Molecular Mechanisms and Physiological Changes behind Benign Tracheal and Subglottic Stenosis in Adults
Abstract
:1. Introduction
2. Molecular Basis of Abnormal Scarring of the Trachea and Subglottic Area
2.1. The Wound Healing Process in Human Trachea
2.2. Fibroblasts Function in Laryngotracheal Stenosis
2.3. Role of Immune Dysregulation in the Pathogenesis of Iatrogenic LTS
2.4. Immune Response in the Pathogenesis of Idiopathic Subglottic Stenosis
2.5. TGF-Β in the Development of LTS
2.6. Role of Hypoxia as Promoter of Laryngotracheal Scarring
2.7. Genetic Susceptibility to Aberrant Tracheal Healing
2.8. Programmed Cell Death Protein 1 Pathway in LTS
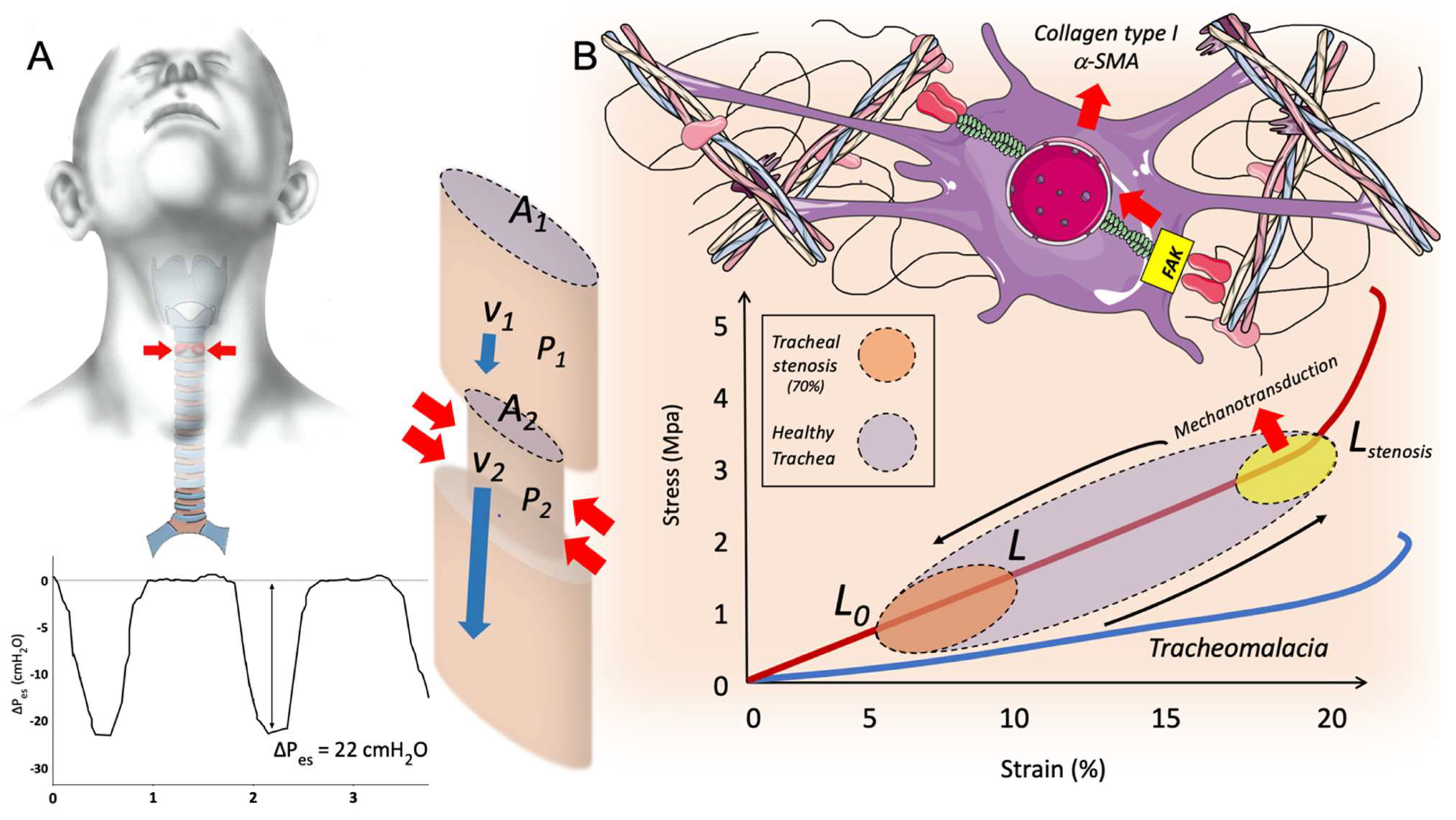
3. Physical Stimuli and Mechanotransduction in the Pathogenesis of Tracheal Stenosis
3.1. Mechanical Behaviour of the Trachea
3.2. Physiological Changes in LTS
3.3. Respiratory Drive in LTS
3.4. Mechanotransduction in Tracheal Scarring
4. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LTS | Laryngotracheal stenosis |
| iSGS | Idiopathic subglottic stenosis |
| ECM | Extracellular matrix |
| GPCR | G-protein coupled receptor |
| MMPS | Matrix metalloproteinases |
| PMPS | Platelet microbicidal proteins |
| VEGF | Vascular endothelial growth factor |
| α-SMA | Alpha-smooth muscle actin |
| IPF | Idiopathic pulmonary fibrosis |
| mRNA | Messenger RNA |
| PGE2 | Prostaglandin E2 |
| TGF-β | Tumour necrosis factor β |
| FGF | Fibroblast growth factor |
| PDGF | Platelet-derived growth factor |
| LTCS | Laryngotracheal complex |
| SCID | Severe combined immunodeficiency |
| IFN- γ | interferon-γ |
| iSGS | Idiopathic subglottic stenosis |
| SMADs | Small mothers against decapentaplegic |
| EMT | Epithelial-to-mesenchymal transition |
| FAK | Focal adhesion kinase |
| BAD | Associated death promoter |
| HIF-1 | Hypoxia-inducible factor-1 |
| PDGFs | Platelet derived growth factors |
| SNPs | Single nucleotide polymorphism |
| BCL-2 | B-cell lymphoma 2 |
| Tregs | Regulatory T cells |
| TM | Tracheomalacia |
| ΔP | Pressure fall |
| ΔPes | Delta oesophageal pressure |
| NFATc | Nuclear factor of activated T cell |
| mTOR | Mammalian target of rapamycin |
References
- Hall, S.R.; Allen, C.T.; Merati, A.L.; Mayerhoff, R.M. Evaluating the utility of serological testing in laryngotracheal stenosis. Laryngoscope 2017, 127, 1408–1412. [Google Scholar] [CrossRef] [PubMed]
- Siciliani, A.; Rendina, E.A.; Ibrahim, M. State of the art in tracheal surgery: A brief literature review. Multidiscip. Respir. Med. 2018, 13, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandour, M.; Remacle, M.; Van De Heyning, P.; Elwany, S.; Tantawy, A.; Gaafar, A. Chronic subglottic and tracheal stenosis: Endoscopic management vs. surgical reconstruction. Eur. Arch. Oto-Rhino-Laryngol. 2003, 260, 374–380. [Google Scholar] [CrossRef] [PubMed]
- DesJardins-Park, H.E.; Mascharak, S.; Chinta, M.S.; Wan, D.C.; Longaker, M.T. The Spectrum of Scarring in Craniofacial Wound Repair. Front. Physiol. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.W.; Gallant-Behm, C.; Wiebe, C.; Mak, K.; Hart, D.A.; Larjava, H.; Häkkinen, L. Wound healing in oral mucosa results in reduced scar formation as compared with skin: Evidence from the red Duroc pig model and humans. Wound Repair Regen. 2009, 17, 717–729. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R. Platelets: At the nexus of antimicrobial defence. Nat. Rev. Microbiol. 2014, 12, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Wietecha, M.S.; DiPietro, L.A. Therapeutic Approaches to the Regulation of Wound Angiogenesis. Adv. Wound Care 2013, 2, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, R.W.D.; Vickaryous, M.K.; Viloria-Petit, A.M. Signalling by Transforming Growth Factor Beta Isoforms in Wound Healing and Tissue Regeneration. J. Dev. Biol. 2016, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Rinkevich, Y. Scars or Regeneration?—Dermal Fibroblasts as Drivers of Diverse Skin Wound Responses. Int. J. Mol. Sci. 2020, 21, 617. [Google Scholar] [CrossRef] [Green Version]
- Rinkevich, Y.; Walmsley, G.G.; Hu, M.S.; Maan, Z.N.; Newman, A.M.; Drukker, M.; Januszyk, M.; Krampitz, G.W.; Gurtner, G.C.; Lorenz, H.P.; et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015, 348, aaa2151. [Google Scholar] [CrossRef] [Green Version]
- Zou, M.-L.; Teng, Y.-Y.; Wu, J.-J.; Liu, S.-Y.; Tang, X.-Y.; Jia, Y.; Chen, Z.-H.; Zhang, K.-W.; Sun, Z.-L.; Li, X.; et al. Fibroblasts: Heterogeneous Cells With Potential in Regenerative Therapy for Scarless Wound Healing. Front. Cell Dev. Biol. 2021, 9, 713605. [Google Scholar] [CrossRef]
- Wernig, G.; Chen, S.-Y.; Cui, L.; Van Neste, C.; Tsai, J.M.; Kambham, N.; Vogel, H.; Natkunam, Y.; Gilliland, D.G.; Nolan, G.; et al. Unifying mechanism for different fibrotic diseases. Proc. Natl. Acad. Sci. USA 2017, 114, 4757–4762. [Google Scholar] [CrossRef] [Green Version]
- Macauley, S.P.; Schultz, G.S.; Bruckner, B.A.; Krawetz, S.A.; Yang, T.P. Effects of transforming growth factor-β1 on extracellular matrix gene expression by human fibroblasts from a laryngeal stenotic lesion. Wound Repair Regen. 1996, 4, 269–277. [Google Scholar] [CrossRef]
- Singh, T.; Sandulache, V.C.; Otteson, T.D.; Barsic, M.; Klein, E.C.; Dohar, J.E.; Hebda, P.A. Subglottic stenosis (SGS) examined as a fibrotic airway mucosal response to injury characterized by altered mucosal fibroblast activity. Arch. Otolaryngol.-Head Neck Surg. 2010, 136, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Sandulache, V.; Parekh, A.; Li-Korotky, H.; Dohar, J.E.; Hebda, P.A. Prostaglandin E2 inhibition of keloid fibroblast migration, contraction, and transforming growth factor (TGF)-β1–induced collagen synthesis. Wound Repair Regen. 2007, 15, 122–133. [Google Scholar] [CrossRef]
- Sandulache, V.C.; Chafin, J.B.; Li-Korotky, H.-S.; Otteson, T.D.; Dohar, J.E.; Hebda, P.A. Elucidating the Role of Interleukin 1β and Prostaglandin E2 in Upper Airway Mucosal Wound Healing. Arch. Otolaryngol.-Head Neck Surg. 2007, 133, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Samad, I.; Motz, K.; Yin, L.X.; Duvvuri, M.V.; Ding, D.; Namba, D.R.; Elisseeff, J.H.; Horton, M.R.; Hillel, A.T. Metabolic variations in normal and fibrotic human laryngotracheal-derived fibroblasts: A Warburg-like effect. Laryngoscope 2017, 127, E107–E113. [Google Scholar] [CrossRef] [Green Version]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Xie, N.; Tan, Z.; Banerjee, S.; Cui, H.; Ge, J.; Liu, R.-M.; Bernard, K.; Thannickal, V.J.; Liu, G. Glycolytic Reprogramming in Myofibroblast Differentiation and Lung Fibrosis. Am. J. Respir. Crit. Care Med. 2015, 192, 1462–1474. [Google Scholar] [CrossRef] [Green Version]
- Lina, I.; Tsai, H.; Ding, D.; Davis, R.; Motz, K.M.; Hillel, A.T. Characterization of Fibroblasts in Iatrogenic Laryngotracheal Stenosis and Type II Diabetes Mellitus. Laryngoscope 2021, 131, 1570–1577. [Google Scholar] [CrossRef]
- Lina, I.A.; Berges, A.; Ospino, R.; Davis, R.J.; Motz, K.M.; Tsai, H.-W.; Collins, S.; Hillel, A.T. Identifying Phenotypically Distinct Fibroblast Subsets in Type 2 Diabetes–Associated Iatrogenic Laryngotracheal Stenosis. Otolaryngol.-Head Neck Surg. 2021, 2021, 01945998211014790. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef] [Green Version]
- Gelbard, A.; Katsantonis, N.-G.; Mizuta, M.; Newcomb, D.; Rotsinger, J.; Rousseau, B.; Daniero, J.; Edell, E.S.; Ekbom, D.C.; Kasperbauer, J.L.; et al. Idiopathic subglottic stenosis is associated with activation of the inflammatory IL-17A/IL-23 axis. Laryngoscope 2016, 126, E356–E361. [Google Scholar] [CrossRef]
- Morrison, R.J.; Katsantonis, N.-G.; Motz, K.M.; Hillel, A.T.; Garrett, C.G.; Netterville, J.; Wootten, C.T.; Majka, S.M.; Blackwell, T.S.; Drake, W.P.; et al. Pathologic Fibroblasts in Idiopathic Subglottic Stenosis Amplify Local Inflammatory Signals. Otolaryngol.-Head Neck Surg. 2019, 160, 107–115. [Google Scholar] [CrossRef]
- Ghosh, A.; Malaisrie, N.; Leahy, K.P.; Singhal, S.; Einhorn, E.; Howlett, P.; Cohen, N.; Mirza, N. Cellular Adaptive Inflammation Mediates Airway Granulation in a Murine Model of Subglottic Stenosis. Otolaryngol.-Head Neck Surg. 2011, 144, 927–933. [Google Scholar] [CrossRef]
- Hillel, A.T.; Ding, D.; Samad, I.; Murphy, M.K.; Motz, K. T-Helper 2 Lymphocyte Immunophenotype Is Associated with Iatrogenic Laryngotracheal Stenosis. Laryngoscope 2019, 129, 177–186. [Google Scholar] [CrossRef]
- Gieseck, R.L.; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef]
- Wynn, T.A. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairfax, K.; Nascimento, M.; Huang, S.C.-C.; Everts, B.; Pearce, E.J. Th2 responses in schistosomiasis. Semin. Immunopathol. 2012, 34, 863–871. [Google Scholar] [CrossRef]
- Jakubzick, C.; Choi, E.S.; Carpenter, K.J.; Kunkel, S.L.; Evanoff, H.; Martinez, F.J.; Flaherty, K.R.; Toews, G.B.; Colby, T.V.; Travis, W.D.; et al. Human Pulmonary Fibroblasts Exhibit Altered Interleukin-4 and Interleukin-13 Receptor Subunit Expression in Idiopathic Interstitial Pneumonia. Am. J. Pathol. 2004, 164, 1989–2001. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Smigiel, K.S.; Parks, W.C. Macrophages, Wound Healing, and Fibrosis: Recent Insights. Curr. Rheumatol. Rep. 2018, 20, 17. [Google Scholar] [CrossRef]
- Hillel, A.T.; Samad, I.; Ma, G.; Ding, D.; Sadtler, K.; Powell, J.D.; Lane, A.P.; Horton, M.R. Dysregulated Macrophages Are Present in Bleomycin-Induced Murine Laryngotracheal Stenosis. Otolaryngol.-Head Neck Surg. 2015, 153, 244–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motz, K.; Lina, I.; Murphy, M.K.; Drake, V.; Davis, R.; Tsai, H.-W.; Feeley, M.; Yin, L.X.; Ding, D.; Hillel, A. M2 Macrophages Promote Collagen Expression and Synthesis in Laryngotracheal Stenosis Fibroblasts. Laryngoscope 2021, 131, E346–E353. [Google Scholar] [CrossRef] [PubMed]
- Schupp, J.; Binder, H.; Jäger, B.; Cillis, G.; Zissel, G.; Müller-Quernheim, J.; Prasse, A. Macrophage Activation in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. PLoS ONE 2015, 10, e0116775. [Google Scholar] [CrossRef] [Green Version]
- Hulsmans, M.; Sam, F.; Nahrendorf, M. Monocyte and macrophage contributions to cardiac remodeling. J. Mol. Cell. Cardiol. 2016, 93, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Sutton, C.E.; Mielke, L.A.; Mills, K.H.G. IL-17-producing γδ T cells and innate lymphoid cells. Eur. J. Immunol. 2012, 42, 2221–2231. [Google Scholar] [CrossRef]
- Hillel, A.T.; Tang, S.; Carlos, C.; Skarlupka, J.H.; Gowda, M.; Yin, L.X.; Motz, K.; Currie, C.R.; Suen, G.; Thibeault, S.L. Laryngotracheal Microbiota in Adult Laryngotracheal Stenosis. mSphere 2019, 4, e00211–e00219. [Google Scholar] [CrossRef] [Green Version]
- Gelbard, A.; Katsantonis, N.-G.; Mizuta, M.; Newcomb, D.; Rotsinger, J.; Rousseau, B.; Daniero, J.J.; Edell, E.S.; Ekbom, D.C.; Kasperbauer, J.L.; et al. Molecular analysis of idiopathic subglottic stenosis for Mycobacterium species. Laryngoscope 2017, 127, 179–185. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B.; Lagares, D. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef]
- Scioscia, K.A.; Miller, F.; April, M.; Gruber, B.L. Growth Factors in Subglottic Stenosis. Ann. Otol. Rhinol. Laryngol. 1996, 105, 936–943. [Google Scholar] [CrossRef]
- Dillard, D.G.; Gal, A.A.; White, S.; Roman-Rodriguez, J.; Jacobs, I.N. Transforming Growth Factor and Neutralizing Antibodies in Subglottic Stenosis. Ann. Otol. Rhinol. Laryngol. 2001, 110, 393–400. [Google Scholar] [CrossRef]
- Simpson, C.B.; White, S.; McGuff, H.S. Anti-Transforming Growth Factor Beta as a Treatment for Laryngotracheal Stenosis in a Canine Model. Laryngoscope 2008, 118, 546–551. [Google Scholar] [CrossRef]
- Touat, L.; Fournier, C.; Ramon, P.; Salleron, J.; Durocher, A.; Nseir, S. Intubation-related tracheal ischemic lesions: Incidence, risk factors, and outcome. Intensive Care Med. 2013, 39, 575–582. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T. Hypoxia in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 553–562. [Google Scholar] [CrossRef]
- Goodwin, J.; Choi, H.; Hsieh, M.-H.; Neugent, M.L.; Ahn, J.-M.; Hayenga, H.N.; Singh, P.K.; Shackelford, D.B.; Lee, I.-K.; Shulaev, V.; et al. Targeting Hypoxia-Inducible Factor-1α/Pyruvate Dehydrogenase Kinase 1 Axis by Dichloroacetate Suppresses Bleomycin-induced Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 58, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Copple, B.L.; Bai, S.; Burgoon, L.D.; Moon, J.-O. Hypoxia-inducible factor-1α regulates the expression of genes in hypoxic hepatic stellate cells important for collagen deposition and angiogenesis. Liver Int. 2011, 31, 230–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Distler, J.H.W.; Jüngel, A.; Pileckyte, M.; Zwerina, J.; Michel, B.A.; Gay, R.E.; Kowal-Bielecka, O.; Matucci-Cerinic, M.; Schett, G.; Marti, H.H.; et al. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum. 2007, 56, 4203–4215. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.J.; Copple, B.L. Role of Hypoxia-Inducible Factors in the Development of Liver Fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 589–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Motz, K.M.; Samad, I.; Duvvuri, M.; Murphy, M.; Ding, D.; Hillel, A.T. Fibroblasts in Hypoxic Conditions Mimic Laryngotracheal Stenosis. Otolaryngol.-Head Neck Surg. 2017, 156, 886–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinn, J.; Kimura, K.S.; Campbell, B.; Lowery, A.S.; Wootten, C.T.; Garrett, C.G.; Francis, D.O.; Hillel, A.T.; Du, L.; Casey, J.D.; et al. Incidence and Outcomes of Acute Laryngeal Injury After Prolonged Mechanical Ventilation. Crit. Care Med. 2019, 47, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Rovó, L.; Széll, M.; Bella, Z.; Korsós, A.; Kemény, L.; Jóri, J. The −509 C/T genotype of TGFβ1 might contribute to the pathogenesis of benign airway stenosis. Otolaryngol.-Head Neck Surg. 2010, 142, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Anis, M.M.; Zhao, Z.; Khurana, J.; Krynetskiy, E.; Soliman, A.M.S. Translational genomics of acquired laryngotracheal stenosis. Laryngoscope 2014, 124, E175–E179. [Google Scholar] [CrossRef] [PubMed]
- Anis, M.; Krynetskaia, N.; Zhao, Z.; Krynetskiy, E.; Soliman, A.M.S. Determining Candidate Single Nucleotide Polymorphisms in Acquired Laryngotracheal Stenosis. Laryngoscope 2018, 128, E111–E116. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Braun, N.A.; Celada, L.J.; Herazo-Maya, J.D.; Abraham, S.; Shaginurova, G.; Sevin, C.M.; Grutters, J.; Culver, D.A.; Dworski, R.; Sheller, J.; et al. Blockade of the Programmed Death-1 Pathway Restores Sarcoidosis CD4+T-Cell Proliferative Capacity. Am. J. Respir. Crit. Care Med. 2014, 190, 560–571. [Google Scholar] [CrossRef] [Green Version]
- Celada, L.J.; Kropski, J.A.; Herazo-Maya, J.D.; Luo, W.; Creecy, A.; Abad, A.T.; Chioma, O.S.; Lee, G.; Hassell, N.E.; Shaginurova, G.I.; et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci. Transl. Med. 2018, 10, eaar8356. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Liu, X.; Liang, J.; Habiel, D.M.; Vrishika, K.; Coelho, A.L.; Deng, N.; Xie, T.; Wang, Y.; Liu, N.; et al. PD-L1 on invasive fibroblasts drives fibrosis in a humanized model of idiopathic pulmonary fibrosis. JCI Insight 2019, 4, e125326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, R.J.; Lina, I.; Ding, D.; Engle, E.L.; Taube, J.; Gelbard, A.; Hillel, A.T. Increased Expression of PD-1 and PD-L1 in Patients With Laryngotracheal Stenosis. Laryngoscope 2021, 131, 967–974. [Google Scholar] [CrossRef]
- Davis, R.J.; Lina, I.; Green, B.; Engle, E.L.; Motz, K.; Ding, D.; Taube, J.M.; Gelbard, A.; Hillel, A.T. Quantitative Assessment of the Immune Microenvironment in Patients With Iatrogenic Laryngotracheal Stenosis. Otolaryngol.-Head Neck Surg. 2021, 164, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Jung, M.; Choudhury, M.; Leof, E.B. Transforming growth factor beta induces fibroblasts to express and release the immunomodulatory protein PD-L1 into extracellular vesicles. FASEB J. 2020, 34, 2213–2226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Z.; Ochoa, I.; Li, Z.-Y.; Liao, Z.; Lin, Y.; Doblaré, M. Study on Tracheal Collapsibility, Compliance, and Stress by Considering Nonlinear Mechanical Property of Cartilage. Ann. Biomed. Eng. 2009, 37, 2380–2389. [Google Scholar] [CrossRef]
- Marchioni, A.; Tonelli, R.; Cerri, S.; Castaniere, I.; Andrisani, D.; Gozzi, F.; Bruzzi, G.; Manicardi, L.; Moretti, A.; Demurtas, J.; et al. Pulmonary Stretch and Lung Mechanotransduction: Implications for Progression in the Fibrotic Lung. Int. J. Mol. Sci. 2021, 22, 6443. [Google Scholar] [CrossRef]
- Rains, J.K.; Bert, J.L.; Roberts, C.R.; Paré, P.D. Mechanical properties of human tracheal cartilage. J. Appl. Physiol. 1992, 72, 219–225. [Google Scholar] [CrossRef]
- Trabelsi, O.; del Palomar, A.P.; López-Villalobos, J.; Ginel, A.; Doblaré, M. Experimental characterization and constitutive modeling of the mechanical behavior of the human trachea. Med. Eng. Phys. 2010, 32, 76–82. [Google Scholar] [CrossRef] [PubMed]
- McCormack, G.S.; Moreno, R.H.; Hogg, J.C.; Paré, P.D. Lung mechanics in papain-treated rabbits. J. Appl. Physiol. 1986, 60, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.G.; Fraser, R.B.; Nadel, J.A. Effect of changing airway mechanics on maximum expiratory flow. J. Appl. Physiol. 1975, 38, 1012–1021. [Google Scholar] [CrossRef]
- Hollister, S.J.; Hollister, M.P.; Hollister, S.K. Computational modeling of airway instability and collapse in tracheomalacia. Respir. Res. 2017, 18, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, E.L.; Bock, J.M.; Zdanski, C.J.; Kimbell, J.S.; Garcia, G.J.M. Relationship between degree of obstruction and airflow limitation in subglottic stenosis. Laryngoscope 2017, 128, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Brouns, M.; Jayaraju, S.T.; Lacor, C.; De Mey, J.; Noppen, M.; Vincken, W.; Verbanck, S. Tracheal stenosis: A flow dynamics study. J. Appl. Physiol. 2007, 102, 1178–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, T.; Carpenter, D.; Cohen, S.; Witsell, D.; Frank-Ito, D.O. Investigating the effects of laryngotracheal stenosis on upper airway aerodynamics. Laryngoscope 2018, 128, E141–E149. [Google Scholar] [CrossRef] [PubMed]
- Argent, A.C.; Newth, C.J.L.; Klein, M. The mechanics of breathing in children with acute severe croup. Intensive Care Med. 2008, 34, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, R.; Fantini, R.; Tabbì, L.; Castaniere, I.; Pisani, L.; Pellegrino, M.R.; Della Casa, G.; D’Amico, R.; Girardis, M.; Nava, S.; et al. Early Inspiratory Effort Assessment by Esophageal Manometry Predicts Noninvasive Ventilation Outcome in De Novo Respiratory Failure. A Pilot Study. Am. J. Respir. Crit. Care Med. 2020, 202, 558–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, M.; Kallet, R.H.; Ware, L.B.; Matthay, M.A. Negative-Pressure Pulmonary Edema. Chest 2016, 150, 927–933. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Edwards, G.A.; Martin, D.; Huang, H.; Crichton, M.L.; Kendall, M.A.F. Allometric scaling of skin thickness, elasticity, viscoelasticity to mass for micro-medical device translation: From mice, rats, rabbits, pigs to humans. Sci. Rep. 2017, 7, 15885. [Google Scholar] [CrossRef]
- Biewener, A.A. Biomechanical consequences of scaling. J. Exp. Biol. 2005, 208 Pt 9, 1665–1676. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, R.; Okai, K.; Tokumura, F.; Mori, K.; Ohmori, Y.; Huang, C.; Hyakusoku, H.; Akaishi, S. The relationship between skin stretching/contraction and pathologic scarring: The important role of mechanical forces in keloid generation. Wound Repair Regen. 2012, 20, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Harn, H.I.-C.; Wang, Y.-K.; Hsu, C.-K.; Ho, Y.-T.; Huang, Y.-W.; Chiu, W.-T.; Lin, H.-H.; Cheng, C.-M.; Tang, M.-J. Mechanical coupling of cytoskeletal elasticity and force generation is crucial for understanding the migrating nature of keloid fibroblasts. Exp. Dermatol. 2015, 24, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Sgalla, G.; Cocconcelli, E.; Tonelli, R.; Richeldi, L. Novel drug targets for idiopathic pulmonary fibrosis. Expert Rev Respir Med. 2016, 10, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mih, J.D.; Shea, B.S.; Kho, A.T.; Sharif, A.S.; Tager, A.M.; Tschumperlin, D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010, 190, 693–706. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Kwon, S.H.; Henn, D.; Kuehlmann, B.A.; Tevlin, R.; Bonham, C.A.; Griffin, M.; Trotsyuk, A.A.; Borrelli, M.R.; Noishiki, C.; et al. Disrupting biological sensors of force promotes tissue regeneration in large organisms. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Ruwanpura, S.M.; Thomas, B.J.; Bardin, P.G. Pirfenidone: Molecular Mechanisms and Potential Clinical Applications in Lung Disease. Am. J. Respir. Cell Mol. Biol. 2020, 62, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Zuñiga, J.R.; Silva-Martínez, M.; Jasso-Victoria, R.; Baltazares-Lipp, M.; Hernández-Jiménez, C.; Buendía-Roldan, I.; Jasso-Arenas, J.; Martínez-Salas, A.; Calyeca-Gómez, J.; Guzmán-Cedillo, A.E.; et al. Effects of Pirfenidone and Collagen-Polyvinylpyrrolidone on Macroscopic and Microscopic Changes, TGF-β1 Expression, and Collagen Deposition in an Experimental Model of Tracheal Wound Healing. BioMed Res. Int. 2017, 2017, 6471071. [Google Scholar] [CrossRef] [Green Version]
- Türkmen, E.; Pata, Y.S. Prevention of tracheal stenosis with pirfenidone after tracheotomy: An experimental study. Laryngoscope 2019, 129, E178–E186. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Fang, X.; Liu, Y.; Zhao, S.; Yu, Z.; Tang, Y.; Wu, P. Antifibrotic Role of Nintedanib in Tracheal Stenosis after a Tracheal Wound. Laryngoscope 2021, 131, E2496–E2505. [Google Scholar] [CrossRef]
- Colak, S.; Ten Dijke, P. Targeting TGF-β Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Platé, M.; Guillotin, D.; Chambers, R.C. The promise of mTOR as a therapeutic target pathway in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2020, 29, 200269. [Google Scholar] [CrossRef] [PubMed]
- Namba, D.R.; Ma, G.; Samad, I.; Ding, D.; Pandian, V.; Powell, J.D.; Horton, M.R.; Hillel, A.T. Rapamycin Inhibits Human Laryngotracheal Stenosis–derived Fibroblast Proliferation, Metabolism, and Function in Vitro. Otolaryngol.-Head Neck Surg. 2015, 152, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, M.; Motz, K.; Murphy, M.; Feeley, M.; Ding, D.; Lee, A.; Elisseeff, J.H.; Hillel, A.T. Engineering an immunomodulatory drug-eluting stent to treat laryngotracheal stenosis. Biomater. Sci. 2019, 7, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, D.; Araki, K.; Tanaka, N.; Suzuki, H.; Tomifuji, M.; Yamashita, T.; Matsushita, K.; Shimada, H.; Shiotani, A. Tacrolimus prevents laryngotracheal stenosis in an acute-injury rat model. Laryngoscope 2015, 125, E210–E215. [Google Scholar] [CrossRef] [PubMed]
- Shlomi, D.; Peled, N.; Shitrit, D.; Bendayan, D.; Amital, A.; Kramer, M.R. Protective Effect of Immunosuppression on Granulation Tissue Formation in Metallic Airway Stents. Laryngoscope 2008, 118, 1383–1388. [Google Scholar] [CrossRef] [PubMed]

| Study | Treatment | Mechanisms of Action | Study Model | Outcome |
|---|---|---|---|---|
| Olmos-Zuniga et al., 2017 [87] | Pirfenidone | Suppression of TGF-b1 Downregulation of cytokine production Inhibition of fibroblast proliferation | Animal model 40 rats undergoing cervical tracheoplasty Group 1: saline solution Group 2: collagen-polyvinylpyrrolidone Group 3: mitomycin C Group 4: Pirfenidone | The animals treated with collagen-polyvinylpyrrolidone and pirfenidone developed less inflammation and fibrosis than animals in the other study groups |
| Turkmen E et al., 2019 [88] | Pirfenidone | Suppression of TGF-b1 Downregulation of cytokine production Inhibition of fibroblast proliferation | Animal model 14 rats undergoing tracheotomy Group 1: pirfenidone intraperitoneally Group 2: saline solution intraperitoneally | Pirfenidone reduced fibrosis and narrowing of tracheal lumen diameter significantly versus control group |
| Fan Y et al., 2021 [89] | Nintedanib | Blockage of the autophosphorylation with consequent inhibition of downstream signalling cascades of FGFRs, PDGFRs, VEGFRs | Animal model and In vitro study of human cells Post-surgical model of tracheal stenosis in rats Group 1: nintedanib Group 2: saline Tracheal specimens were harvested after 3 weeks | Nintedanib prevented tracheal stenosis, and reduced collagen deposition, the expression of fibrotic marker proteins and CD4+ T-lymphocyte infiltration |
| Namba DR et al., 2015 [92] | Rapamycin | Inhibition of the mammalian target of rapamycin (mTOR) | Controlled in vitro study Fibroblasts isolated from biopsies of 5 patients with LTS were cultured and treated with rapamycin or dimethylsulfoxide or normal controls | Rapamycin significantly decreased proliferation, metabolism and collagen deposition of human LTS fibroblasts compared to dimethylsulfoxide or normal controls |
| Duvvuri M et al., 2019 [93] | Drug-eluting stent containing rapamycin | Inhibition of the mammalian target of rapamycin (mTOR) | Animal model and in vitro study Mice | In vitro, rapamycin stent decreased collagen-1 deposition and fibroblasts cell proliferation In vivo, rapamycin stent reduced lamina propria thickness and collagen 1, collagen-3, TGF-b and a-SMA expression |
| Mizokami D et al., 2015 [94] | Tacrolimus | Inhibition of calcineurin with suppression of T-cell activation | Animal model 19 rats with acute tracheal injury Group 1: control Group 2: high-dose tacrolimus Group 3: low-dose tacrolimus | Low dose of tacrolimus prevented laryngotracheal stenosis compared to the untreated animals |
| Dillard DG et al., 2001 [46] | Anti-human neutralizing antibodies to TGF-b1 | TGF-b inhibition | Animal model Laryngotracheal injury in rats Group 1: osmotic pump infusion of TGF-b1 Group 2: pump infusion of neutralizing antibodies Group 3: control | TGF-b1 infusion increased the expression of ECM proteins compared to control Neutralizing antibodies decrease ECM protein expression in the in airway |
| Simpson CB et al., 2008 [47] | Anti-human neutralizing antibodies to TGF-b1 | TGF-b inhibition | Animal model Modified canine model of LTS Group 1: saline injection into the injury site Group2: combination of local injection and intravenous anti-TGF-b | Combination of intralesional and intravenous anti-TGF-b resulted in a reduction in tracheal stenosis and an increase survival time compared to control animals |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchioni, A.; Tonelli, R.; Andreani, A.; Cappiello, G.F.; Fermi, M.; Trentacosti, F.; Castaniere, I.; Fantini, R.; Tabbì, L.; Andrisani, D.; et al. Molecular Mechanisms and Physiological Changes behind Benign Tracheal and Subglottic Stenosis in Adults. Int. J. Mol. Sci. 2022, 23, 2421. https://doi.org/10.3390/ijms23052421
Marchioni A, Tonelli R, Andreani A, Cappiello GF, Fermi M, Trentacosti F, Castaniere I, Fantini R, Tabbì L, Andrisani D, et al. Molecular Mechanisms and Physiological Changes behind Benign Tracheal and Subglottic Stenosis in Adults. International Journal of Molecular Sciences. 2022; 23(5):2421. https://doi.org/10.3390/ijms23052421
Chicago/Turabian StyleMarchioni, Alessandro, Roberto Tonelli, Alessandro Andreani, Gaia Francesca Cappiello, Matteo Fermi, Fabiana Trentacosti, Ivana Castaniere, Riccardo Fantini, Luca Tabbì, Dario Andrisani, and et al. 2022. "Molecular Mechanisms and Physiological Changes behind Benign Tracheal and Subglottic Stenosis in Adults" International Journal of Molecular Sciences 23, no. 5: 2421. https://doi.org/10.3390/ijms23052421
APA StyleMarchioni, A., Tonelli, R., Andreani, A., Cappiello, G. F., Fermi, M., Trentacosti, F., Castaniere, I., Fantini, R., Tabbì, L., Andrisani, D., Gozzi, F., Bruzzi, G., Manicardi, L., Moretti, A., Baroncini, S., Samarelli, A. V., Pinelli, M., De Santis, G., Stefani, A., ... Clini, E. (2022). Molecular Mechanisms and Physiological Changes behind Benign Tracheal and Subglottic Stenosis in Adults. International Journal of Molecular Sciences, 23(5), 2421. https://doi.org/10.3390/ijms23052421








