The Diminishment of Novel Endometrial Carcinoma-Derived Stem-like Cells by Targeting Mitochondrial Bioenergetics and MYC
Abstract
:1. Introduction
2. Results
2.1. Parental Tumor Classifications
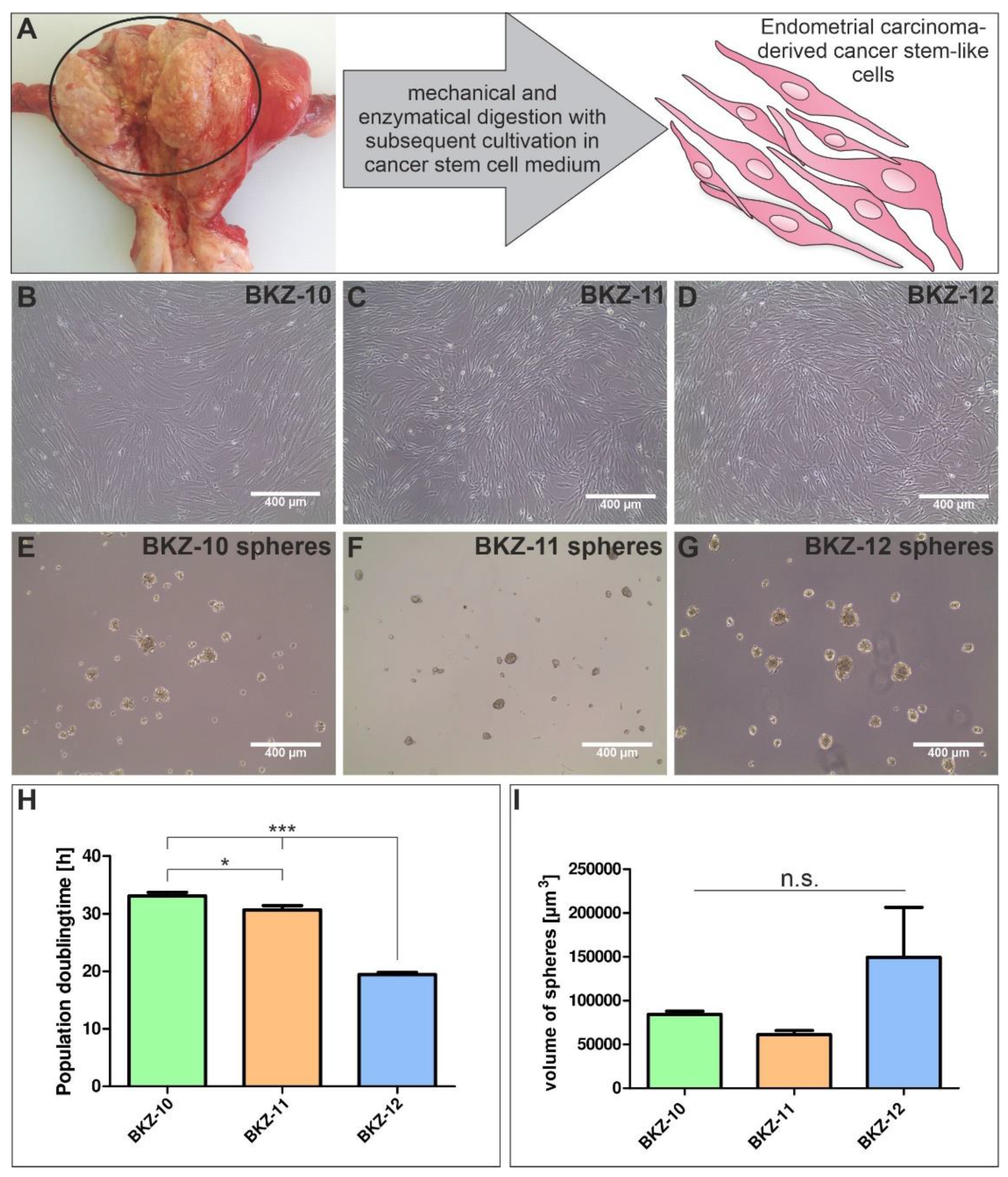
2.2. Successful Isolation of Endometrial Carcinoma-Derived Stem-like Cells
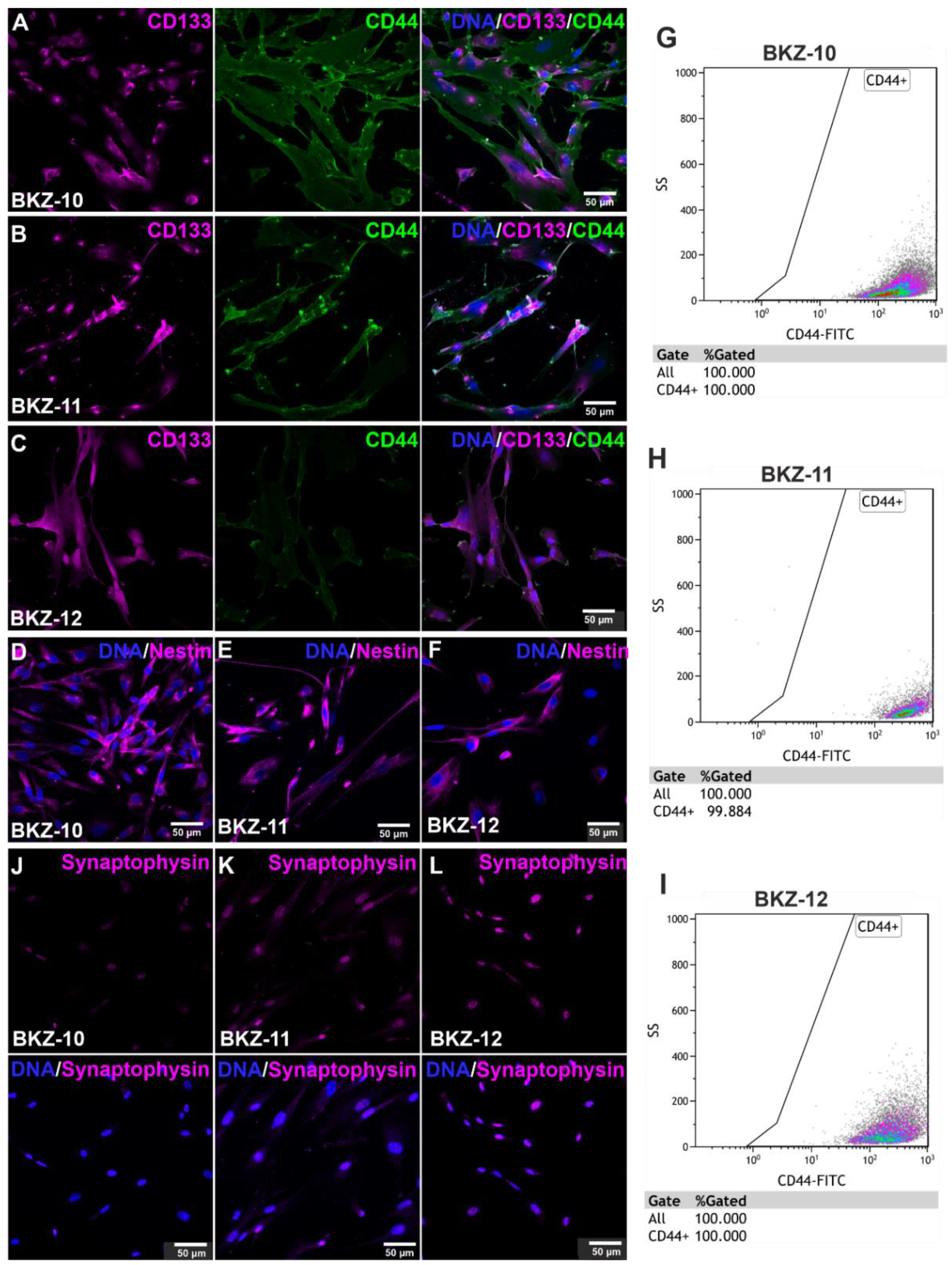
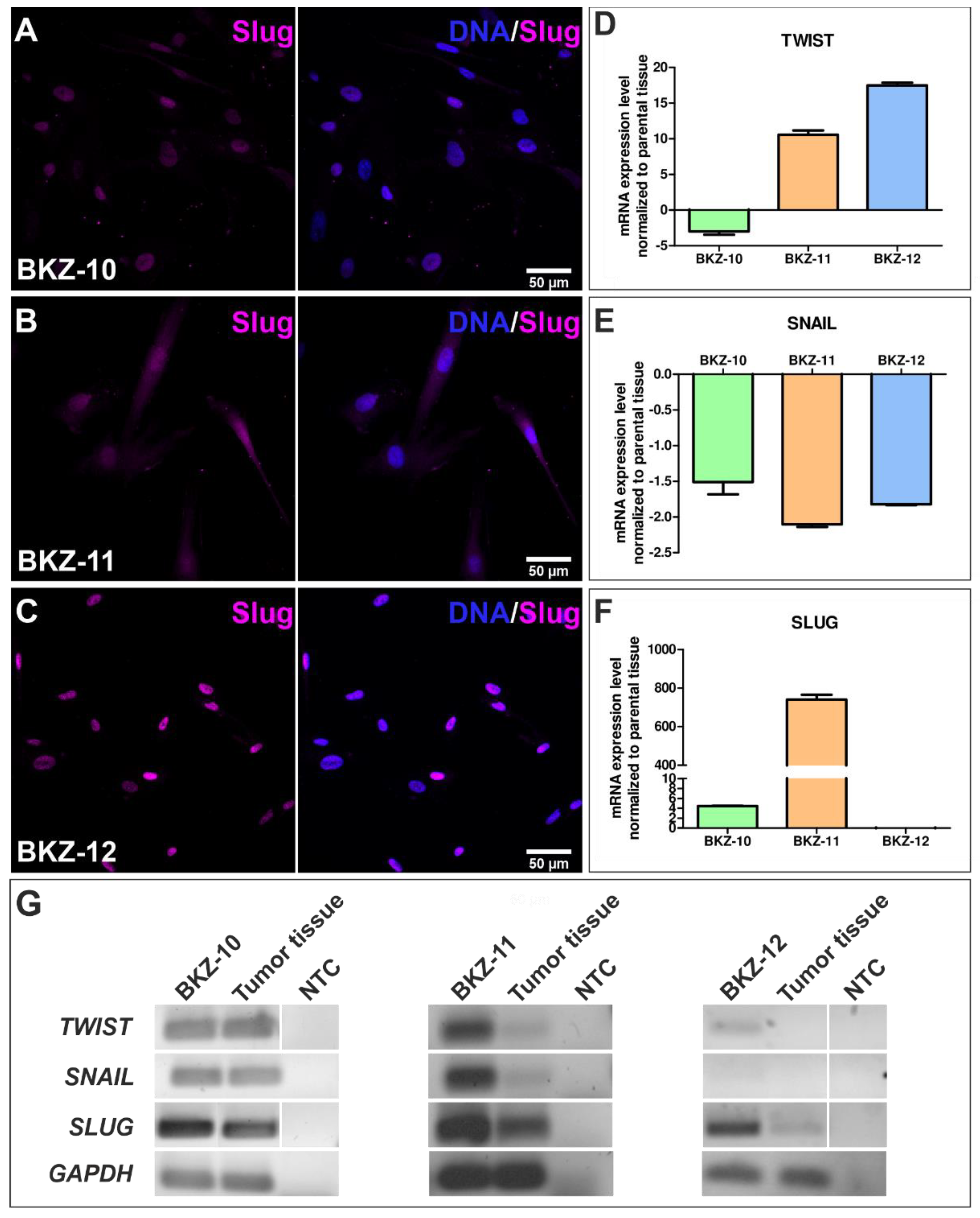
2.3. Endometrial Carcinoma-Derived Stem-like Cells Show an EMT Phentotype
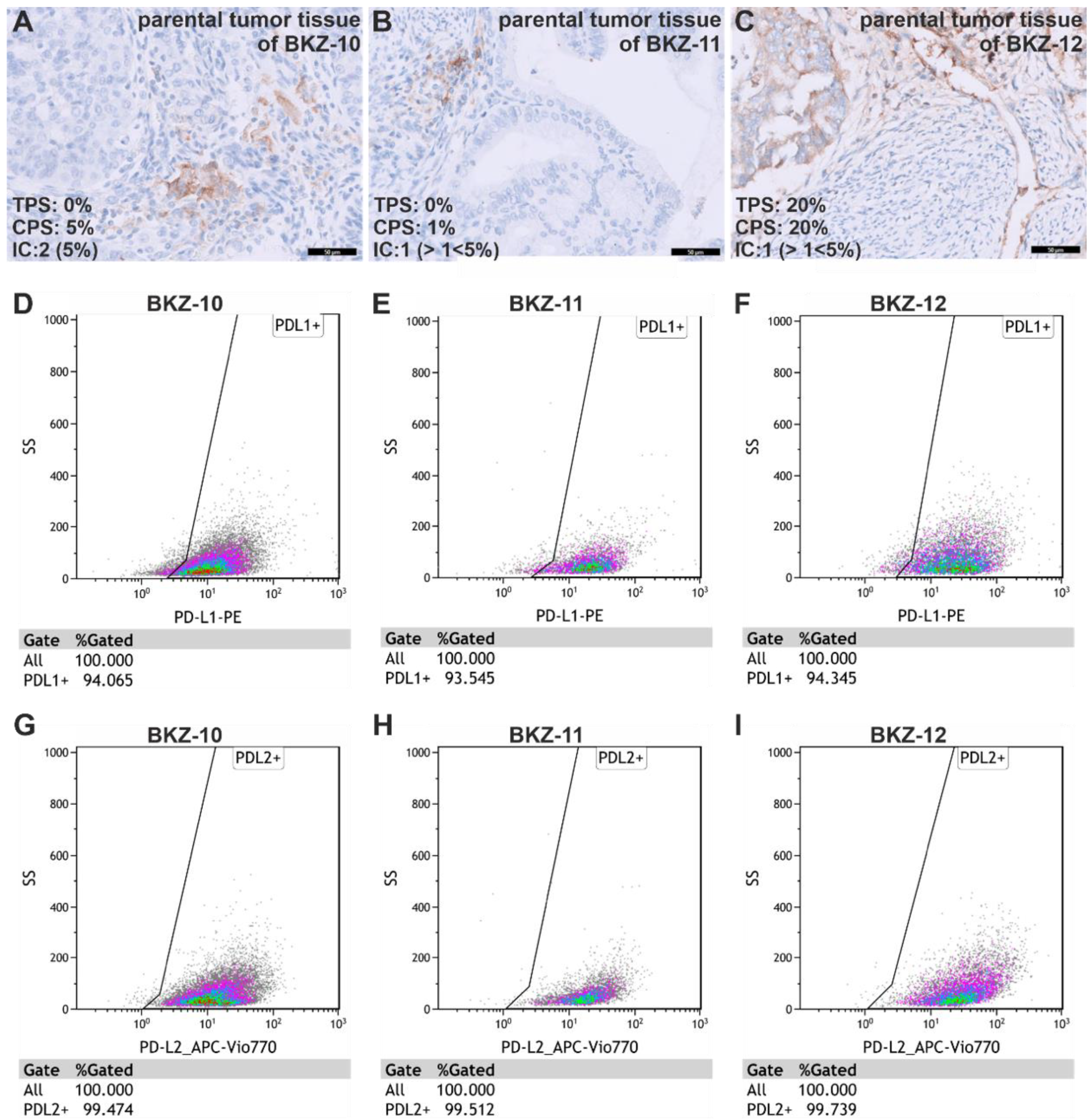
2.4. Endometrial Carcinoma-Derived Stem-like Cells Reveal Immune-Evasive Characteristics
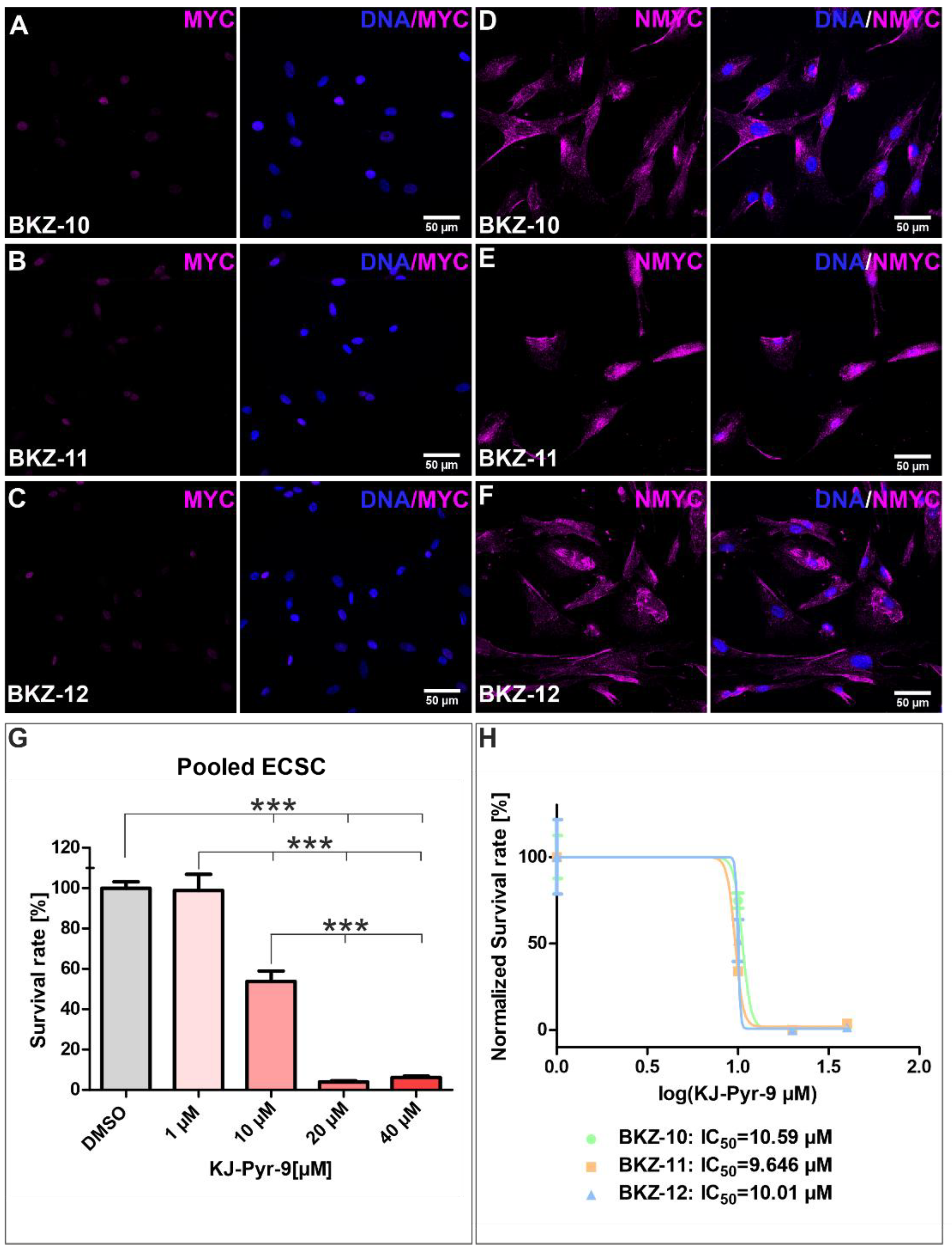
2.5. Impairment of Endometrial Carcinoma-Derived Stem-like Cells by Targeting MYC
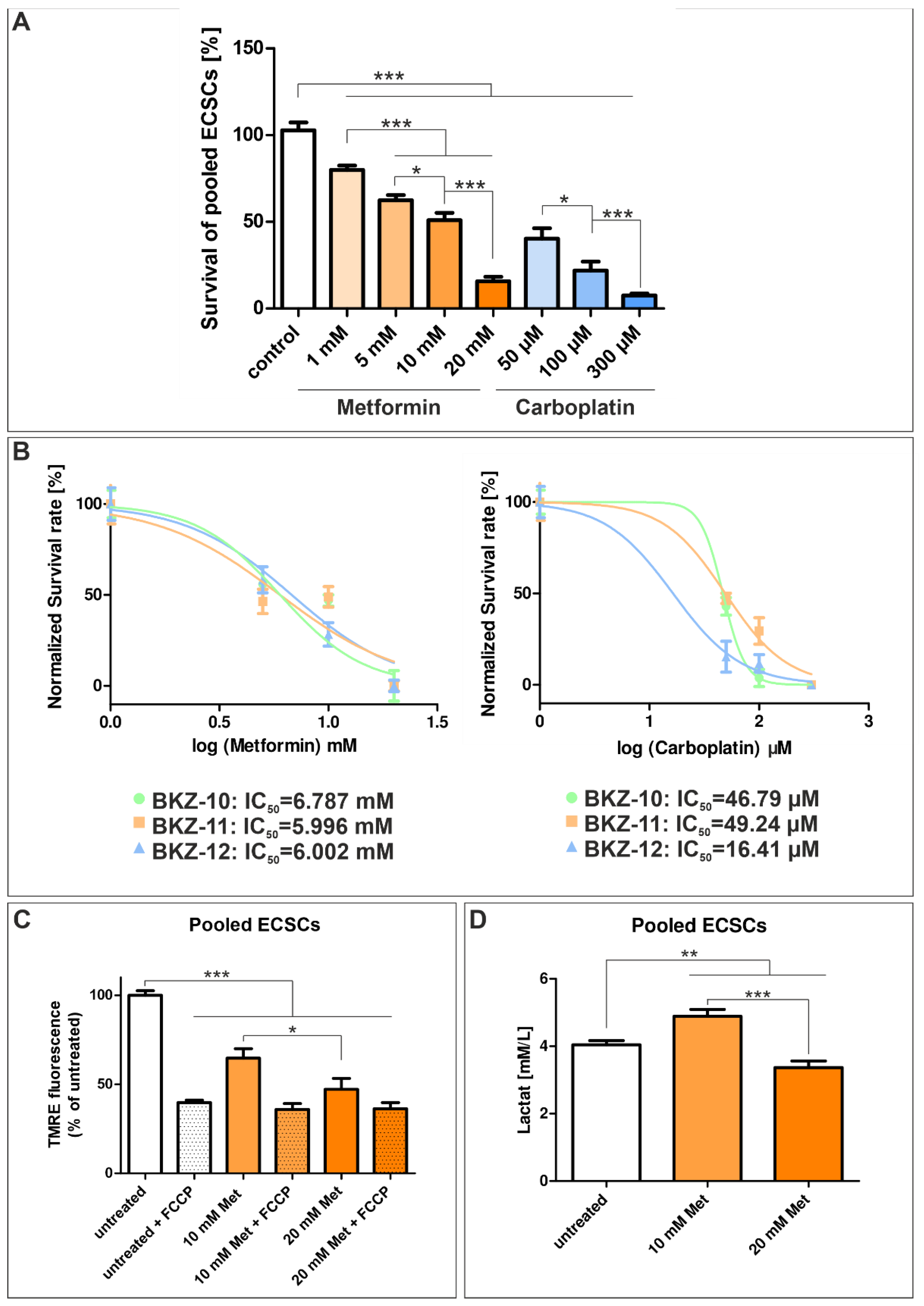
2.6. Metformin Targets the Mitochondrial Metabolism of Endometrial Carcinoma-Derived Stem-like Cells
3. Discussion
4. Materials and Methods
4.1. DNA Extraction and MSI Analysis
4.2. POLE Mutation Analysis
4.3. Endometrial Cancer Stem-like Cell Establishment and Cell Culture
4.4. Immunocytochemistry
4.5. Flow Cytometry
4.6. Polymerase Chain Reaction
4.7. Inhibitor and Drug Treatments
4.8. Mitochondrial Membrane Potential Assay and Lactate Concentration
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer in Women: Burden and Trends. Cancer Epidemiology Biomarkers Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Di Cristofano, A.; Ellenson, L.H. Endometrial carcinoma. Annu. Rev. Pathol. Mech. Dis. 2007, 2, 57–85. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Uterine Cancer. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 1 June 2021).
- Vermij, L.; Smit, V.; Nout, R.; Bosse, T. Incorporation of molecular characteristics into endometrial cancer management. Histopathology 2020, 76, 52–63. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Female Genital Tumours, 5th ed.; International Agency for Research on Cancer World Health Organization: Lyon, France, 2020. [Google Scholar]
- López-Reig, R.; Fernández-Serra, A.; Romero, I.; Zorrero, C.; Illueca, C.; García-Casado, Z.; Poveda, A.; López-Guerrero, J.A. Prognostic classification of endometrial cancer using a molecular approach based on a twelve-gene NGS panel. Sci. Rep. 2019, 9, 18093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizza, E.; Cutillo, G.; Bruno, V.; Sperduti, I.; Mancini, E.; Baiocco, E.; Chiofalo, B.; Cicchillitti, L.; Certelli, C.; Zampa, A.; et al. Pattern of recurrence in patients with endometrial cancer: A retrospective study. Eur. J. Surg. Oncol. 2020, 46, 1697–1702. [Google Scholar] [CrossRef]
- Ito, T.; Zimdahl, B.; Reya, T. aSIRTing control over cancer stem cells. Cancer Cell 2012, 21, 140–142. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Hong, D.; Park, J.Y. Association between Morphological Patterns of Myometrial Invasion and Cancer Stem Cell Markers in Endometrial Endometrioid Carcinoma. Pathol. Oncol. Res. 2019, 25, 123–130. [Google Scholar] [CrossRef]
- Elbasateeny, S.S.; Salem, A.A.; Abdelsalam, W.A.; Salem, R.A. Immunohistochemical expression of cancer stem cell related markers CD44 and CD133 in endometrial cancer. Pathol.-Res. Pract. 2016, 212, 10–16. [Google Scholar] [CrossRef]
- Rutella, S.; Bonanno, G.; Procoli, A.; Mariotti, A.; Corallo, M.; Prisco, M.G.; Eramo, A.; Napoletano, C.; Gallo, D.; Perillo, A.; et al. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin. Cancer Res. 2009, 15, 4299–4311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Yoshida, T.; Okabe, M.; Zhou, K.; Wang, F.; Soko, C.; Saito, S.; Nikaido, T. Isolation of Stem-like Cancer Cells in Primary Endometrial Cancer Using Cell Surface Markers CD133 and CXCR4. Transl. Oncol. 2017, 10, 976–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, D.-C.; Liu, H.-W.; Chang, Y.-H.; Chu, T.-Y. Expression of CD133 in endometrial cancer cells and its implications. J. Cancer 2017, 8, 2142–2153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Zhang, J.; Yang, X.; Fang, C.; Xu, H.; Xi, X. SALL4 as an Epithelial-Mesenchymal Transition and Drug Resistance Inducer through the Regulation of c-Myc in Endometrial Cancer. PLoS ONE 2015, 10, e0138515. [Google Scholar]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Terai, Y.; Kawaguchi, H.; Fujiwara, S.; Yoo, S.; Tsunetoh, S.; Takai, M.; Kanemura, M.; Tanabe, A.; Ohmichi, M. Prognostic impact of EMT (epithelial-mesenchymal-transition)-related protein expression in endometrial cancer. Cancer Biol. Ther. 2013, 14, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Sciacovelli, M.; Frezza, C. Metabolic reprogramming and epithelial-to-mesenchymal transition in cancer. FEBS J. 2017, 284, 3132–3144. [Google Scholar] [CrossRef]
- Ozsvari, B.; Bonuccelli, G.; Sanchez-Alvarez, R.; Foster, R.; Sotgia, F.; Lisanti, M.P. Targeting flavin-containing enzymes eliminates cancer stem cells (CSCs), by inhibiting mitochondrial respiration: Vitamin B2 (Riboflavin) in cancer therapy. Aging 2017, 9, 2610–2628. [Google Scholar] [CrossRef] [Green Version]
- Farnie, G.; Sotgia, F.; Lisanti, M.P. High mitochondrial mass identifies a sub-population of stem-like cancer cells that are chemo-resistant. Oncotarget 2015, 6, 30472–30486. [Google Scholar] [CrossRef] [Green Version]
- Neradil, J.; Veselska, R. Nestin as a marker of cancer stem cells. Cancer Sci. 2015, 106, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, A.A.; Baker, T.M.; Dorjbal, B.; Waheed, S.; Zahn, C.M.; Hamilton, C.A.; Maxwell, G.L.; Syed, V. Nestin suppression attenuates invasive potential of endometrial cancer cells by downregulating TGF-β signaling pathway. Oncotarget 2016, 7, 69733–69748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windmöller, B.A.; Greiner, J.F.W.; Förster, C.; Wilkens, L.; Mertzlufft, F.; Schulte am Esch, J.; Kaltschmidt, B.; Kaltschmidt, C.; Beshay, M. A typical carcinoid of the lung—A case report with pathological correlation and propagation of the cancer stem cell line BKZ1 with synaptophysin expression. Medicine 2019, 98, e18174. [Google Scholar] [CrossRef] [PubMed]
- Schulte am Esch, J.; Windmöller, B.A.; Hanewinkel, J.; Storm, J.; Förster, C.; Wilkens, L.; Krüger, M.; Kaltschmidt, B.; Kaltschmidt, C. Isolation and characterization of two novel colorectal cancer cell lines, containing a subpopulation with potential stem-like properties: Treatment options by MYC/NMYC inhibition. Cancers 2020, 12, 2582. [Google Scholar] [CrossRef]
- Mori, Y.; Yamawaki, K.; Ishiguro, T.; Yoshihara, K.; Ueda, H.; Sato, A.; Ohata, H.; Yoshida, Y.; Minamino, T.; Okamoto, K.; et al. ALDH-Dependent Glycolytic Activation Mediates Stemness and Paclitaxel Resistance in Patient-Derived Spheroid Models of Uterine Endometrial Cancer. Stem Cell Rep. 2019, 13, 730–746. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Cong, Y.; Wang, D.; Sun, Y.; Deng, L.; Liu, Y.; Martin-Trevino, R.; Shang, L.; McDermott, S.P.; Landis, M.D.; et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014, 2, 78–91. [Google Scholar] [CrossRef]
- Kwilas, A.R.; Ardiani, A.; Dirmeier, U.; Wottawah, C.; Schlom, J.; Hodge, J.W. A poxviral-based cancer vaccine targeting the transcription factor twist inhibits primary tumor growth and metastases in a model of metastatic breast cancer and improves survival in a spontaneous prostate cancer model. Oncotarget 2015, 6, 28194–28210. [Google Scholar] [CrossRef] [Green Version]
- Hsu, J.-M.; Xia, W.; Hsu, Y.-H.; Chan, L.-C.; Yu, W.-H.; Cha, J.-H.; Chen, C.-T.; Liao, H.-W.; Kuo, C.-W.; Khoo, K.-H.; et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat. Commun. 2018, 9, 1908. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Tanegashima, T.; Togashi, Y.; Azuma, K.; Kawahara, A.; Ideguchi, K.; Sugiyama, D.; Kinoshita, F.; Akiba, J.; Kashiwagi, E.; Takeuchi, A.; et al. Immune Suppression by PD-L2 against Spontaneous and Treatment-Related Antitumor Immunity. Clin. Cancer Res. 2019, 25, 4808–4819. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Guo, Q.; Li, X.; Yang, X.; Ni, H.; Wang, T.; Zhao, Q.; Liu, H.; Xing, Y.; Xi, T.; et al. MiR-873/PD-L1 axis regulates the stemness of breast cancer cells. EBioMedicine 2019, 41, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Hong, W.; Xue, M.; Jiang, J.; Zhang, Y.; Gao, X. Circular RNA circ-CPA4/let-7 miRNA/PD-L1 axis regulates cell growth, stemness, drug resistance and immune evasion in non-small cell lung cancer (NSCLC). J. Exp. Clin. Cancer Res. 2020, 39, 149. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gibbons, D.L.; Goswami, S.; Cortez, M.A.; Ahn, Y.-H.; Byers, L.A.; Zhang, X.; Yi, X.; Dwyer, D.; Lin, W.; et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014, 5, 5241. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, Y.; Li, J.; Zheng, X.; Zhou, Q.; Turner, A.; Wu, C.; Lu, B.; Jiang, J. PD-L1 Expression Promotes Epithelial to Mesenchymal Transition in Human Esophageal Cancer. Cell. Physiol. Biochem. 2017, 42, 2267–2280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, T.; Ahn, R.; Yang, K.; Zhu, X.; Fu, Z.; Morin, G.; Bramley, R.; Cliffe, N.C.; Xue, Y.; Kuasne, H.; et al. CD44 Promotes PD-L1 Expression and Its Tumor-Intrinsic Function in Breast and Lung Cancers. Cancer Res. 2020, 80, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, H.; Wang, X.; Zhao, C.; Wang, H. CD44, a marker of cancer stem cells, is positively correlated with PD-L1 expression and immune cells infiltration in lung adenocarcinoma. Cancer Cell Int. 2020, 20, 583. [Google Scholar] [CrossRef]
- Marinkovic, D.; Marinkovic, T. The new role for an old guy: MYC as an immunoplayer. J. Cell. Physiol. 2021, 236, 3234–3243. [Google Scholar] [CrossRef]
- Eilers, M.; Eisenman, R.N. Myc’s broad reach. Genes Dev. 2008, 22, 2755–2766. [Google Scholar] [CrossRef] [Green Version]
- Witte, K.E.; Hertel, O.; Windmöller, B.A.; Helweg, L.P.; Höving, A.L.; Knabbe, C.; Busche, T.; Greiner, J.F.W.; Kalinowski, J.; Noll, T.; et al. Nanopore Sequencing Reveals Global Transcriptome Signatures of Mitochondrial and Ribosomal Gene Expressions in Various Human Cancer Stem-like Cell Populations. Cancers 2021, 13, 1136. [Google Scholar] [CrossRef]
- Hart, J.R.; Garner, A.L.; Yu, J.; Ito, Y.; Sun, M.; Ueno, L.; Rhee, J.-K.; Baksh, M.M.; Stefan, E.; Hartl, M.; et al. Inhibitor of MYC identified in a Kröhnke pyridine library. Proc. Natl. Acad. Sci. USA 2014, 111, 12556–12561. [Google Scholar] [CrossRef] [Green Version]
- Windmöller, B.A.; Beshay, M.; Helweg, L.P.; Flottmann, C.; Beermann, M.; Förster, C.; Wilkens, L.; Greiner, J.F.W.; Kaltschmidt, C.; Kaltschmidt, B. Novel Primary Human Cancer Stem-like Cell Populations from Non-Small Cell Lung Cancer: Inhibition of Cell Survival by Targeting NF-κB and MYC Signaling. Cells 2021, 10, 1024. [Google Scholar] [CrossRef] [PubMed]
- Akram, T.; Maseelall, P.; Fanning, J. Carboplatin and paclitaxel for the treatment of advanced or recurrent endometrial cancer. Am. J. Obstet. Gynecol. 2005, 192, 1365–1367. [Google Scholar] [CrossRef]
- Bao, B.; Wang, Z.; Ali, S.; Ahmad, A.; Azmi, A.S.; Sarkar, S.H.; Banerjee, S.; Kong, D.; Li, Y.; Thakur, S.; et al. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev. Res. 2012, 5, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhang, P.; Wang, H.; Hou, D.; Li, W.; Xiao, G.; Li, C. Inhibitory effects of metformin at low concentration on epithelial-mesenchymal transition of CD44+CD117+ ovarian cancer stem cells. Stem Cell Res. Ther. 2015, 6, 262. [Google Scholar] [CrossRef] [Green Version]
- Kitson, S.J.; Rosser, M.; Fischer, D.P.; Marshall, K.M.; Clarke, R.B.; Crosbie, E.J. Targeting Endometrial Cancer Stem Cell Activity with Metformin Is Inhibited by Patient-Derived Adipocyte-Secreted Factors. Cancers 2019, 11, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Wang, Y.-L.; Yu, L.; Hu, Q.; Ji, L.; Zhang, Y.; Liao, Q.-P. Metformin promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. J. Steroid Biochem. Mol. Biol. 2011, 126, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Zhao, Y.; Wang, X.; Qi, Y.; Zhou, C.; Hua, Y.; Hou, J.; Jiang, S.-W. Culture characters, genetic background, estrogen/progesterone receptor expression, and tumorigenic activities of frequently used sixteen endometrial cancer cell lines. Clin. Chim. Acta 2019, 489, 225–232. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Zhou, X.; Li, Y.; Liu, T.; Yin, X.; Zhang, B. Role of metformin in inhibiting estrogen-induced proliferation and regulating ERα and ERβ expression in human endometrial cancer cells. Oncol. Lett. 2017, 14, 4949–4956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Qi, S.; Zhao, X.; Li, M.; Ding, S.; Lu, J.; Zhang, H. Metformin inhibits 17β-estradiol-induced epithelial-to-mesenchymal transition via βKlotho-related ERK1/2 signaling and AMPKα signaling in endometrial adenocarcinoma cells. Oncotarget 2016, 7, 21315–21331. [Google Scholar] [CrossRef]
- Laskov, I.; Abou-Nader, P.; Amin, O.; Philip, C.-A.; Beauchamp, M.-C.; Yasmeen, A.; Gotlieb, W.H. Metformin Increases E-cadherin in Tumors of Diabetic Patients with Endometrial Cancer and Suppresses Epithelial-Mesenchymal Transition in Endometrial Cancer Cell Lines. Int. J. Gynecol. Cancer 2016, 26, 1213–1221. [Google Scholar] [CrossRef]
- Gao, Z.-Y.; Liu, Z.; Bi, M.-H.; Zhang, J.-J.; Han, Z.-Q.; Han, X.; Wang, H.-Y.; Sun, G.-P.; Liu, H. Metformin induces apoptosis via a mitochondria-mediated pathway in human breast cancer cells in vitro. Exp. Ther. Med. 2016, 11, 1700–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [Green Version]
- Sancho, P.; Burgos-Ramos, E.; Tavera, A.; Kheir, T.B.; Jagust, P.; Schoenhals, M.; Barneda, D.; Sellers, K.; Campos-Olivas, R.; Graña, O.; et al. MYC/PGC-1α Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metab. 2015, 22, 590–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, E.M.; Walter, P.; Jackson, A.; Clark, L.; Franasiak, J.; Bolac, C.; Havrilesky, L.J.; Secord, A.A.; Moore, D.T.; Gehrig, P.A.; et al. Metformin is associated with improved survival in endometrial cancer. Gynecol. Oncol. 2014, 132, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Laganà, A.S.; Nigro, A.; La Rosa, V.L.; Rossetti, P.; Rapisarda, A.M.C.; La Vignera, S.; Condorelli, R.A.; Corrado, F.; Buscema, M.; et al. Peroxisome Proliferator-Activated Receptor Modulation during Metabolic Diseases and Cancers: Master and Minions. PPAR Res. 2016, 2016, 6517313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laganà, A.S.; Vitale, S.G.; Nigro, A.; Sofo, V.; Salmeri, F.M.; Rossetti, P.; Rapisarda, A.M.C.; La Vignera, S.; Condorelli, R.A.; Rizzo, G.; et al. Pleiotropic Actions of Peroxisome Proliferator-Activated Receptors (PPARs) in Dysregulated Metabolic Homeostasis, Inflammation and Cancer: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2016, 17, 999. [Google Scholar] [CrossRef] [Green Version]
- Berg, K.D.; Glaser, C.L.; Thompson, R.E.; Hamilton, S.R.; Griffin, C.A.; Eshleman, J.R. Detection of microsatellite instability by fluorescence multiplex polymerase chain reaction. J. Mol. Diagn. 2000, 2, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Schiemann, U.; Müller-Koch, Y.; Gross, M.; Daum, J.; Lohse, P.; Baretton, G.; Muders, M.; Mussack, T.; Kopp, R.; Holinski-Feder, E. Extended microsatellite analysis in microsatellite stable, MSH2 and MLH1 mutation-negative HNPCC patients: Genetic reclassification and correlation with clinical features. Digestion 2004, 69, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Stenzinger, A.; Pfarr, N.; Endris, V.; Penzel, R.; Jansen, L.; Wolf, T.; Herpel, E.; Warth, A.; Klauschen, F.; Kloor, M.; et al. Mutations in POLE and survival of colorectal cancer patients—Link to disease stage and treatment. Cancer Med. 2014, 3, 1527–1538. [Google Scholar] [CrossRef]
- Yu, S.; Shao, H.; Ban, X.; Zhang, H.; You, Y.; Zhou, N.; Mao, X.; Zhao, H.; Chen, J.; Lu, Z. Detection of POLE Subtypes in High-Grade Endometrioid Carcinoma by BaseScope-ISH Assay. Front. Oncol. 2019, 9, 831. [Google Scholar] [CrossRef]
- Walia, V.; Elble, R.C. Enrichment for breast cancer cells with stem/progenitor properties by differential adhesion. Stem Cells Dev. 2010, 19, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Morata-Tarifa, C.; Jiménez, G.; García, M.A.; Entrena, J.M.; Griñán-Lisón, C.; Aguilera, M.; Picon-Ruiz, M.; Marchal, J.A. Low adherent cancer cell subpopulations are enriched in tumorigenic and metastatic epithelial-to-mesenchymal transition-induced cancer stem-like cells. Sci. Rep. 2016, 6, 18772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Malley, D.P.; Yang, Y.; Boisot, S.; Sudarsanam, S.; Wang, J.-F.; Chizhevsky, V.; Zhao, G.; Arain, S.; Weiss, L.M. Immunohistochemical detection of PD-L1 among diverse human neoplasms in a reference laboratory: Observations based upon 62,896 cases. Mod. Pathol. 2019, 32, 929–942. [Google Scholar] [CrossRef]


| Donor of | P53-IHC | Mismatch–Repair | POLE Status | Classification | Prognosis |
|---|---|---|---|---|---|
| BKZ-10 | Not aberrant | MSS | Wild type | NSMP EC = no specific molecular profile | Intermediate to excellent |
| BKZ-11 | Not aberrant | MSI-L | Wild type | MMR-deficient EC | Intermediate |
| BKZ-12 | Not aberrant | MSS | Wild type | NSMP EC = no specific molecular profile | Intermediate to excellent |
| Target Gene | Sequence |
|---|---|
| Beta-Aktin (AKTB) forward | TCCCTGGAGAAGAGCTACGA |
| AKTB reverse | AGCACTGTGTTGGCGTACAG |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward | CATGAGAAGTATGACAACAGCCT |
| GAPDH reverse | AGTCCTTCCACGTATACCAAAGT |
| Ribosomal protein lateral stalk subunit P0 (RPLP0) forward | TGGGCAAGAACACCATGATG |
| RPLP0 reverse | AGTTTCTCCAGAGCTGGGTTGT |
| Snail (SNAI1) forward | CCCAATCGGAAGCCTAACTA |
| SNAI1 reverse | GGACAGAGTCCCAGATGAGC |
| Slug (SNAI2) forward | TCGGACCCACACATTACCTT |
| SNAI2 reverse | TTGGAGCAGTTTTTGCACTG |
| Twist-related protein 1 (TWIST1) forward | GTCCGCAGTCTTACGAGGAG |
| TWIST1 reverse | CCAGTTGAGGGTCTGAATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helweg, L.P.; Windmöller, B.A.; Burghardt, L.; Storm, J.; Förster, C.; Wethkamp, N.; Wilkens, L.; Kaltschmidt, B.; Banz-Jansen, C.; Kaltschmidt, C. The Diminishment of Novel Endometrial Carcinoma-Derived Stem-like Cells by Targeting Mitochondrial Bioenergetics and MYC. Int. J. Mol. Sci. 2022, 23, 2426. https://doi.org/10.3390/ijms23052426
Helweg LP, Windmöller BA, Burghardt L, Storm J, Förster C, Wethkamp N, Wilkens L, Kaltschmidt B, Banz-Jansen C, Kaltschmidt C. The Diminishment of Novel Endometrial Carcinoma-Derived Stem-like Cells by Targeting Mitochondrial Bioenergetics and MYC. International Journal of Molecular Sciences. 2022; 23(5):2426. https://doi.org/10.3390/ijms23052426
Chicago/Turabian StyleHelweg, Laureen P., Beatrice A. Windmöller, Leonie Burghardt, Jonathan Storm, Christine Förster, Nils Wethkamp, Ludwig Wilkens, Barbara Kaltschmidt, Constanze Banz-Jansen, and Christian Kaltschmidt. 2022. "The Diminishment of Novel Endometrial Carcinoma-Derived Stem-like Cells by Targeting Mitochondrial Bioenergetics and MYC" International Journal of Molecular Sciences 23, no. 5: 2426. https://doi.org/10.3390/ijms23052426
APA StyleHelweg, L. P., Windmöller, B. A., Burghardt, L., Storm, J., Förster, C., Wethkamp, N., Wilkens, L., Kaltschmidt, B., Banz-Jansen, C., & Kaltschmidt, C. (2022). The Diminishment of Novel Endometrial Carcinoma-Derived Stem-like Cells by Targeting Mitochondrial Bioenergetics and MYC. International Journal of Molecular Sciences, 23(5), 2426. https://doi.org/10.3390/ijms23052426










