Serial Passaging of RAW 264.7 Cells Modulates Intracellular AGE Formation and Downregulates RANKL-Induced In Vitro Osteoclastogenesis
Abstract
:1. Introduction
2. Results
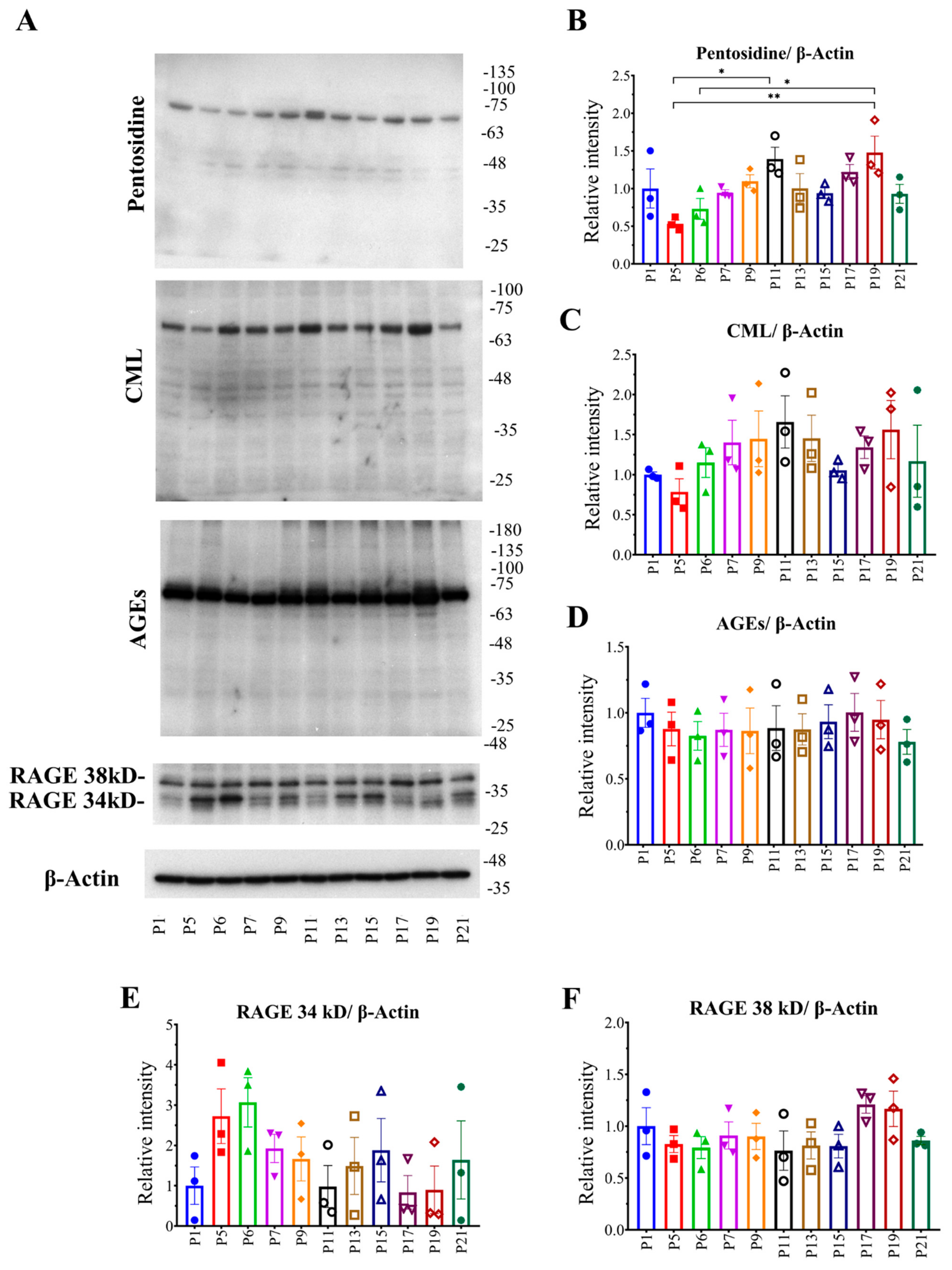
2.1. Relationship between Passage Number of RAW 264.7 Cells with Intracellular AGE Formation and Accumulation
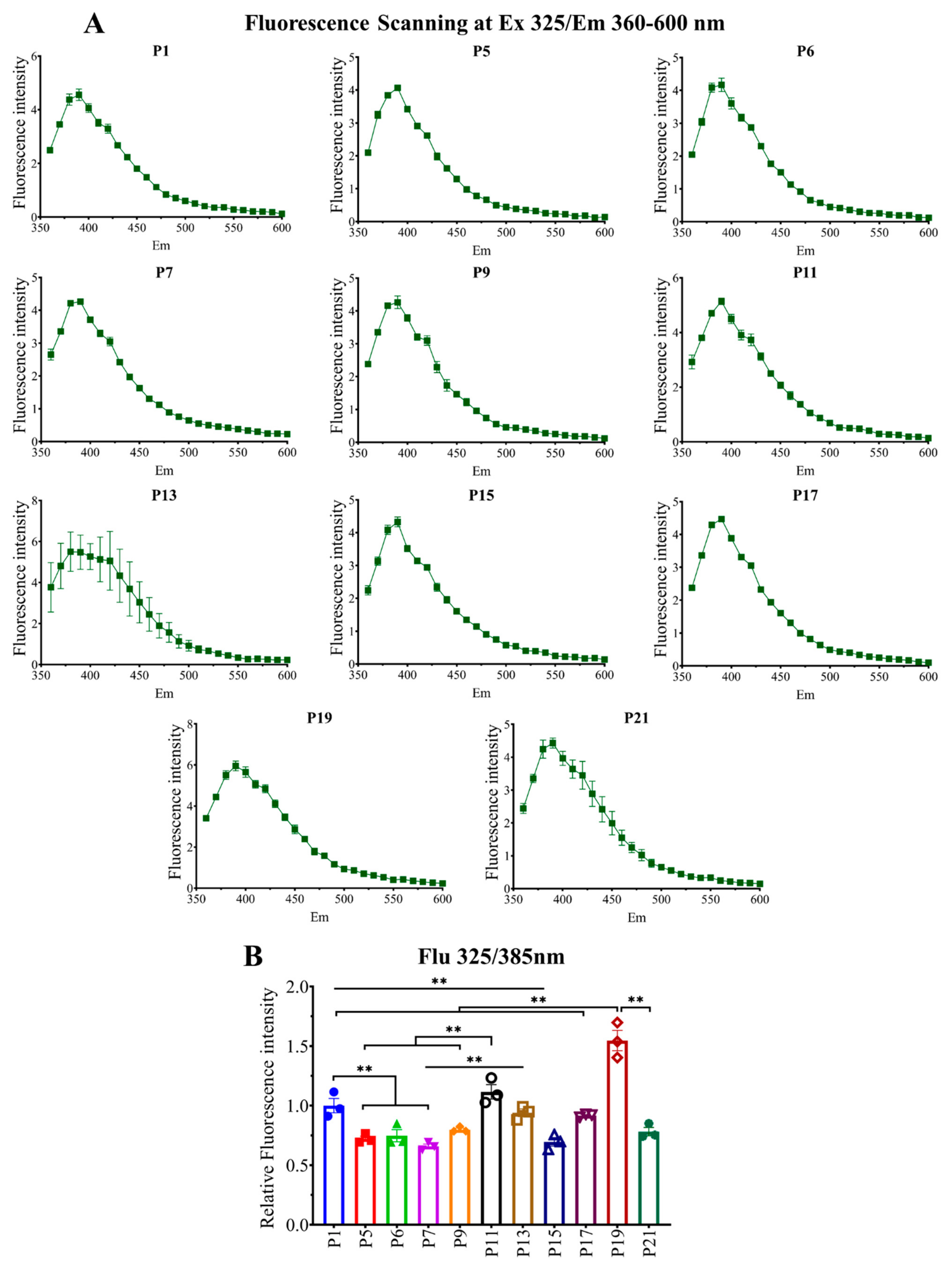
2.2. Relationship between Passage Number of RAW264.7 Cells with Intracellular Fluorescent AGE Formation and Accumulation
2.3. Increasing Passage Number Significantly Inhibited Osteoclastogenic Differentiation
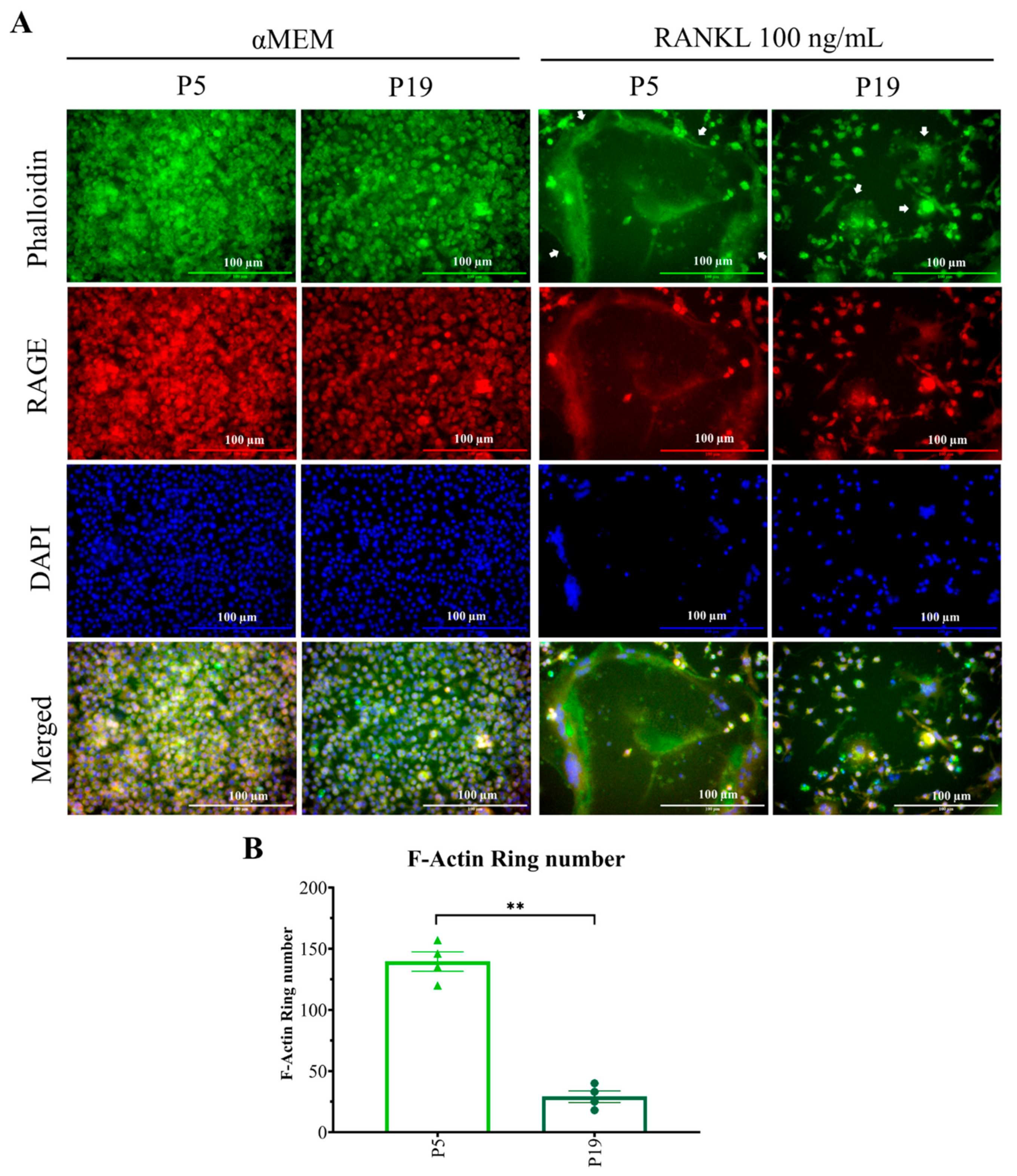
2.4. Higher Passage Number Reduced F-Actin Ring Size Significantly
2.5. Higher Passage Number Reduced Osteoclastogenic Gene Expression
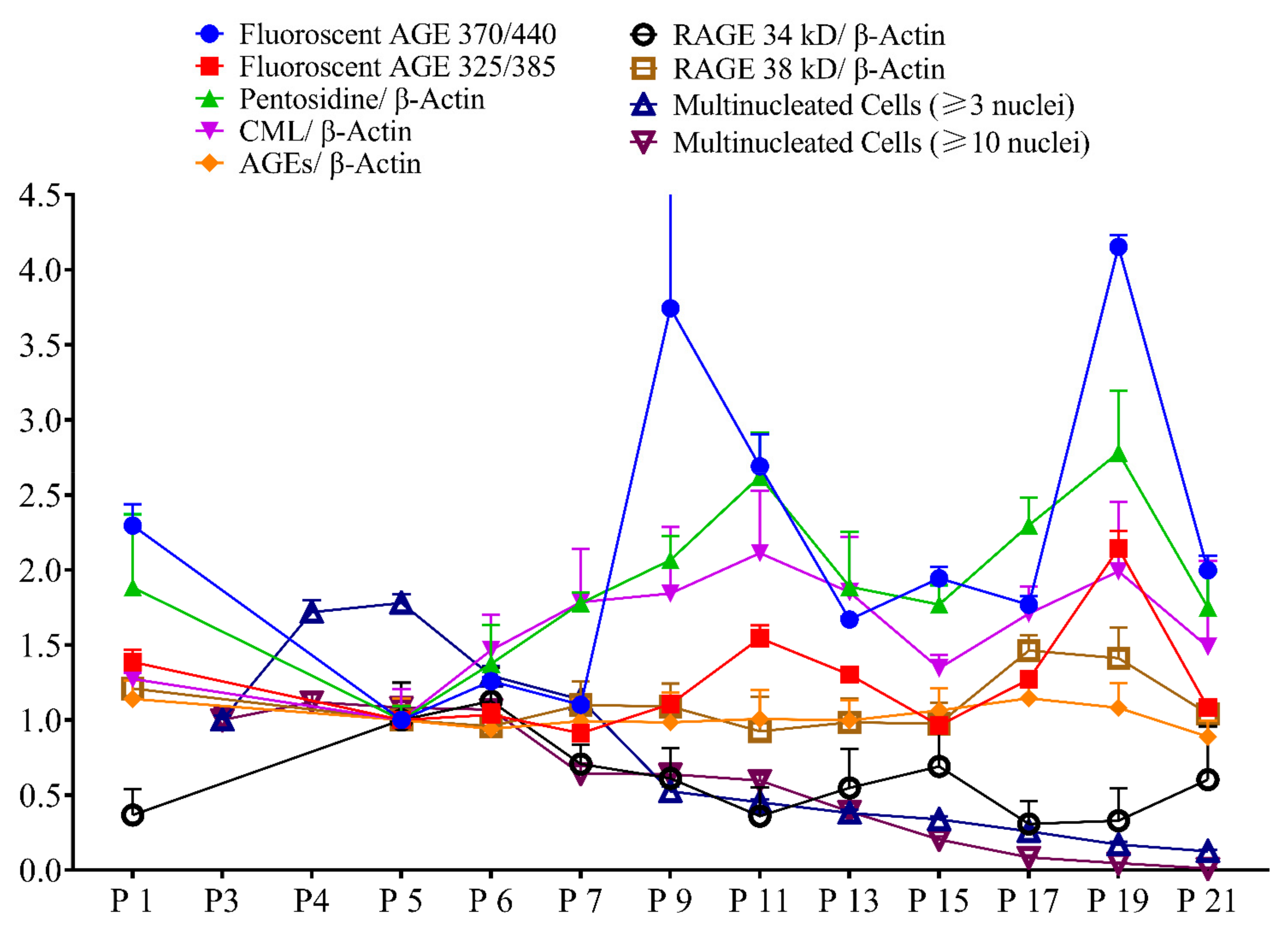
2.6. Correlation Study
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. TRAP Staining
4.3. Isolation of Total RNA and q-PCR
4.4. Protein Extraction and Western Blot Analysis
4.5. Fluorescence Scanning and Measurement
4.6. Immunofluorescence Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbott, A. Biology’s new dimension. Nature 2003, 424, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Bulletin, T. Passage Number Effects in Cell Lines. Available online: https://www.atcc.org/resources/technical-documents/passage-number-effects-in-cell-lines (accessed on 13 January 2022).
- Haridas, P.; McGovern, J.A.; Kashyap, A.S.; McElwain, D.L.S.; Simpson, M.J. Standard melanoma-associated markers do not identify the MM127 metastatic melanoma cell line. Sci. Rep. 2016, 6, 24569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, E.L.; Mitsui, Y. The relationship between in vitro cellular aging and in vivo human age. Proc. Natl. Acad. Sci. USA 1976, 73, 3584–3588. [Google Scholar] [CrossRef] [Green Version]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Foster, S.A.; Galloway, D.A. Human papillomavirus type 16 E7 alleviates a proliferation block in early passage human mammary epithelial cells. Oncogene 1996, 12, 1773–1779. [Google Scholar] [PubMed]
- Rubin, H. Cell aging in vivo and in vitro. Mech. Ageing Dev. 1997, 98, 1–35. [Google Scholar] [CrossRef]
- Lin, H.K.; Hu, Y.C.; Yang, L.; Altuwaijri, S.; Chen, Y.T.; Kang, H.Y.; Chang, C. Suppression Versus Induction of Androgen Receptor Functions by the Phosphatidylinositol 3-Kinase/Akt Pathway in Prostate Cancer LNCaP Cells with Different Passage Numbers. J. Biol. Chem. 2003, 278, 50902–50907. [Google Scholar] [CrossRef] [Green Version]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Collin-Osdoby, P.; Osdoby, P. RANKL-Mediated Osteoclast Formation from Murine RAW 264.7 cells BT—Bone Research Protocols. In Bone Research Protocols; Helfrich, M.H., Ralston, S.H., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 816, pp. 187–202. ISBN 9781617794155. [Google Scholar]
- Mamun-Or-Rashid, A.N.M.; Takabe, W.; Yagi, M.; Yonei, Y. Melatonin and astaxanthin modulate RANKL-induced TRAP activity in RAW264.7 cells in an opposite fashion. Glycative Stress Res. 2019, 6, 135–141. [Google Scholar]
- Mamun-Or-Rashid, A.N.M.; Takabe, W.; Yagi, M.; Yonei, Y. Glycated-HSA inhibits osteoclastogenesis in RAW264.7 cells depending on the glycating agents via downregulating RANKL-signaling. Glycative Stress Res. 2017, 4, 217–231. [Google Scholar]
- Mamun-Or-Rashid, A.N.M.; Takabe, W.; Yagi, M.; Yonei, Y. Glycated-proteins modulate RANKL-induced osteoclastogenesis in RAW264.7 cells. Glycative Stress Res. 2017, 4, 232–239. [Google Scholar]
- Mamun-Or-Rashid, A.N.M.; Takabe, W.; Yagi, M.; Yonei, Y. RANKL regulates RAW264.7 cell osteoclastogenesis in a manner independent of M-CSF, dependent on FBS, media content and cell density. Glycative Stress Res. 2017, 4, 40–52. [Google Scholar]
- Mamun-Or-Rashid, A.N.M.; Lucy, T.T.; Yagi, M.; Yonei, Y. Inhibitory Effects of Astaxanthin on CML-HSA-Induced Inflammatory and RANKL-Induced Osteoclastogenic Gene Expression in RAW 264.7 Cells. Biomedicines 2021, 10, 54. [Google Scholar] [CrossRef]
- Mamun-Or-Rashid, A.N.M.; Takabe, W.; Yonei, Y. Melatonin has no direct effect on inflammatory gene expression in CML-HSA stimulated RAW264.7 cells. Glycative Stress Res. 2016, 3, 141–151. [Google Scholar]
- Sato, K.; Yagi, M.; Umehara, H.; Yonei, Y. Establishment of a model for evaluating tumor necrosis factor-α production by cultured RAW264.7 in response to glycation stress. Glycative Stress Res. 2014, 1, 1–7. [Google Scholar]
- Sato, K.; Yagi, M.; Takabe, W.; Yonei, Y. Inhibitory effect of plant extract on tumor necrosis factor-α formation from carboxymethyllysine stimulated macrophages. Glycative Stress Res. 2015, 2, 191–196. [Google Scholar]
- Ghanem, A.A.; Elewa, A.; Arafa, L.F. Pentosidine and N-carboxymethyl-lysine: Biomarkers for type 2 diabetic retinopathy. Eur. J. Ophthalmol. 2011, 21, 48–54. [Google Scholar] [CrossRef]
- Sanguineti, R.; Puddu, A.; Mach, F.; Montecucco, F.; Viviani, G.L. Advanced glycation end products play adverse proinflammatory activities in osteoporosis. Mediat. Inflamm. 2014, 2014, 975872. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Dicarbonyl stress, protein glycation and the unfolded protein response. Glycoconj. J. 2021, 38, 331–340. [Google Scholar] [CrossRef]
- Ni, J.; Yuan, X.; Gu, J.; Yue, X.; Gu, X.; Nagaraj, R.H.; Crabb, J.W. Plasma protein pentosidine and carboxymethyllysine, biomarkers for age-related macular degeneration. Mol. Cell. Proteom. 2009, 8, 1921–1933. [Google Scholar] [CrossRef] [Green Version]
- Hein, G.; Wiegand, R.; Lehmann, G.; Stein, G.; Franke, S. Advanced glycation end-products pentosidine and N -carboxymethyllysine are elevated in serum of patients with osteoporosis. Rheumatology 2003, 42, 1242–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci. Rep. 2016, 6, 27848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Marumo, K. New treatment strategy against osteoporosis: Advanced glycation end products as a factor for poor bone quality. Glycative Stress Res. 2015, 2, 1–14. [Google Scholar]
- Barzilay, J.I.; Bůžková, P.; Zieman, S.J.; Kizer, J.R.; Djoussé, L.; Ix, J.H.; Tracy, R.P.; Siscovick, D.S.; Cauley, J.A.; Mukamal, K.J.; et al. Circulating levels of carboxy-methyl-lysine (CML) are associated with hip fracture risk: The cardiovascular health study HHS Public Access. J. Bone Min. Res. 2014, 29, 1061–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, M.; Nakamura, Y.; Suzuki, T.; Miyazaki, A.; Takahashi, J.; Saito, M.; Shiraki, M. Pentosidine and carboxymethyl-lysine associate differently with prevalent osteoporotic vertebral fracture and various bone markers. Sci. Rep. 2020, 10, 22090. [Google Scholar] [CrossRef] [PubMed]
- Franke, S.; Sommer, M.; Rüster, C.; Bondeva, T.; Marticke, J.; Hofmann, G.; Hein, G.; Wolf, G. Advanced glycation end products induce cell cycle arrest and proinflammatory changes in osteoarthritic fibroblast-like synovial cells. Arthritis Res. Ther. 2009, 11, R136. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wake, H.; Morioka, Y.; Liu, K.; Teshigawara, K.; Shibuya, M.; Zhou, J.; Mori, S.; Takahashi, H.; Nishibori, M. Phagocytosis of Advanced Glycation End Products (AGEs) in Macrophages Induces Cell Apoptosis. Oxid. Med. Cell. Longev. 2017, 2017, 8419035. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Chang, Y.; Li, Y.; Chen, S.; Chen, Y.; Ye, N.; Dai, D.; Sun, Y. Advanced glycation end products promote the proliferation and migration of primary rat vascular smooth muscle cells via the upregulation of BAG3. Int. J. Mol. Med. 2017, 39, 1242–1254. [Google Scholar] [CrossRef]
- Sareen, N.; Sequiera, G.L.; Chaudhary, R.; Abu-El-Rub, E.; Chowdhury, S.R.; Sharma, V.; Surendran, A.; Moudgil, M.; Fernyhough, P.; Ravandi, A.; et al. Early passaging of mesenchymal stem cells does not instigate significant modifications in their immunological behavior. Stem Cell Res. Ther. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Zuo, J.; Holliday, L.S. Specialized roles for actin in osteoclasts: Unanswered questions and therapeutic opportunities. Biomolecules 2019, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Han, J.Y.; Xi, C.X.; Xie, J.X.; Feng, X.; Wang, C.Y.; Mei, L.; Xiong, W.C. HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE. J. Bone Miner. Res. 2008, 23, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Immel, D.; Xi, C.X.; Bierhaus, A.; Feng, X.; Mei, L.; Nawroth, P.; Stern, D.M.; Xiong, W.C. Regulation of osteoclast function and bone mass by RAGE. J. Exp. Med. 2006, 203, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Hamadneh, L.; Al-Majawleh, M.; Jarrar, Y.; Shraim, S.; Hasan, M.; Abu-Irmaileh, B. Culturing conditions highly affect DNA methylation and gene expression levels in MCF7 breast cancer cell line. In Vitro Cell. Dev. Biol.-Anim. 2018, 54, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wu, X.; Qin, X.-M.; Li, Z. Uncovering the Effect of Passage Number on HT29 Cell Line Based on the Cell Metabolomic Approach. J. Proteome Res. 2021, 20, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Peterson, W.J.; Tachiki, K.H.; Yamaguchi, D.T. Serial passage of MC3T3-E1 cells down-regulates proliferation during osteogenesis in vitro. Cell Prolif. 2004, 37, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Kinarivala, N.; Shah, K.; Abbruscato, T.J.; Trippier, P.C. Passage Variation of PC12 Cells Results in Inconsistent Susceptibility to Externally Induced Apoptosis. ACS Chem. Neurosci. 2016, 8, 82–88. [Google Scholar] [CrossRef]
- Chennazhy, K.P.; Krishnan, L.K. Effect of passage number and matrix characteristics on differentiation of endothelial cells cultured for tissue engineering. Biomaterials 2005, 26, 5658–5667. [Google Scholar] [CrossRef]
- Kwist, K.; Bridges, W.C.; Burg, K.J.L. The effect of cell passage number on osteogenic and adipogenic characteristics of D1 cells. Cytotechnology 2016, 68, 1661–1667. [Google Scholar] [CrossRef]
- Abdul-Hamid, N.A.; Abas, F.; Maulidiani, M.; Ismail, I.S.; Tham, C.L.; Swarup, S.; Umashankar, S. NMR metabolomics for evaluating passage number and harvesting effects on mammalian cell metabolome. Anal. Biochem. 2019, 576, 20–32. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]






| Primer Name | Forward | Reverse |
|---|---|---|
| NFATc1 | GGA GCG GAG AAA CTT TGC G | GTG ACA CTA GGG GAC ACA TAA CT |
| TRAP | GCG ACC ATT GTT AGC CAC ATA CG | CGT TGA TGT CGC ACA GAG GGA T |
| CTSK | GAA GAA GAC TCA CCA GAA GCA G | TCC AGG TTA TGG GCA GAG ATT |
| Atp6v0 | ACG GTG ATG TCA CAG CAG ACG T | CCT CTG GAT AGA GCC TGC CGC A |
| GAPDH | AGG TCG GTG TGA ACG GAT TTG | TGT AGA CCA TGT AGT TGA GGT CA |
| MMP9 | CTG GAC AGC CAG ACA CTA AAG | CTC GCG GCA AGT CTT CAG AG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucy, T.T.; Mamun-Or-Rashid, A.N.M.; Yagi, M.; Yonei, Y. Serial Passaging of RAW 264.7 Cells Modulates Intracellular AGE Formation and Downregulates RANKL-Induced In Vitro Osteoclastogenesis. Int. J. Mol. Sci. 2022, 23, 2371. https://doi.org/10.3390/ijms23042371
Lucy TT, Mamun-Or-Rashid ANM, Yagi M, Yonei Y. Serial Passaging of RAW 264.7 Cells Modulates Intracellular AGE Formation and Downregulates RANKL-Induced In Vitro Osteoclastogenesis. International Journal of Molecular Sciences. 2022; 23(4):2371. https://doi.org/10.3390/ijms23042371
Chicago/Turabian StyleLucy, Tanzima Tarannum, A. N. M. Mamun-Or-Rashid, Masayuki Yagi, and Yoshikazu Yonei. 2022. "Serial Passaging of RAW 264.7 Cells Modulates Intracellular AGE Formation and Downregulates RANKL-Induced In Vitro Osteoclastogenesis" International Journal of Molecular Sciences 23, no. 4: 2371. https://doi.org/10.3390/ijms23042371
APA StyleLucy, T. T., Mamun-Or-Rashid, A. N. M., Yagi, M., & Yonei, Y. (2022). Serial Passaging of RAW 264.7 Cells Modulates Intracellular AGE Formation and Downregulates RANKL-Induced In Vitro Osteoclastogenesis. International Journal of Molecular Sciences, 23(4), 2371. https://doi.org/10.3390/ijms23042371







