapid Replication and Propagation of Dengue and Japanese Encephalitis Viruses. EBioMedicine 2019, 39, 332–347. [Google Scholar] [CrossRef] [Green Version]
- Fragnoud, R.; Flamand, M.; Reynier, F.; Buchy, P.; Duong, V.; Pachot, A.; Paranhos-Baccala, G.; Bedin, F. Differential Proteomic Analysis of Virus-Enriched Fractions Obtained from Plasma Pools of Patients with Dengue Fever or Severe Dengue. BMC Infect. Dis. 2015, 15, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Cloutier, N.; Paré, A.; Farndale, R.W.; Schumacher, H.R.; Nigrovic, P.A.; Lacroix, S.; Boilard, E. Platelets Can Enhance Vascular Permeability. Blood 2012, 120, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Marcoux, G.; Laroche, A.; Hasse, S.; Bellio, M.; Mbarik, M.; Tamagne, M.; Allaeys, I.; Zufferey, A.; Lévesque, T.; Rebetz, J.; et al. Platelet EVs Contain an Active Proteasome Involved in Protein Processingfor Antigen Presentation via MHC-I Molecules. Blood 2021, 138, 2607–2620. [Google Scholar] [CrossRef] [PubMed]
- French, S.L.; Butov, K.R.; Allaeys, I.; Canas, J.; Morad, G.; Davenport, P.; Laroche, A.; Trubina, N.M.; Italiano, J.E., Jr.; Moses, M.A.; et al. Platelet-Derived Extracellular Vesicles Infiltrate and Modify the Bone Marrow during Inflammation. Blood Adv. 2020, 4, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Milasan, A.; Tessandier, N.; Tan, S.; Brisson, A.; Boilard, E.; Martel, C. Extracellular Vesicles Are Present in Mouse Lymph and Their Level Differs in Atherosclerosis. J. Extracell. Vesicles 2016, 5, 31427. [Google Scholar] [CrossRef] [Green Version]
- Tessandier, N.; Melki, I.; Cloutier, N.; Allaeys, I.; Miszta, A.; Tan, S.; Milasan, A.; Michel, S.; Benmoussa, A.; Lévesque, T.; et al. Platelets Disseminate Extracellular Vesicles in Lymph in Rheumatoid Arthritis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 929–942. [Google Scholar] [CrossRef]
- Guerreiro, E.M.; Vestad, B.; Steffensen, L.A.; Aass, H.C.D.; Saeed, M.; Øvstebø, R.; Costea, D.E.; Galtung, H.K.; Søland, T.M. Efficient Extracellular Vesicle Isolation by Combining Cell Media Modifications, Ultrafiltration, and Size-Exclusion Chromatography. PLoS ONE 2018, 13, e0204276. [Google Scholar] [CrossRef] [Green Version]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A Comparison of Methods for the Isolation and Separation of Extracellular Vesicles from Protein and Lipid Particles in Human Serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Weber, S.R.; Zhao, Y.; Chen, H.; Sundstrom, J.M. Chapter 2—Methods for Exosome Isolation and Characterization. In Exosomes; Edelstein, L., Smythies, J., Quesenberry, P., Noble, D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 23–38. ISBN 978-0-12-816053-4. [Google Scholar]
- Zhang, J.; Yuan, T.; Wang, J.H.-C. Moderate Treadmill Running Exercise Prior to Tendon Injury Enhances Wound Healing in Aging Rats. Oncotarget 2016, 7, 8498–8512. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Shang-Chun, G.; Pei, H.; Chang-Qing, Z.; Bing-Fang, Z. Applications of Leukocyte- and Platelet-Rich Plasma (L-PRP) in Trauma Surgery. Curr. Pharm. Biotechnol. 2012, 13, 1173–1184. [Google Scholar]
- Martino, M.M.; Tortelli, F.; Mochizuki, M.; Traub, S.; Ben-David, D.; Kuhn, G.A.; Müller, R.; Livne, E.; Eming, S.A.; Hubbell, J.A. Engineering the Growth Factor Microenvironment with Fibronectin Domains to Promote Wound and Bone Tissue Healing. Sci. Transl. Med. 2011, 3, 100ra89. [Google Scholar] [CrossRef] [Green Version]
- Losi, P.; Briganti, E.; Errico, C.; Lisella, A.; Sanguinetti, E.; Chiellini, F.; Soldani, G. Fibrin-Based Scaffold Incorporating VEGF- and BFGF-Loaded Nanoparticles Stimulates Wound Healing in Diabetic Mice. Acta Biomater. 2013, 9, 7814–7821. [Google Scholar] [CrossRef]
- Beanes, S.R.; Dang, C.; Soo, C.; Ting, K. Skin Repair and Scar Formation: The Central Role of TGF-β. Expert Rev. Mol. Med. 2003, 5, 1–22. [Google Scholar] [CrossRef]
- Masieri, L.; Sessa, F.; Mari, A.; Campi, R.; Cito, G.; Verrienti, P.; Nozzoli, C.; Saccardi, R.; Sforza, S.; Di Maida, F.; et al. Intravesical Application of Platelet-Rich Plasma in Patients with Persistent Haemorrhagic Cystitis after Hematopoietic Stem Cell Transplantation: A Single-Centre Preliminary Experience. Int. Urol. Nephrol. 2019, 51, 1715–1720. [Google Scholar] [CrossRef]
- Karina, K.; Rosliana, I.; Rosadi, I.; Sobariah, S.; Christoffel, L.M.; Novariani, R.; Rosidah, S.; Fatkhurohman, N.; Hertati, Y.; Puspitaningrum, N.; et al. Phase I/II Clinical Trial of Autologous Activated Platelet-Rich Plasma (AaPRP) in the Treatment of Severe Coronavirus Disease 2019 (COVID-19) Patients. Int. J. Inflamm. 2021, 2021, e5531873. [Google Scholar] [CrossRef]
- Farghali, H.A.; AbdElKader, N.A.; AbuBakr, H.O.; Ramadan, E.S.; Khattab, M.S.; Salem, N.Y.; Emam, I.A. Corneal Ulcer in Dogs and Cats: Novel Clinical Application of Regenerative Therapy Using Subconjunctival Injection of Autologous Platelet-Rich Plasma. Front. Vet. Sci. 2021, 8, 123. [Google Scholar] [CrossRef]
- Henschler, R.; Gabriel, C.; Schallmoser, K.; Burnouf, T.; Koh, M.B.C. Human Platelet Lysate Current Standards and Future Developments. Transfusion 2019, 59, 1407–1413. [Google Scholar] [CrossRef]
- Burnouf, T.; Strunk, D.; Koh, M.B.C.; Schallmoser, K. Human Platelet Lysate: Replacing Fetal Bovine Serum as a Gold Standard for Human Cell Propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Muthu, S.; Khanna, M.; Jain, R.; Talagavadi Channaiah, A.; Muthukanagaraj, P.; Siddesh, S.E.; Gulati, A.; Satish, A.; Jeyaraman, N.; et al. Platelet Lysate for COVID-19 Pneumonia—A Newer Adjunctive Therapeutic Avenue. Stem Cell Investig. 2021, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Schallmoser, K.; Henschler, R.; Gabriel, C.; Koh, M.B.C.; Burnouf, T. Production and Quality Requirements of Human Platelet Lysate: A Position Statement from the Working Party on Cellular Therapies of the International Society of Blood Transfusion. Trends Biotechnol. 2020, 38, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liang, J.-X.; Li, S.-H.; Huang, S.; Yan, J.-X.; Xiao, L.-L.; Song, J.-X.; Liu, H.-W. Allogeneic Platelet-Rich Plasma Therapy as an Effective and Safe Adjuvant Method for Chronic Wounds. J. Surg. Res. 2020, 246, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Machhi, J.; Shahjin, F.; Das, S.; Patel, M.; Abdelmoaty, M.M.; Cohen, J.D.; Singh, P.A.; Baldi, A.; Bajwa, N.; Kumar, R.; et al. A Role for Extracellular Vesicles in SARS-CoV-2 Therapeutics and Prevention. J. Neuroimmune Pharm. 2021, 16, 270–288. [Google Scholar] [CrossRef]
- Wu, J.; Piao, Y.; Liu, Q.; Yang, X. Platelet-Rich Plasma-Derived Extracellular Vesicles: A Superior Alternative in Regenerative Medicine? Cell Prolif. 2021, 54, e13123. [Google Scholar] [CrossRef]
- Soleymani, S.; Yari, F.; Bolhassani, A.; Bakhshandeh, H. Platelet Microparticles: An Effective Delivery System for Anti-Viral Drugs. J. Drug Deliv. Sci. Technol. 2019, 51, 290–296. [Google Scholar] [CrossRef]
- Lovisolo, F.; Carton, F.; Gino, S.; Migliario, M.; Renò, F. Platelet Rich Plasma-Derived Microvesicles Increased in Vitro Wound Healing. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9658–9664. [Google Scholar]
- Torreggiani, E.; Perut, F.; Roncuzzi, L.; Zini, N.; Baglìo, S.R.; Baldini, N. Exosomes: Novel Effectors of Human Platelet Lysate Activity. Eur. Cell Mater. 2014, 28, 137–151; discussion 151. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Fan, Q.; Xu, J.; Bai, J.; Han, X.; Dong, Z.; Zhou, X.; Liu, Z.; Gu, Z.; Wang, C. Calming Cytokine Storm in Pneumonia by Targeted Delivery of TPCA-1 Using Platelet-Derived Extracellular Vesicles. Matter 2020, 3, 287–301. [Google Scholar] [CrossRef]
- Ma, Q.; Bai, J.; Xu, J.; Dai, H.; Fan, Q.; Fei, Z.; Chu, J.; Yao, C.; Shi, H.; Zhou, X.; et al. Reshaping the Inflammatory Environment in Rheumatoid Arthritis Joints by Targeting Delivery of Berberine with Platelet-Derived Extracellular Vesicles. Adv. NanoBiomed Res. 2021, 1, 2100071. [Google Scholar] [CrossRef]
- Ma, Q.; Fan, Q.; Han, X.; Dong, Z.; Xu, J.; Bai, J.; Tao, W.; Sun, D.; Wang, C. Platelet-Derived Extracellular Vesicles to Target Plaque Inflammation for Effective Anti-Atherosclerotic Therapy. J. Control. Release 2021, 329, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-C.; Tao, S.-C.; Yin, W.-J.; Qi, X.; Yuan, T.; Zhang, C.-Q. Exosomes Derived from Platelet-Rich Plasma Promote the Re-Epithelization of Chronic Cutaneous Wounds via Activation of YAP in a Diabetic Rat Model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, S.-C.; Yuan, T.; Rui, B.-Y.; Zhu, Z.-Z.; Guo, S.-C.; Zhang, C.-Q. Exosomes Derived from Human Platelet-Rich Plasma Prevent Apoptosis Induced by Glucocorticoid-Associated Endoplasmic Reticulum Stress in Rat Osteonecrosis of the Femoral Head via the Akt/Bad/Bcl-2 Signal Pathway. Theranostics 2017, 7, 733–750. [Google Scholar] [CrossRef]
- Iyer, S.R.; Scheiber, A.L.; Yarowsky, P.; Henn, R.F.; Otsuru, S.; Lovering, R.M. Exosomes Isolated From Platelet-Rich Plasma and Mesenchymal Stem Cells Promote Recovery of Function After Muscle Injury. Am. J. Sports Med. 2020, 48, 2277–2286. [Google Scholar] [CrossRef]
- Lopez, E.; Srivastava, A.K.; Burchfield, J.; Wang, Y.-W.; Cardenas, J.C.; Togarrati, P.P.; Miyazawa, B.; Gonzalez, E.; Holcomb, J.B.; Pati, S.; et al. Platelet-Derived- Extracellular Vesicles Promote Hemostasis and Prevent the Development of Hemorrhagic Shock. Sci. Rep. 2019, 9, 17676. [Google Scholar] [CrossRef] [Green Version]
- Vozel, D.; Božič, D.; Jeran, M.; Jan, Z.; Pajnič, M.; Pađen, L.; Steiner, N.; Kralj-Iglič, V.; Battelino, S. Autologous Platelet- and Extracellular Vesicle-Rich Plasma Is an Effective Treatment Modality for Chronic Postoperative Temporal Bone Cavity Inflammation: Randomized Controlled Clinical Trial. Front. Bioeng. Biotechnol. 2021, 9, 677541. [Google Scholar] [CrossRef]
- Ferreira, P.M.; Bozbas, E.; Tannetta, S.D.; Alroqaiba, N.; Zhou, R.; Crawley, J.T.B.; Gibbins, J.M.; Jones, C.I.; Ahnström, J.; Yaqoob, P. Mode of Induction of Platelet-Derived Extracellular Vesicles Is a Critical Determinant of Their Phenotype and Function. Sci. Rep. 2020, 10, 18061. [Google Scholar] [CrossRef]
- Nyam-Erdene, A.; Nebie, O.; Delila, L.; Buée, L.; Devos, D.; Chou, S.-Y.; Blum, D.; Burnouf, T. Characterization and Chromatographic Isolation of Platelet Extracellular Vesicles from Human Platelet Lysates for Applications in Neuroregenerative Medicine. ACS Biomater. Sci. Eng. 2021, 7, 5823–5835. [Google Scholar] [CrossRef]
- Bhan, A.; Ansari, K.; Chen, M.Y.; Jandial, R. Human Induced Pluripotent Stem Cell-Derived Platelets Loaded with Lapatinib Effectively Target HER2+ Breast Cancer Metastasis to the Brain. Sci. Rep. 2021, 11, 16866. [Google Scholar] [CrossRef]
- Han, X.; Chen, J.; Chu, J.; Liang, C.; Ma, Q.; Fan, Q.; Liu, Z.; Wang, C. Platelets as Platforms for Inhibition of Tumor Recurrence Post-Physical Therapy by Delivery of Anti-PD-L1 Checkpoint Antibody. J. Control. Release 2019, 304, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, S.; Wang, Z. High Yield, Scalable and Remotely Drug-Loaded Neutrophil-Derived Extracellular Vesicles (EVs) for Anti-Inflammation Therapy. Biomaterials 2017, 135, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.-T.; Wang, B.; Lv, L.-L.; Liu, B.-C. Extracellular Vesicle-Based Nanotherapeutics: Emerging Frontiers in Anti-Inflammatory Therapy. Theranostics 2020, 10, 8111–8129. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes Facilitate Therapeutic Targeting of Oncogenic KRAS in Pancreatic Cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Li, C.; Zhang, Y.; Zhang, D.; Otterbein, L.E.; Jin, Y. Caveolin-1 Selectively Regulates MicroRNA Sorting into Microvesicles after Noxious Stimuli. J. Exp. Med. 2019, 216, 2202–2220. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Wei, M.; Gao, X.; Zhao, L.; Shi, R.; Sun, W.; Duan, Y.; Yang, G.; Yuan, L. In Vitro and in Vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with MiRNA or CRISPR/DCas9. Nano Lett. 2019, 19, 19–28. [Google Scholar] [CrossRef]
- Kalinec, G.M.; Gao, L.; Cohn, W.; Whitelegge, J.P.; Faull, K.F.; Kalinec, F. Extracellular Vesicles From Auditory Cells as Nanocarriers for Anti-Inflammatory Drugs and Pro-Resolving Mediators. Front. Cell. Neurosci. 2019, 13, 530. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.-T.; Lv, L.-L.; Wang, B.; Cao, J.-Y.; Feng, Y.; Li, Z.-L.; Wu, M.; Wang, F.-M.; Wen, Y.; Zhou, L.-T.; et al. Employing Macrophage-Derived Microvesicle for Kidney-Targeted Delivery of Dexamethasone: An Efficient Therapeutic Strategy against Renal Inflammation and Fibrosis. Theranostics 2019, 9, 4740–4755. [Google Scholar] [CrossRef]
- Mo, L.-H.; Han, H.-Y.; Jin, Q.-R.; Song, Y.-N.; Wu, G.-H.; Zhang, Y.; Yang, L.-T.; Liu, T.; Liu, Z.-G.; Feng, Y.; et al. T Cell Activator-Carrying Extracellular Vesicles Induce Antigen-Specific Regulatory T Cells. Clin. Exp. Immunol. 2021, 206, 129–140. [Google Scholar] [CrossRef]
- Veilleux, V.; Mallet Gauthier, È.; Jougleux, J.-L.; Boudreau, L.; Robichaud, G. Breast Cancer Processes Are Modulated by Platelet-Derived Microparticles. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Haghbeen, M.; Hashemi Tayer, A.; Kamravan, M.; Sotoodeh Jahromi, A. Platelet-Derived Procoagulant Microparticles as Blood-Based Biomarker of Breast Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, J.; Liu, Y.; Wu, J.; Yuan, Y.; Wang, C.; Fang, X.; Li, H. Prediction of the Therapeutic Effects of Pembrolizumab and Nivolumab in Advanced Non-Small Cell Lung Cancer by Platelet-Derived Microparticles in Circulating Blood. Technol. Cancer Res. Treat. 2021, 20, 1533033821997817. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.-E.; El-Baba, M.D.; Saxena, P.; Ausländer, S.; Tan, K.R.; Fussenegger, M. Designer Exosomes Produced by Implanted Cells Intracerebrally Deliver Therapeutic Cargo for Parkinson’s Disease Treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piffoux, M.; Nicolás-Boluda, A.; Mulens-Arias, V.; Richard, S.; Rahmi, G.; Gazeau, F.; Wilhelm, C.; Silva, A.K.A. Extracellular Vesicles for Personalized Medicine: The Input of Physically Triggered Production, Loading and Theranostic Properties. Adv. Drug Deliv. Rev. 2019, 138, 247–258. [Google Scholar] [CrossRef]
- Gudbergsson, J.M.; Jønsson, K.; Simonsen, J.B.; Johnsen, K.B. Systematic Review of Targeted Extracellular Vesicles for Drug Delivery—Considerations on Methodological and Biological Heterogeneity. J. Control. Release 2019, 306, 108–120. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering Hybrid Exosomes by Membrane Fusion with Liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef] [Green Version]
- Piffoux, M.; Silva, A.K.A.; Wilhelm, C.; Gazeau, F.; Tareste, D. Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems. ACS Nano 2018, 12, 6830–6842. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage Exosomes as Natural Nanocarriers for Protein Delivery to Inflamed Brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Pawlowski, C.L.; Li, W.; Sun, M.; Ravichandran, K.; Hickman, D.; Kos, C.; Kaur, G.; Sen Gupta, A. Platelet Microparticle-Inspired Clot-Responsive Nanomedicine for Targeted Fibrinolysis. Biomaterials 2017, 128, 94–108. [Google Scholar] [CrossRef]
- Cheng, Q.; Shi, X.; Han, M.; Smbatyan, G.; Lenz, H.-J.; Zhang, Y. Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J. Am. Chem. Soc. 2018, 140, 16413–16417. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T.; et al. Engineered Exosomes With Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e008737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Yun, N.; Mun, D.; Kang, J.-Y.; Lee, S.-H.; Park, H.; Park, H.; Joung, B. Cardiac-Specific Delivery by Cardiac Tissue-Targeting Peptide-Expressing Exosomes. Biochem. Biophys. Res. Commun. 2018, 499, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface Functionalized Exosomes as Targeted Drug Delivery Vehicles for Cerebral Ischemia Therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Speidl, W.S.; Exner, M.; Amighi, J.; Kastl, S.P.; Zorn, G.; Maurer, G.; Wagner, O.; Huber, K.; Minar, E.; Wojta, J.; et al. Complement Component C5a Predicts Future Cardiovascular Events in Patients with Advanced Atherosclerosis. Eur. Heart J. 2005, 26, 2294–2299. [Google Scholar] [CrossRef]
- Patzelt, J.; Verschoor, A.; Langer, H.F. Platelets and the Complement Cascade in Atherosclerosis. Front. Physiol. 2015, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- Rondina, M.T.; Tatsumi, K.; Bastarache, J.A.; Mackman, N. Microvesicle Tissue Factor Activity and Interleukin-8 Levels Are Associated with Mortality in Patients with Influenza A/H1N1 Infection. Crit. Care Med. 2016, 44, e574–e578. [Google Scholar] [CrossRef]
- Oseasohn, R.; Adelson, L.; Kaji, M. Clinicopathologic Study of Thirty-Three Fatal Cases of Asian Influenza. N. Engl. J. Med. 1959, 260, 509–518. [Google Scholar] [CrossRef]
- Hahn, S.; Giaglis, S.; Chowdhury, C.S.; Chowdury, C.S.; Hösli, I.; Hasler, P. Modulation of Neutrophil NETosis: Interplay between Infectious Agents and Underlying Host Physiology. In Seminars in Immunopathology; Springer: Berlin, Germany, 2013; Volume 35, pp. 439–453. [Google Scholar]
- Szebeni, J.; Alving, C.R.; Rosivall, L.; Bünger, R.; Baranyi, L.; Bedöcs, P.; Tóth, M.; Barenholz, Y. Animal Models of Complement-Mediated Hypersensitivity Reactions to Liposomes and Other Lipid-Based Nanoparticles. J. Liposome Res. 2007, 17, 107–117. [Google Scholar] [CrossRef]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A Stress Reaction in Blood Triggered by Nanomedicines and Biologicals. Mol. Immunol. 2014, 61, 163–173. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Huang, C.-C.; Changou, C.A.; Lu, L.-S.; Goubran, H.; Burnouf, T. Clinical-Grade Cryopreserved Doxorubicin-Loaded Platelets: Role of Cancer Cells and Platelet Extracellular Vesicles Activation Loop. J. Biomed. Sci. 2020, 27, 45. [Google Scholar] [CrossRef] [Green Version]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef] [PubMed]


| Disease/ Condition | Model | Source of pEVs | Application to Viral Diseases | Reference (s) |
|---|---|---|---|---|
| HIV-1 | In vitro Hela and U266 cells | Platelet concentrate, differential centrifugation. “PMPs”/PMVs | HIV and encapsulation of other anti-viral drugs for other viruses. | Soleymani, S. et al., 2019 |
| Wound healing | In vitro HaCaT cell monolayers. | PRP-MVs | Viral diseases associated with aberrant hemostasis and thrombosis issues, such as dengue virus, Ebola, and severe SARS-CoV-2. | Lovisolo, F. et al., 2020 |
| Cancer (HER2+ breast-to brain-metastasis) | Mouse | Indirect in vivo generation of pEVs via hiPSC-generated platelets infused into mice. | Cancer-inducing viral diseases. | Bhan, A. et al., 2021 |
| Cancer | Mouse | Transplant of engineered platelets with monoclonal antibodies on their surface. In vivo generation of pEVs with these monoclonal antibodies upon platelet activation. | Cancer-inducing viral diseases. | Han, X. et al., 2019 |
| Pneumonia | Mouse | Mouse PRP, PRP-EVs | Pulmonary inflammation diseases, such as influenza and SARS-CoV-2. | Ma, Q.; Fan, Q. et al., 2020 |
| Atherosclerosis | Mouse | Mouse PRP, PRP-EVs | Ma, Q.; Fan, Q. et al., 2021 | |
| Rheumatoid Arthritis | Mouse | Mouse PRP, PRP-EVs | Ma, Q.; Bai, J. et al., 2021 | |
| Diabetes/chronic wounds | Rat | Human PRP, PRP-Exos | Viral diseases with aberrant hemostasis and thrombosis issues, such as dengue virus, Ebola, and severe SARS-CoV-2. | Guo, S. et al., 2017 Tao, S. et al., 2017 |
| Muscle Injury | Rat | PRP-Exos | Iyer, S. et al., 2020 | |
| Hemorrhagic Shock | Rat (liver trauma) | PRP-EVs | Viral diseases with aberrant hemostasis and thrombosis issues, such as dengue virus, Ebola, and severe SARS-CoV-2. | Lopez, E. et al., 2019 |
| Chronic Postoperative Temporal Bone Cavity Inflammation | Human Clinical Trials | PVRP | Vozel, D. et al., 2021 NCT04281901 (clinicaltrials.gov) | |
| Chronic Middle Ear Infections | Human Clinical Trials | PVRP | NCT04761562 (clinicaltrials.gov) | |
| Chronic Lower Back Pain | Human Clinical Trials | PRP-Exos | NCT04849429 (clinicaltrials.gov) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostermeier, B.; Soriano-Sarabia, N.; Maggirwar, S.B. Platelet-Released Factors: Their Role in Viral Disease and Applications for Extracellular Vesicle (EV) Therapy. Int. J. Mol. Sci. 2022, 23, 2321. https://doi.org/10.3390/ijms23042321
Ostermeier B, Soriano-Sarabia N, Maggirwar SB. Platelet-Released Factors: Their Role in Viral Disease and Applications for Extracellular Vesicle (EV) Therapy. International Journal of Molecular Sciences. 2022; 23(4):2321. https://doi.org/10.3390/ijms23042321
Chicago/Turabian StyleOstermeier, Brita, Natalia Soriano-Sarabia, and Sanjay B. Maggirwar. 2022. "Platelet-Released Factors: Their Role in Viral Disease and Applications for Extracellular Vesicle (EV) Therapy" International Journal of Molecular Sciences 23, no. 4: 2321. https://doi.org/10.3390/ijms23042321
APA StyleOstermeier, B., Soriano-Sarabia, N., & Maggirwar, S. B. (2022). Platelet-Released Factors: Their Role in Viral Disease and Applications for Extracellular Vesicle (EV) Therapy. International Journal of Molecular Sciences, 23(4), 2321. https://doi.org/10.3390/ijms23042321






