Ab Initio Insight into the Interaction of Metal-Decorated Fluorinated Carbon Fullerenes with Anti-COVID Drugs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Atomistic Models of Fluorinated Fullerenes
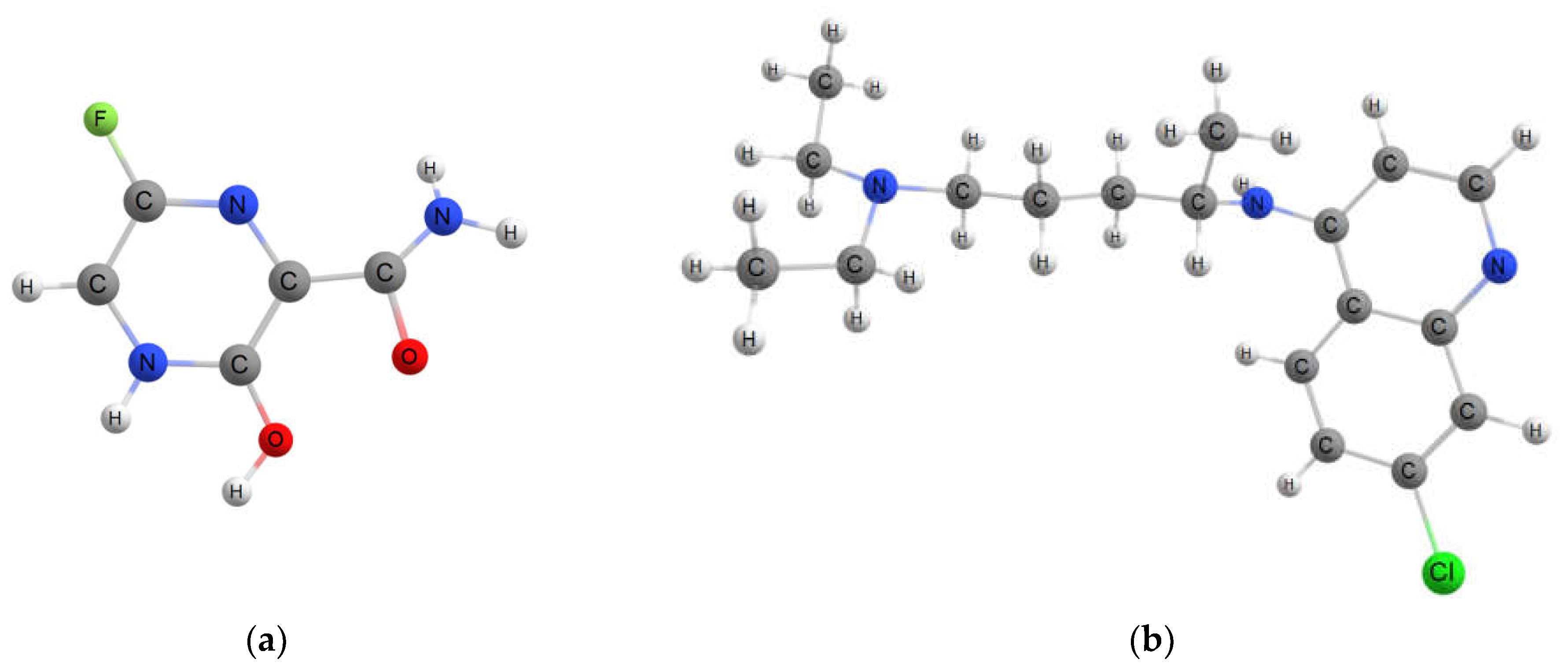
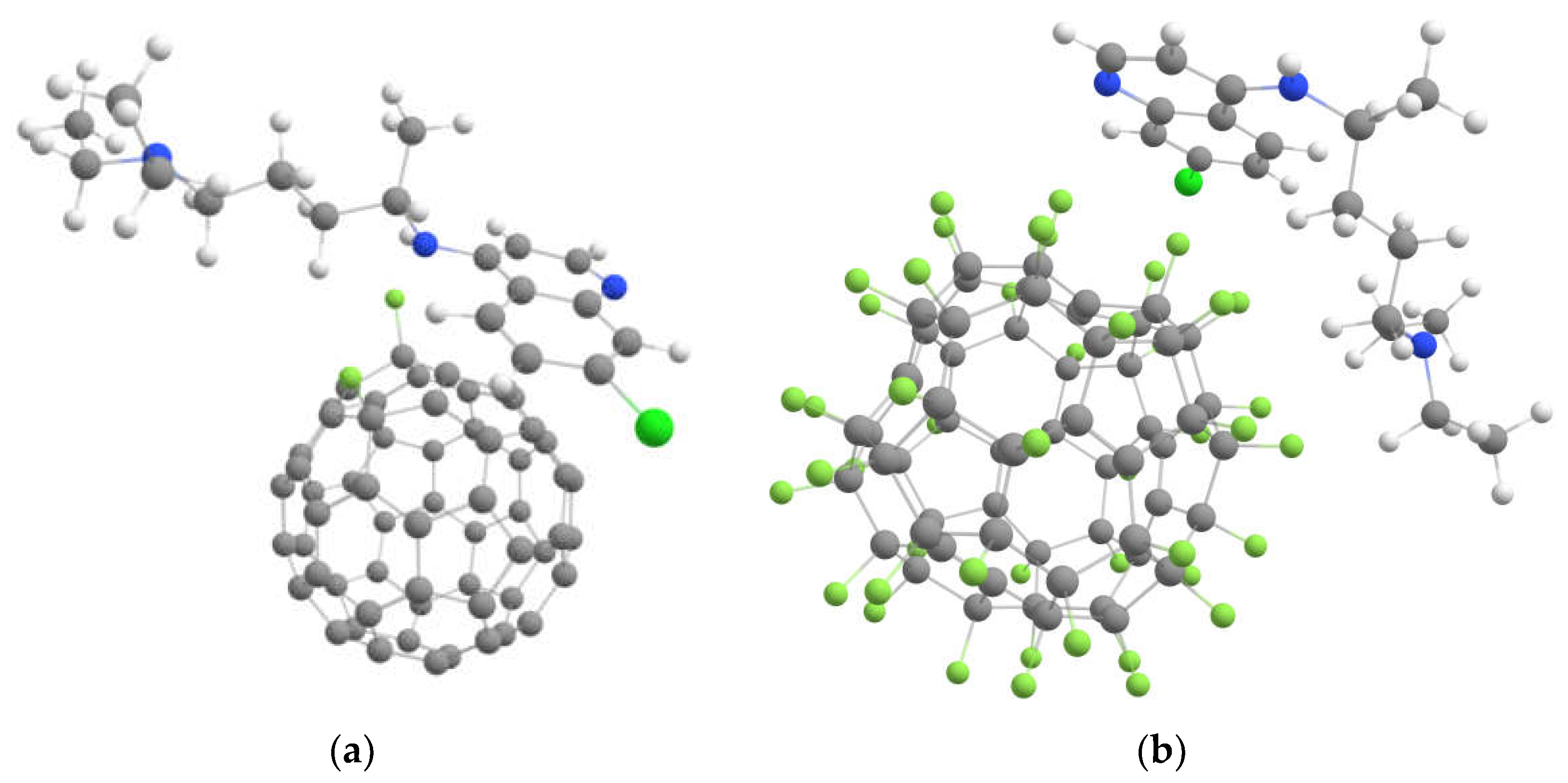
2.2. Interaction of Drugs Molecules with the Pristine and Fluorinated Fullerenes
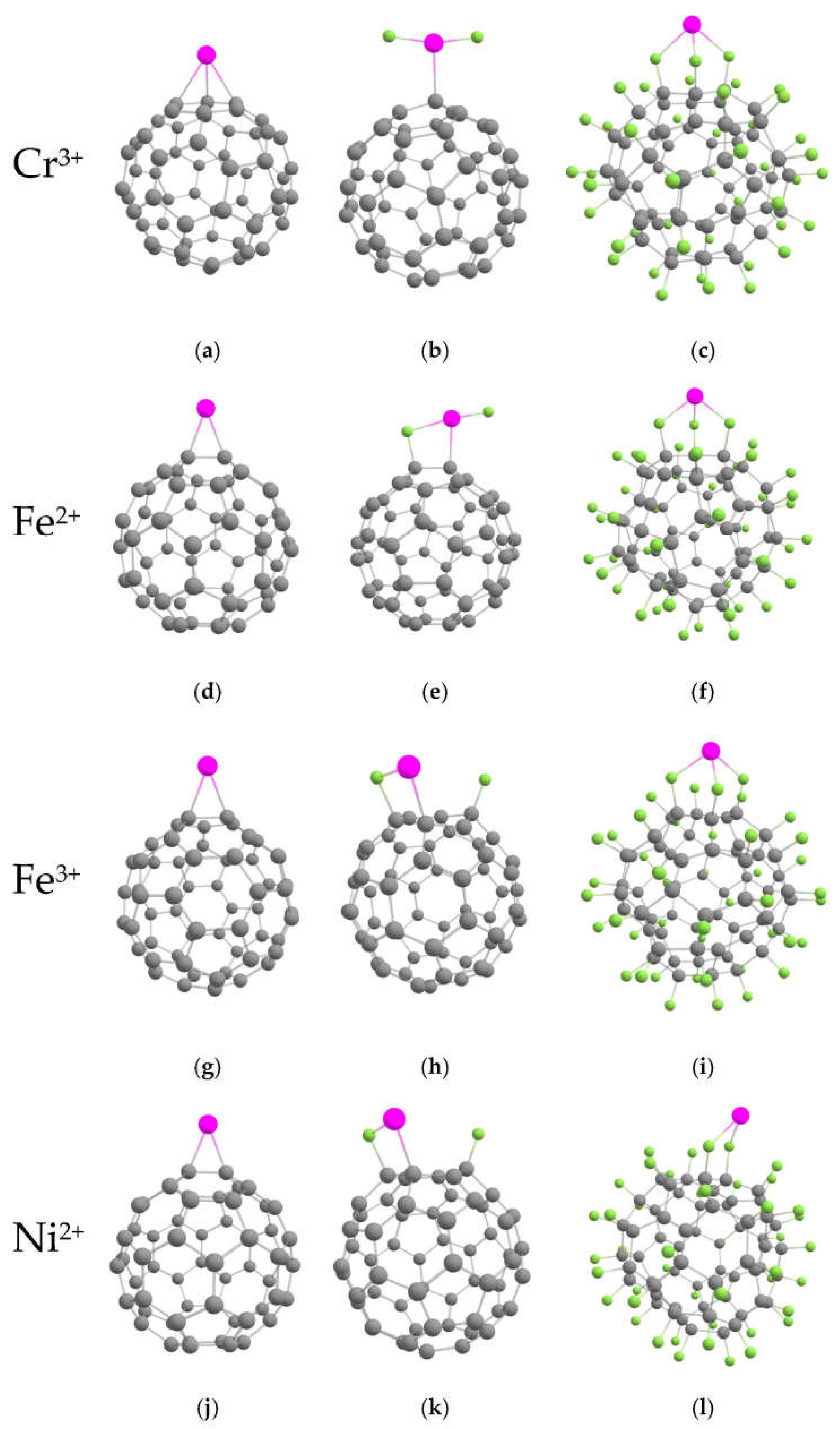
2.3. Interaction of Metals Ions Molecules with the Pristine and Fluorinated Fullerenes
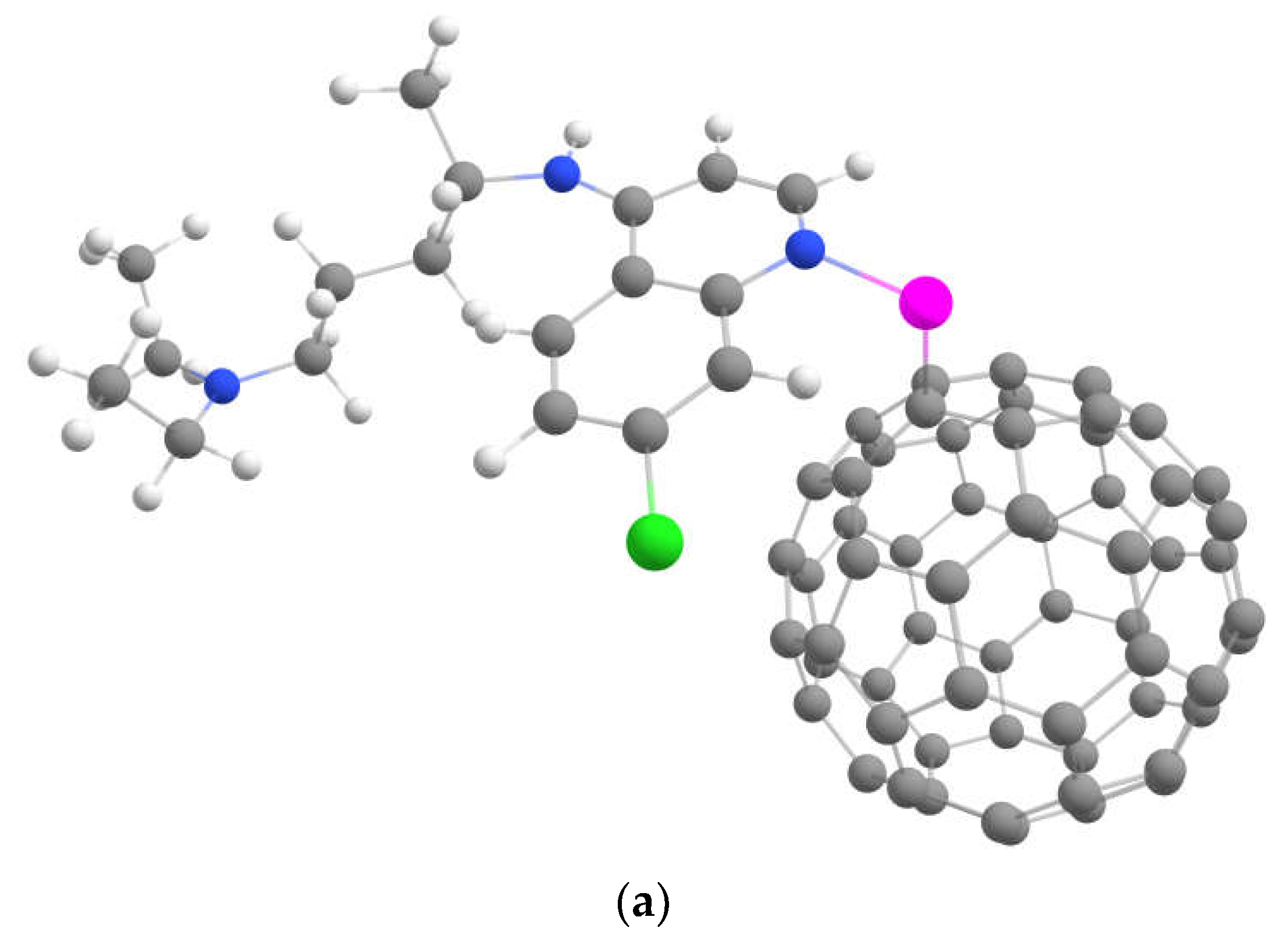
2.4. Loading of Chloroquine on Metal-Decorated Fluorinated Fullerenes
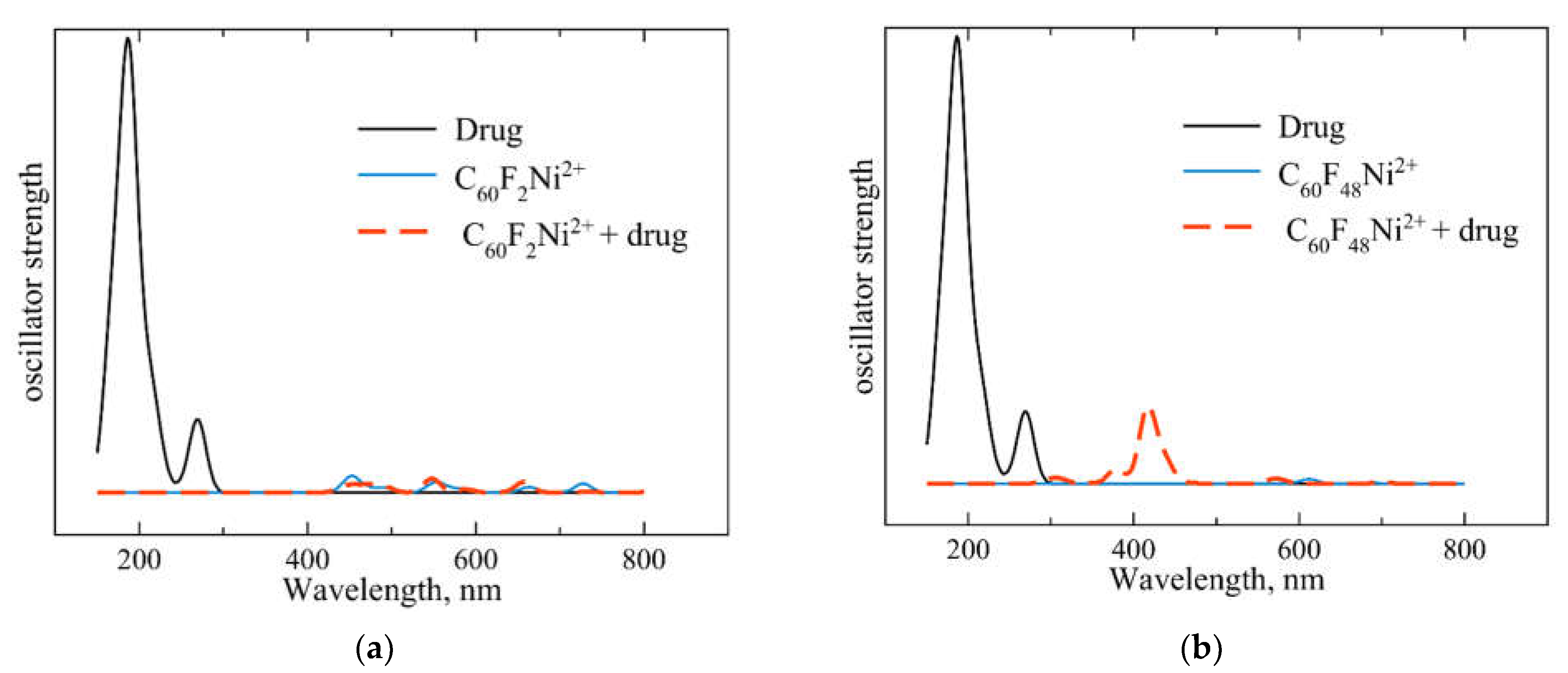
2.5. Spectral Fingerprints of the C60F2Ni2+-Chloroquine and C60F48Ni2+-Chloroquine Complexes
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veclani, D.; Tolazzi, M.; Melchior, A. Molecular Interpretation of Pharmaceuticals’ Adsorption on Carbon Nanomaterials: Theory Meets Experiments. Processes 2020, 8, 642. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical Engineers Get to Revisit an Old Friend. Materials Today 2017, 20, 460. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ke, X.; Zhu, Z.; Zhu, F.; Ruan, M.; Chen, H.; Huang, R.; Zheng, L. A New Carbon Solid Made of the World’s Smallest Caged Fullerene C20. Phys. Lett. A 2001, 280, 351–356. [Google Scholar] [CrossRef]
- Katin, K.P.; Podlivaev, A.I. Dynamic Characteristics of the Low-Temperature Decomposition of the C20 Fullerene. Phys. Solid State 2010, 52, 436. [Google Scholar] [CrossRef] [Green Version]
- Rysaeva, L.K.; Lobzenko, I.P.; Baimova, J.A.; Dmitriev, S.V.; Zhou, K. Modeling C540-C20 Fullerene Collisions. Rev. Adv. Mater. Sci. 2018, 57, 143. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Raza, K. C60-Fullerenes as Drug Delivery Carriers for Anticancer Agents: Promises and Hurdles. Pharm. Nanotech. 2018, 5, 169. [Google Scholar] [CrossRef]
- Staykov, A.; Ooishi, Y.; Ishihara, T. Immobilizing Metal Nanoparticles on Single Wall Nanotubes. Effect of Surface Curvature. J. Phys. Chem. C 2014, 118, 8907. [Google Scholar] [CrossRef]
- Prudkovskiy, V.S.; Katin, K.P.; Maslov, M.M.; Puech, P.; Yakimova, R.; Deligeorgis, G. Efficient Cleaning of Graphene from Residual Lithographic Polymers by Ozone Treatment. Carbon 2016, 109, 221. [Google Scholar] [CrossRef]
- Mesarič, T.; Baweja, L.; Drašler, B.; Drobne, D.; Makovec, D.; Dušak, P.; Dhawan, A.; Sepčić, K. Effects of Surface Curvature and Surface Characteristics of Carbon-Based Nanomaterials on the Adsorption and Activity of Acetylcholinesterase. Carbon 2013, 62, 222. [Google Scholar] [CrossRef]
- Andrievsky, G.; Klochkov, V.; Derevyanchenko, L. Is the C60 Fullerene Molecule Toxic? Fuller. Nanotub. Carbon Nanostruct. 2005, 13, 363. [Google Scholar] [CrossRef]
- Kolosnjaj, J.; Szwarc, H.; Moussa, F. Toxicity Studies of Fullerenes and Derivatives. In Bio-Applications of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2007; pp. 168–180. [Google Scholar] [CrossRef]
- Spohn, P.; Hirsch, C.; Hasler, F.; Bruinink, A.; Krug, H.F.; Wick, P. C60 Fullerene: A Powerful Antioxidant or a Damaging Agent? The Importance of an in-Depth Material Characterization Prior to Toxicity Assays. Environ. Pollut. 2009, 157, 1134. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J. Fullerenes in Electrochemical Catalytic and Affinity Biosensing: A Review. C 2017, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Sharoyko, V.V.; Ageev, S.V.; Meshcheriakov, A.A.; Akentiev, A.V.; Noskov, B.A.; Rakipov, I.T.; Charykov, N.A.; Kulenova, N.A.; Shaimardanova, B.K.; Podolsky, N.E.; et al. Physicochemical Study of Water-Soluble C60(OH)24 Fullerenol. J. Mol. Liq. 2020, 311, 113360. [Google Scholar] [CrossRef]
- Akentiev, A.V.; Gorniaia, S.B.; Isakov, N.A.; Lebedev, V.T.; Milyaeva, O.Y.; Sedov, V.P.; Semenov, K.N.; Timoshen, K.A.; Noskov, B.A. Surface Properties of Fullerenol C60(OH)20 Solutions. J. Mol. Liq. 2020, 306, 112904. [Google Scholar] [CrossRef]
- Gökpek, Y.; Bilge, M.; Bilge, D.; Alver, Ö.; Parlak, C. Adsorption Mechanism, Structural and Electronic Properties: 4-Phenylpyridine & Undoped or Doped (B or Si) C60. J. Mol. Liq. 2017, 238, 225. [Google Scholar] [CrossRef]
- Muz, İ.; Göktaş, F.; Kurban, M. A Density Functional Theory Study on Favipiravir Drug Interaction with BN-Doped C60 Heterofullerene. Phys. E 2022, 135, 114950. [Google Scholar] [CrossRef]
- Rad, A.S.; Shahavi, M.H.; Esfahani, M.R.; Darvishinia, N.; Ahmadizadeh, S. Are Nickel- and Titanium- Doped Fullerenes Suitable Adsorbents for Dopamine in an Aqueous Solution? Detailed DFT and AIM Studies. J. Mol. Liq. 2021, 322, 114942. [Google Scholar] [CrossRef]
- Bagheri Novir, S.; Aram, M.R. Quantum Mechanical Simulation of Chloroquine Drug Interaction with C60 Fullerene for Treatment of COVID-19. Chem. Phys. Lett. 2020, 757, 137869. [Google Scholar] [CrossRef]
- Parlak, C.; Alver, Ö. A Density Functional Theory Investigation on Amantadine Drug Interaction with Pristine and B, Al, Si, Ga, Ge Doped C60 Fullerenes. Chem. Phys. Lett. 2017, 678, 85. [Google Scholar] [CrossRef]
- Muz, İ.; Kurban, M. A First-Principles Evaluation on the Interaction of 1,3,4-Oxadiazole with Pristine and B-, Al-, Ga-Doped C60 Fullerenes. J. Mol. Liq. 2021, 335, 116181. [Google Scholar] [CrossRef]
- Baei, M.T.; Soltani, A.; Rajabzadeh, H.; Tazikeh-Lemeski, E. Structural and Electronic Properties of XY-Doped (AlN, AlP, GaN, GaP) C58 Fullerenes: A DFT Study. Russ. J. Inorg. Chem. 2017, 62, 1067–1076. [Google Scholar] [CrossRef]
- Podlivaev, A.I.; Katin, K.P.; Lobanov, D.A.; Openov, L.A. Specific Features of the Stone-Wales Transformation in the C20 and C36 Fullerenes. Phys. Solid State 2011, 53, 215. [Google Scholar] [CrossRef]
- Khosravanian, A.; Moslehipour, A.; Ashrafian, H. A Review on Bioimaging, Biosensing, and Drug Delivery Systems Based on Graphene Quantum Dots. Prog. Chem. Biochem. Res. 2021, 4, 44. [Google Scholar] [CrossRef]
- Antonova, I.; Nebogatikova, N.; Zerrouki, N.; Kurkina, I.; Ivanov, A. Flexibility of Fluorinated Graphene-Based Materials. Materials 2020, 13, 1032. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, A.I.; Nebogatikova, N.A.; Kotin, I.A.; Smagulova, S.A.; Antonova, I.V. Resistive Switching Effects in Fluorinated Graphene Films with Graphene Quantum Dots Enhanced by Polyvinyl Alcohol. Nanotechnology 2019, 30, 255701. [Google Scholar] [CrossRef]
- Sun, L.; Gong, P.; Liu, X.; Pang, M.; Tian, M.; Chen, J.; Du, J.; Liu, Z. Fluorinated Carbon Fiber as a Novel Nanocarrier for Cancer Chemo-Photothermal Therapy. J. Mater. Chem. B 2017, 5, 6128. [Google Scholar] [CrossRef]
- Romero-Aburto, R.; Narayanan, T.N.; Nagaoka, Y.; Hasumura, T.; Mitcham, T.M.; Fukuda, T.; Cox, P.J.; Bouchard, R.R.; Maekawa, T.; Kumar, D.S.; et al. Fluorinated Graphene Oxide; a New Multimodal Material for Biological Applications. Adv. Mater. 2013, 25, 5632. [Google Scholar] [CrossRef] [Green Version]
- Rad, A.S.; Ardjmand, M.; Esfahani, M.R.; Khodashenas, B. DFT Calculations towards the Geometry Optimization, Electronic Structure, Infrared Spectroscopy and UV–Vis Analyses of Favipiravir Adsorption on the First-Row Transition Metals Doped Fullerenes; a New Strategy for COVID-19 Therapy. Spectrochim. Acta A 2021, 247, 119082. [Google Scholar] [CrossRef]
- Joshi, S.; Parkar, J.; Ansari, A.; Vora, A.; Talwar, D.; Tiwaskar, M.; Patil, S.; Barkate, H. Role of Favipiravir in the Treatment of COVID-19. Int. J. Infect. Dis. 2021, 102, 501. [Google Scholar] [CrossRef]
- Touret, F.; de Lamballerie, X. Of Chloroquine and COVID-19. Antivir. Res. 2020, 177, 104762. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Ingoglia, G.; Ippolito, M.; Giarratano, A.; Einav, S. A Systematic Review on the Efficacy and Safety of Chloroquine for the Treatment of COVID-19. J. Crit. Care 2020, 57, 279. [Google Scholar] [CrossRef] [PubMed]
- Parlak, C.; Alver, Ö.; Şenyel, M. Computational Study on Favipiravir Adsorption onto Undoped- and Silicon-Decorated C60 Fullerenes. J. Theor. Comput. Chem. 2017, 16, 1750011. [Google Scholar] [CrossRef]
- Soliman, K.A.; Aal, S.A. Theoretical Investigation of Favipiravir Antiviral Drug Based on Fullerene and Boron Nitride Nanocages. Diam. Relat. Mater. 2021, 117, 108458. [Google Scholar] [CrossRef]
- Troyanov, I.S.; Kemnitz, E. Synthesis and Structure of Halogenated Fullerenes. Curr. Org. Chem. 2012, 16, 1060. [Google Scholar] [CrossRef]
- Darwish, A.D.; Avent, A.G.; Street, J.M.; Taylor, R. Electrophilic Substitution of C60F18into Phenols: HF Elimination between OH and a 1,3-Shifted Fluorine Giving Benzofurano [2′,3′:10,26]Hexadecafluoro[60]Fullerene and Derivatives. Org. Biomol. Chem. 2003, 1, 1764. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Uchimaru, T.; Wakisaka, A.; Ono, T. Magnitude and Directionality of Halogen Bond of Benzene with C6F5X, C6H5X, and CF3X (X = I, Br, Cl, and F). J. Phys. Chem. A 2016, 120, 7020–7029. [Google Scholar] [CrossRef]
- Katin, K.P.; Prudkovskiy, V.S.; Maslov, M.M. Molecular Dynamics Simulation of Nickel-coated Graphene Bending. Micro&Nano Lett. 2018, 13, 160. [Google Scholar] [CrossRef]
- Salem, M.A.; Katin, K.P.; Kaya, S.; Kochaev, A.I.; Maslov, M.M. Interaction of Dopants and Functional Groups Adsorbed on the Carbon Fullerenes: Computational Study. Phys. E 2020, 124, 114319. [Google Scholar] [CrossRef]
- Papoular, R.J.; Allouchi, H.; Dzyabchenko, A.V.; Davydov, V.A.; Rakhmanina, A.V.; Boltalina, O.V.; Seppelt, K.; Agafonov, V. High-Resolution X-Ray Powder Diffraction Structure Determination of C60F48. Fuller. Nanotub. Carb. Nanostruct. 2006, 14, 279. [Google Scholar] [CrossRef]
- Hossain, M.R.; Hasan, M.M.; Nishat, M.; Ahmed, F.; Ferdous, T.; Hossain, M.A. DFT and QTAIM Investigations of the Adsorption of Chlormethine Anticancer Drug on the Exterior Surface of Pristine and Transition Metal Functionalized Boron Nitride Fullerene. J. Mol. Liq. 2021, 323, 114627. [Google Scholar] [CrossRef]
- Li, M.; Wei, Y.; Zhang, G.; Wang, F.; Li, M.; Soleymanabadi, H. A DFT Study on the Detection of Isoniazid Drug by Pristine, Si and Al Doped C70 Fullerenes. Phys. E 2020, 118, 113878. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Pople, J.A.; Ratner, M.A.; Windus, T.L. 6-31G* Basis Set for Atoms K through Zn. J. Chem. Phys. 1998, 109, 1223. [Google Scholar] [CrossRef]
- Tanaka, M.; Katouda, M.; Nagase, S. Optimization of RI-MP2 Auxiliary Basis Functions for 6-31G** and 6-311G** Basis Sets for First-, Second-, and Third-Row Elements. J. Comput. Chem. 2013, 34, 2568–2575. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Luehr, N.; Kulik, H.J.; Martínez, T.J. Quantum Chemistry for Solvated Molecules on Graphical Processing Units Using Polarizable Continuum Models. J. Chem. Theory Comput. 2015, 11, 3131–3144. [Google Scholar] [CrossRef] [Green Version]
- Seritan, S.; Bannwarth, C.; Fales, B.S.; Hohenstein, E.G.; Kokkila-Schumacher, S.I.L.; Luehr, N.; Snyder, J.W., Jr.; Song, C.; Titov, A.V.; Ufimtsev, I.S.; et al. TeraChem: Accelerating Electronic Structure and ab Initio Molecular Dynamics with Graphical Processing Units. J. Chem. Phys. 2020, 152, 224110. [Google Scholar] [CrossRef]
- Wang, L.-P.; Song, C. Geometry Optimization Made Simple with Translation and Rotation Coordinates. J. Chem. Phys. 2020, 144, 214108. [Google Scholar] [CrossRef] [Green Version]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51. [Google Scholar] [CrossRef] [Green Version]
- Isborn, C.M.; Luehr, N.; Ufimtsev, I.S.; Martínez, T.J. Excited-State Electronic Structure with Configuration Interaction Singles and Tamm–Dancoff Time-Dependent Density Functional Theory on Graphical Processing Units. J. Chem. Theory Comput. 2011, 7, 1814. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox Interactions of Vitamin C and Iron: Inhibition of the Pro-Oxidant Activity by Deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef] [PubMed]
- Smidstrup, S.; Markussen, T.; Vancraeyveld, P.; Wellendorff, J.; Schneider, J.; Gunst, T.; Verstichel, B.; Stradi, D.; Khomyakov, P.A.; Vej-Hansen, U.G.; et al. Quantum ATK: An integrated platform of electronic and atomic-scale modelling tools. J. Phys.: Cond. Mat. 2020, 32, 015901. [Google Scholar] [CrossRef]


| Eb | Edef (Fullerene) | Edef (Drug) | Eint | D | HOMO | LUMO | Gap | |
|---|---|---|---|---|---|---|---|---|
| C60F2 | – | 0 | – | – | 3.95 | −5.86 | −3.34 | 2.52 |
| C60F2 + drug | 0.41 | 0.01 | 0.04 | 0.46 | 5.61 | −5.54 | −3.28 | 2.26 |
| C60F48 | – | 0 | – | – | 0.17 | −9.51 | −4.15 | 5.36 |
| C60F48 + drug | 0.29 | 0.03 | 0.06 | 0.38 | 7.01 | −5.37 | −4.16 | 1.21 |
| System | Eb | Edef | lC–M (n) | lM–F (m) | D | HOMO | LUMO | Gap |
|---|---|---|---|---|---|---|---|---|
| non-fluorinated fullerene | ||||||||
| C60Cr3+ | 17.94 | 0.18 | 2.265 (3) | – | 18.79 | −7.08 | −6.14 | 0.94 |
| C60Fe2+ | 6.26 | 0.39 | 2.013 (2) | – | 14.85 | −6.85 | −5.83 | 1.02 |
| C60Fe3+ | 21.71 | 0.35 | 2.034 (2) | – | 15.17 | −7.14 | −6.20 | 0.94 |
| C60Ni2+ | 8.69 | 0.44 | 1.928 (2) | – | 9.45 | −6.91 | −6.00 | 0.91 |
| low-fluorinated fullerene | ||||||||
| C60F2Cr3+ | 21.97 | – | 2.150 (1) | 1.684 (2) | 1.87 | −7.13 | −6.90 | 0.23 |
| C60F2Fe2+ | 8.54 | – | 2.046 (1) | 1.836 (2) | 2.84 | −5.96 | −5.03 | 0.93 |
| C60F2Fe3+ | 22.67 | 1.16 | 1.994 (1) | 1.866 (1) | 12.18 | −7.14 | −6.29 | 0.85 |
| C60F2Ni2+ | 9.04 | 0.89 | 1.924 (1) | 1.895 (1) | 10.98 | −6.93 | −5.79 | 1.14 |
| high-fluorinated fullerene | ||||||||
| C60F48Cr3+ | 14.02 | 2.30 | – | 2.023 (3) | 29.81 | −9.37 | −7.94 | 1.43 |
| C60F48Fe2+ | 4.64 | 1.06 | – | 1.918 (3) | 39.71 | −9.92 | −8.30 | 1.62 |
| C60F48Fe3+ | 17.01 | 2.62 | – | 1.921 (3) | 26.77 | −9.40 | −8.47 | 0.93 |
| C60F48Ni2+ | 3.89 | 0.55 | – | 1.930 (2) | 39.48 | −9.92 | −9.56 | 0.36 |
| Eb | Edef (Fullerene) | Edef (Drug) | lM-N | D | HOMO | LUMO | Gap | |
|---|---|---|---|---|---|---|---|---|
| non-fluorinated fullerene | ||||||||
| C60Cr3+ + drug | 5.21 | 0.16 | 0.79 | 1.968 | 39.53 | −7.04 | −4.42 | 2.62 |
| C60Fe2+ + drug | 3.74 | 0.25 | 0.68 | 1.904 | 20.39 | −5.18 | −4.55 | 0.63 |
| C60Fe3+ + drug | 5.20 | 0.23 | 0.84 | 1.909 | 39.81 | −6.58 | −4.67 | 1.91 |
| C60Ni2+ + drug | 2.61 | 0.23 | 0.32 | 1.853 | 28.88 | −5.66 | −5.33 | 0.34 |
| low-fluorinated fullerene | ||||||||
| C60F2Fe3+ + drug | 4.85 | 0.32 | 0.43 | 1.908 | 34.46 | −7.01 | −5.24 | 1.77 |
| C60F2Ni2+ + drug | 3.43 | 0.04 | 0.24 | 1.852 | 15.30 | −6.03 | −5.07 | 0.96 |
| high-fluorinated fullerene | ||||||||
| C60F48Cr3+ + drug | 8.01 | 0.87 | 0.65 | 1.895 | 68.71 | −6.41 | −6.27 | 0.14 |
| C60F48Fe2+ + drug | 4.78 | 0.87 | 0.58 | 1.875 | 44.13 | −5.38 | −4.97 | 0.41 |
| C60F48Fe3+ + drug | 9.32 | 1.06 | 0.80 | 1.868 | 68.96 | −7.10 | −5.32 | 1.78 |
| C60F48Ni2+ + drug | 5.88 | 0.47 | 0.42 | 1.846 | 46.82 | −5.90 | −5.46 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katin, K.P.; Kochaev, A.I.; Kaya, S.; El-Hajjaji, F.; Maslov, M.M. Ab Initio Insight into the Interaction of Metal-Decorated Fluorinated Carbon Fullerenes with Anti-COVID Drugs. Int. J. Mol. Sci. 2022, 23, 2345. https://doi.org/10.3390/ijms23042345
Katin KP, Kochaev AI, Kaya S, El-Hajjaji F, Maslov MM. Ab Initio Insight into the Interaction of Metal-Decorated Fluorinated Carbon Fullerenes with Anti-COVID Drugs. International Journal of Molecular Sciences. 2022; 23(4):2345. https://doi.org/10.3390/ijms23042345
Chicago/Turabian StyleKatin, Konstantin P., Alexey I. Kochaev, Savas Kaya, Fadoua El-Hajjaji, and Mikhail M. Maslov. 2022. "Ab Initio Insight into the Interaction of Metal-Decorated Fluorinated Carbon Fullerenes with Anti-COVID Drugs" International Journal of Molecular Sciences 23, no. 4: 2345. https://doi.org/10.3390/ijms23042345
APA StyleKatin, K. P., Kochaev, A. I., Kaya, S., El-Hajjaji, F., & Maslov, M. M. (2022). Ab Initio Insight into the Interaction of Metal-Decorated Fluorinated Carbon Fullerenes with Anti-COVID Drugs. International Journal of Molecular Sciences, 23(4), 2345. https://doi.org/10.3390/ijms23042345










