Application of a Screen-Printed Sensor Modified with Carbon Nanofibers for the Voltammetric Analysis of an Anticancer Disubstituted Fused Triazinone
Abstract
:1. Introduction
2. Results and Discussion
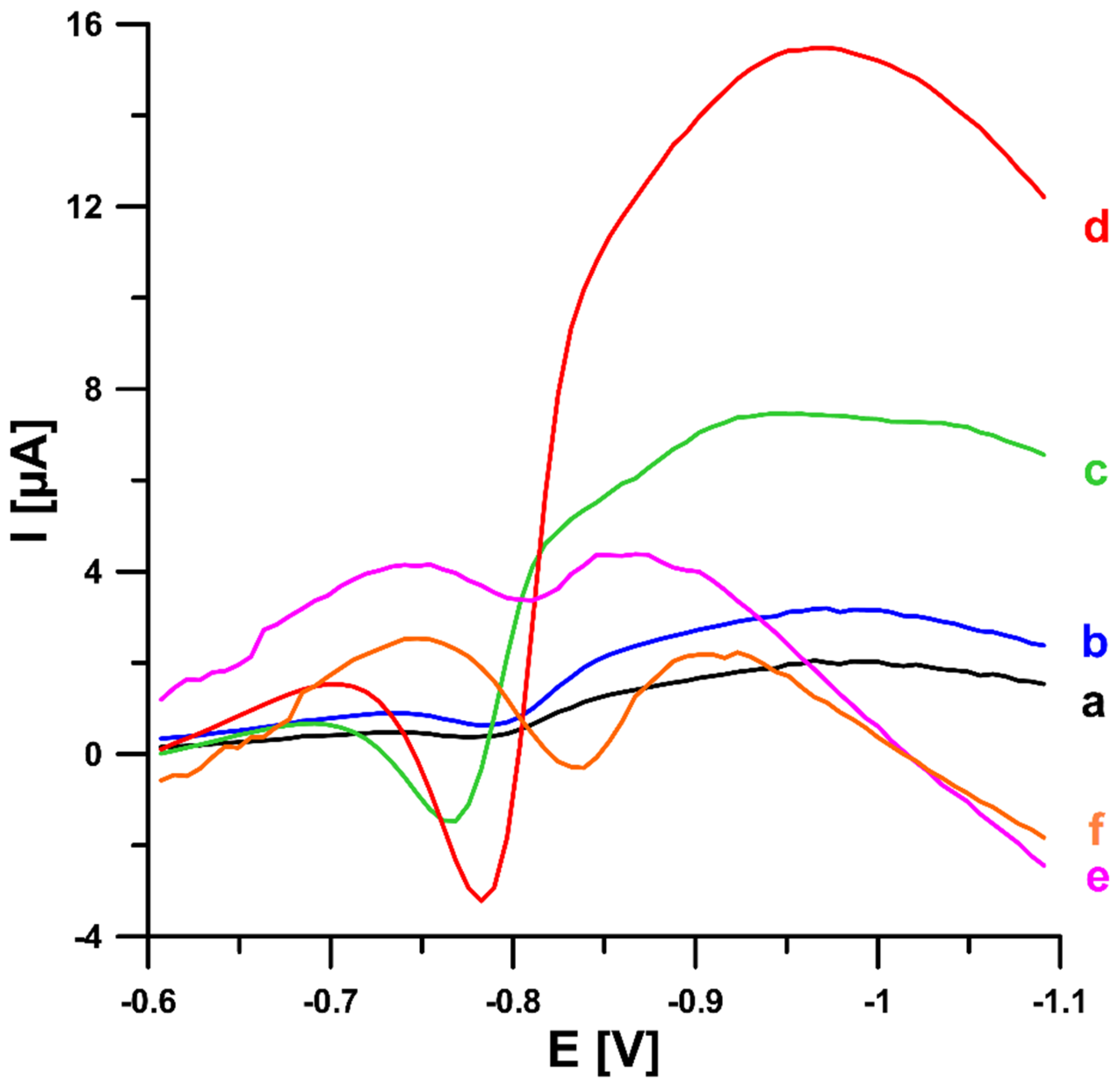
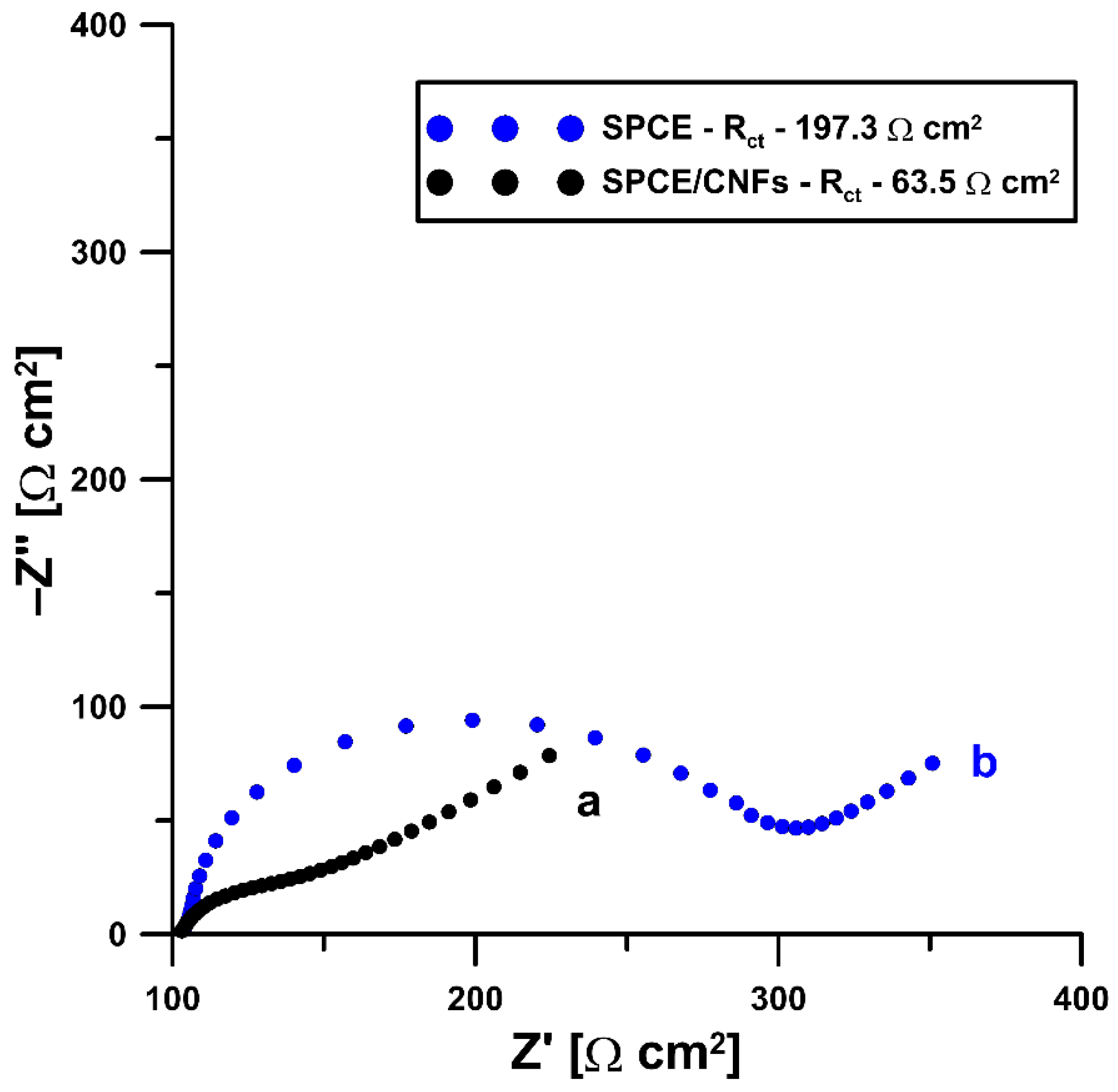
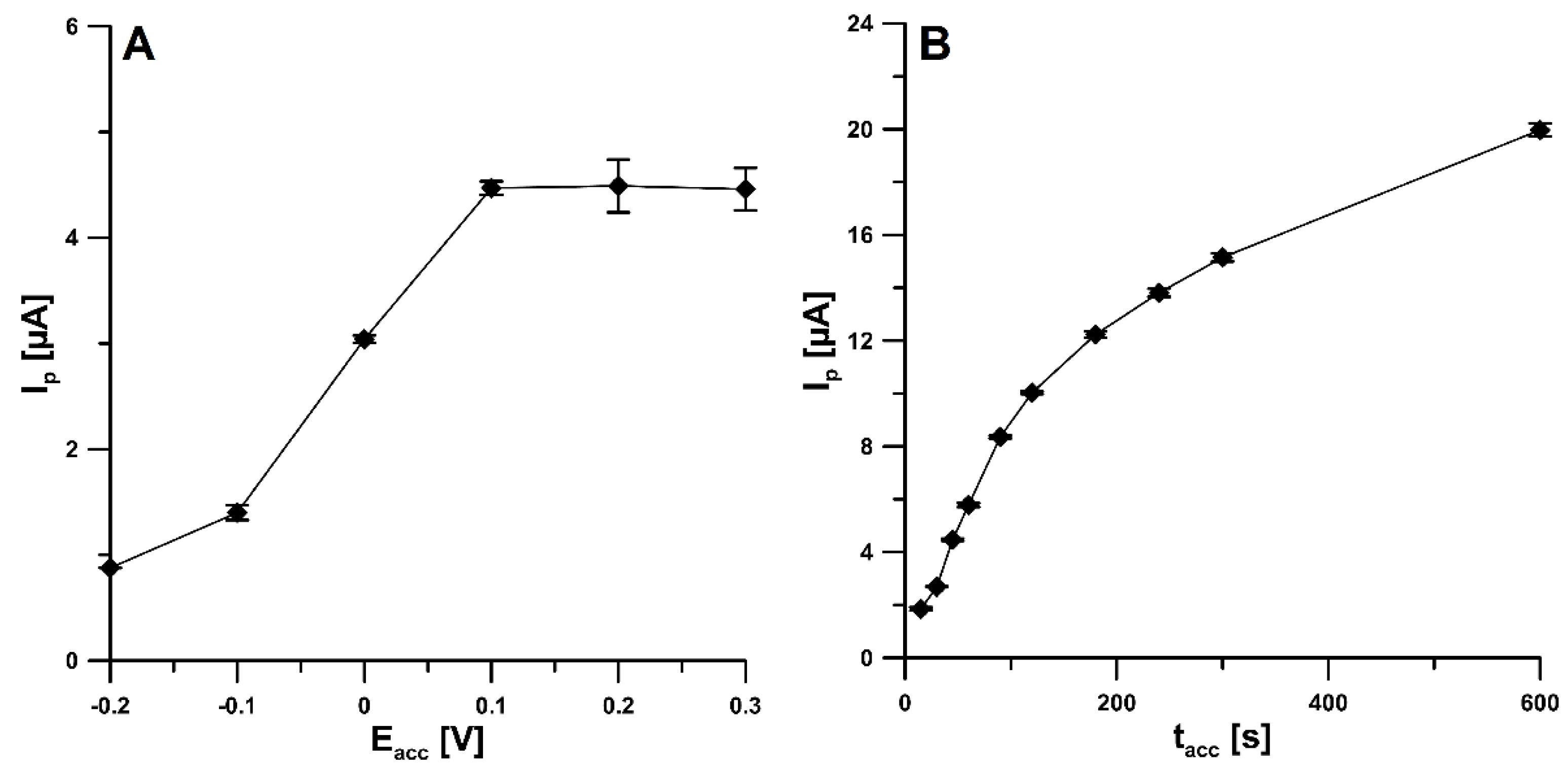
2.1. Initial Studies
2.2. Effect of Type and pH of Supporting Electrolytes
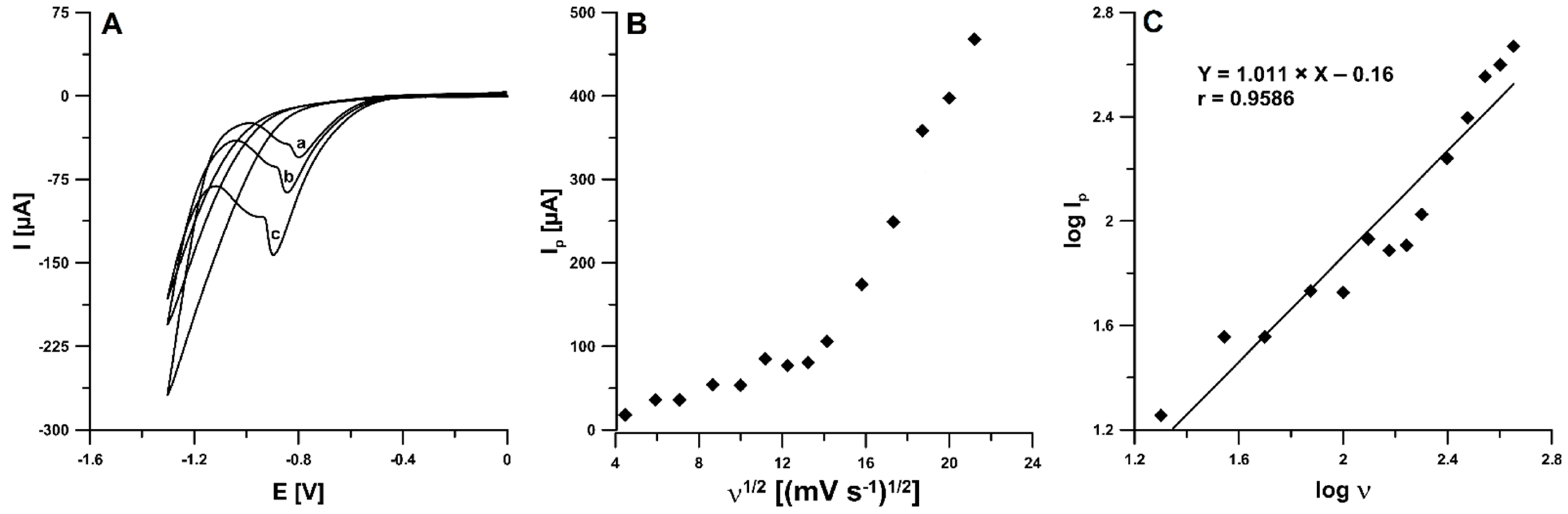
2.3. CV Studies
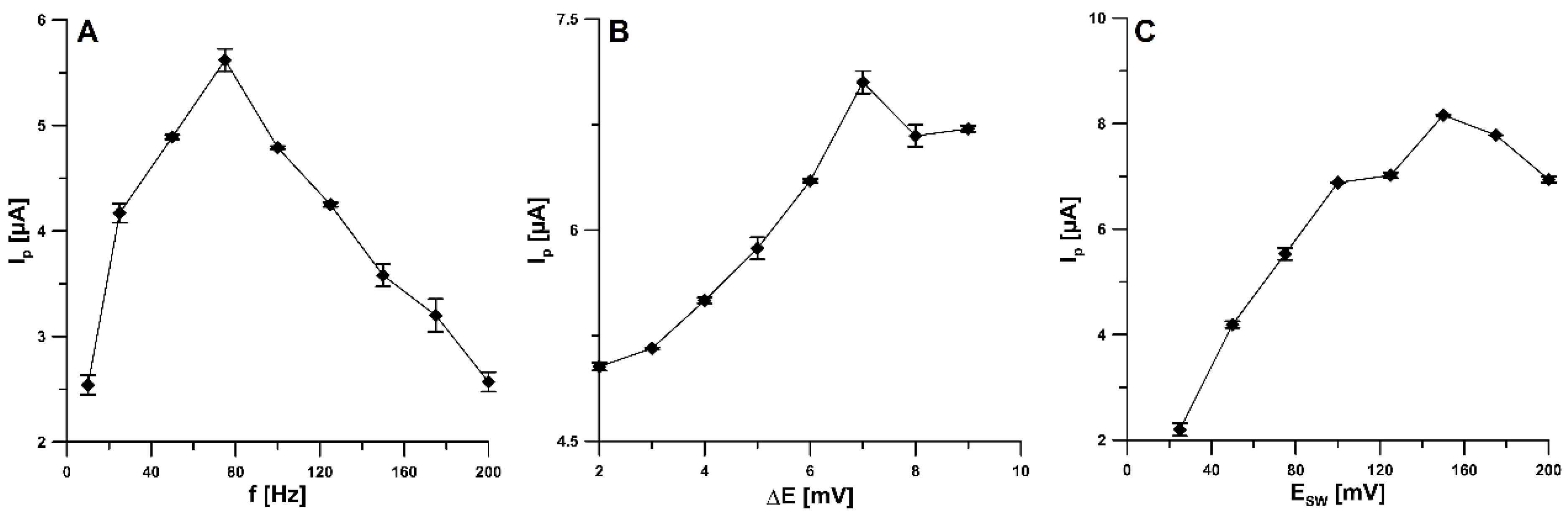
2.4. Effect of SWAdSV Parameters
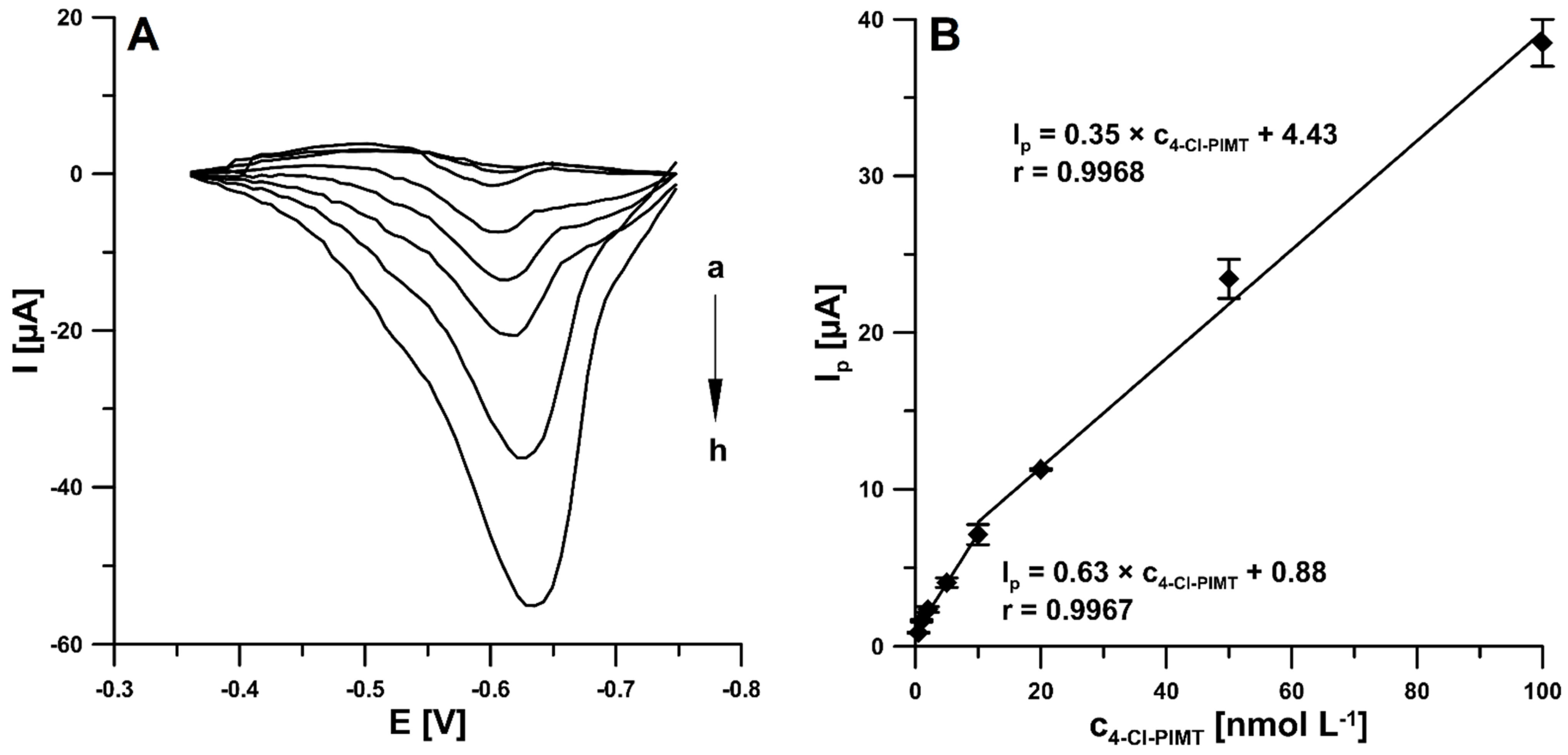
2.5. Analytical Performance
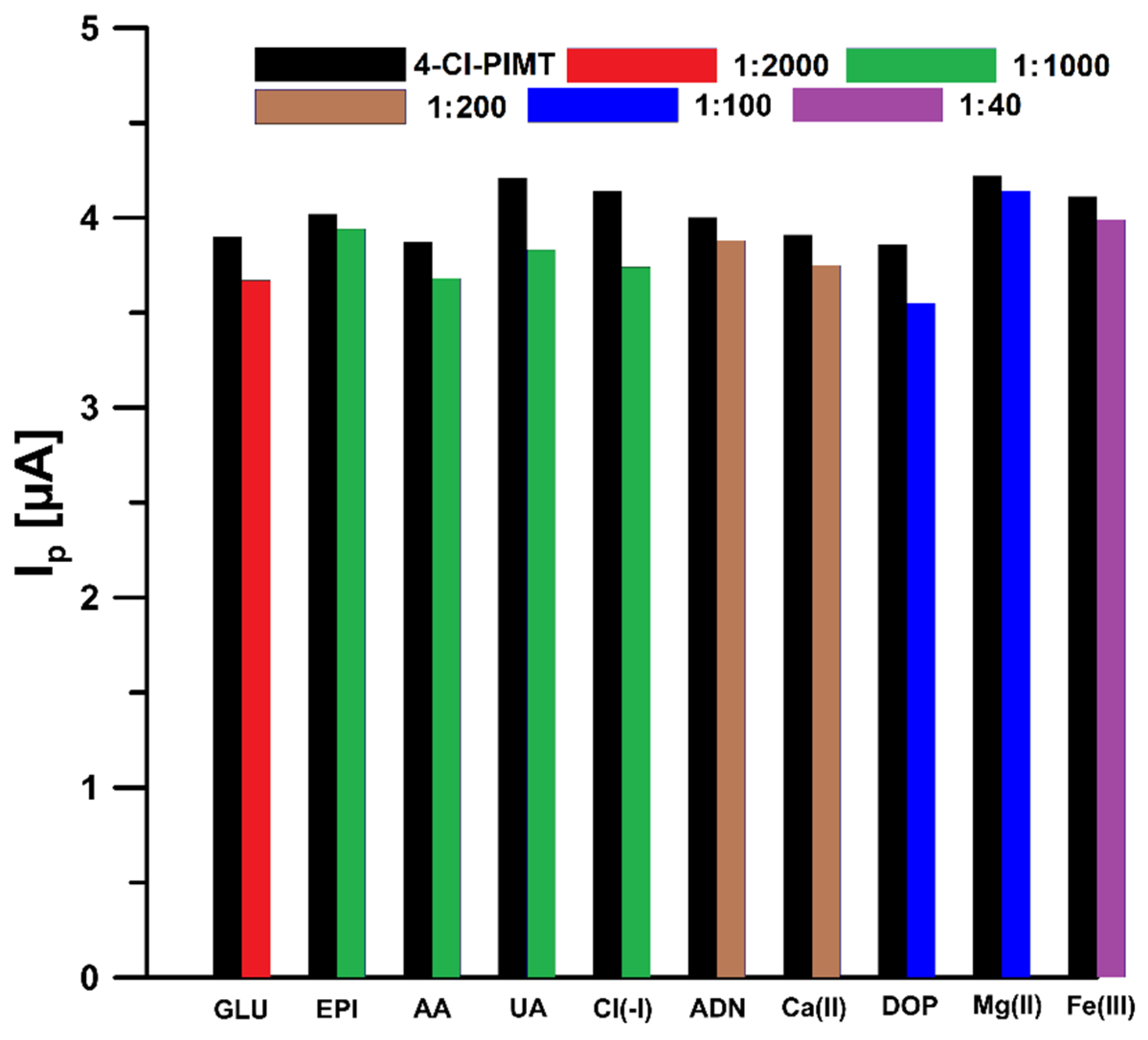
2.6. Selectivity
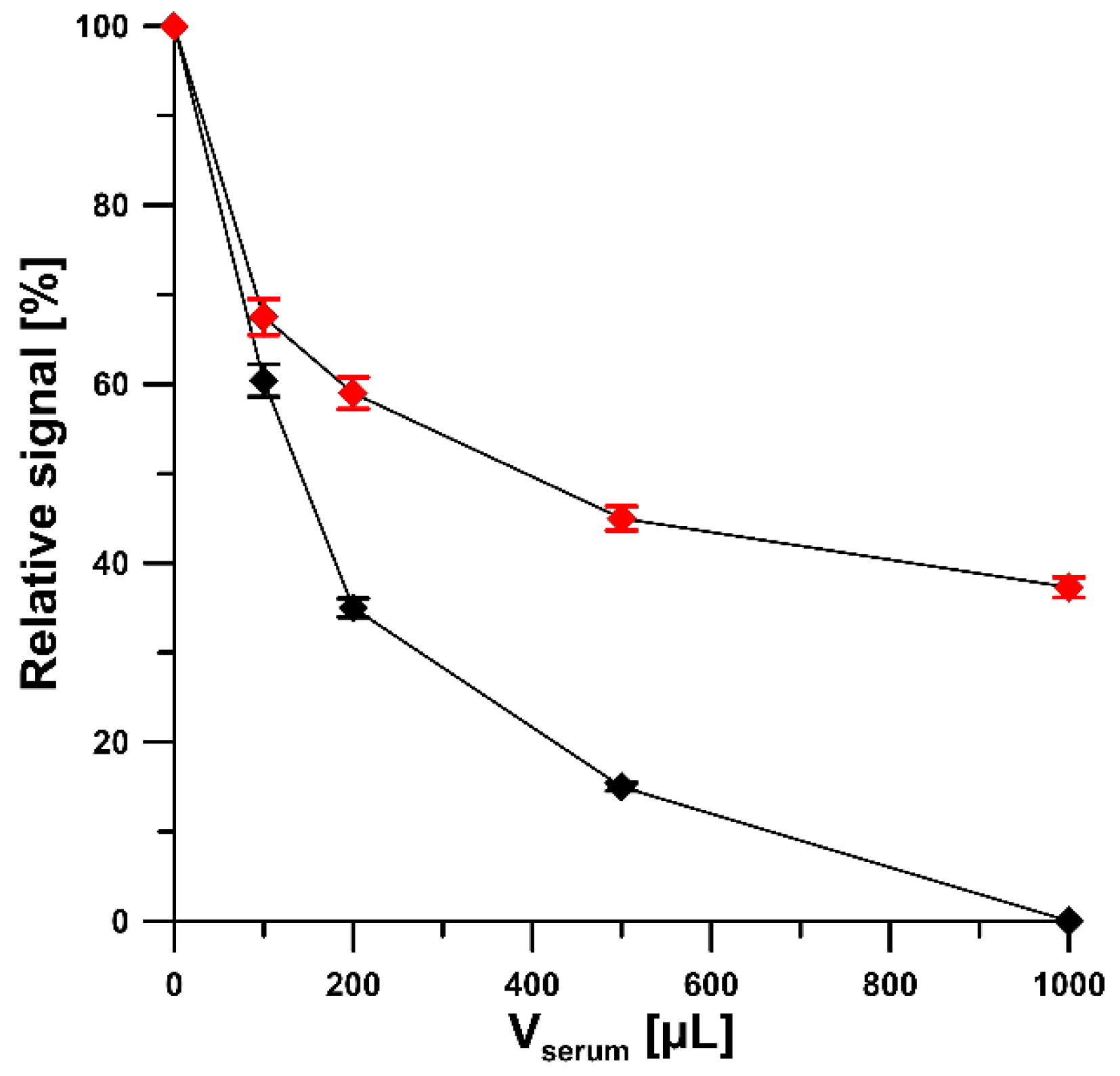
2.7. Serum Sample Analysis
3. Materials and Methods
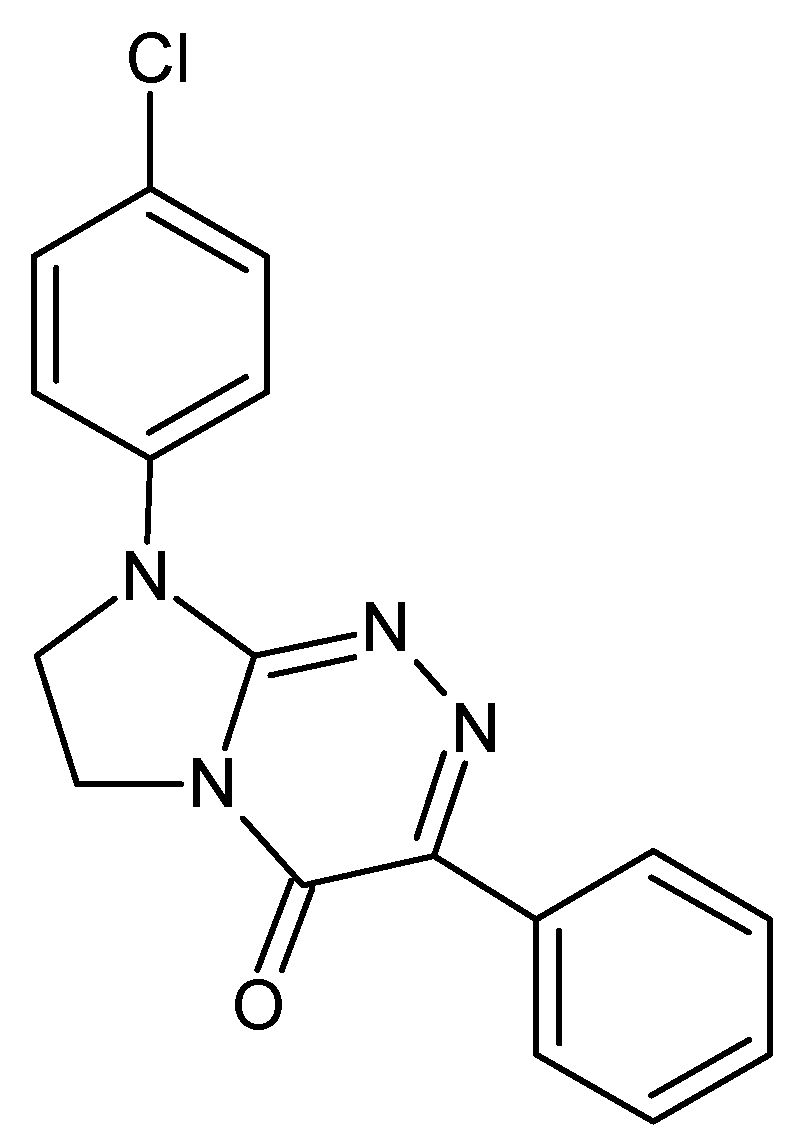
3.1. The Investigated Electroactive Molecule
3.2. Apparatus
3.3. Reagents and Solutions
3.4. 4-Cl-PIMT SWAdSV Analysis
3.5. 4-Cl-PIMT UHPLC-ESI-MS Analysis
3.6. Serum Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sztanke, K.; Pasternak, K.; Sztanke, M.; Kandefer-Szerszeń, M.; Kozioł, A.E.; Dybała, I. Crystal structure, antitumour and antimetastatic activities of disubstituted fused 1,2,4-triazinones. Bioorg. Med. Chem. Lett. 2009, 19, 5095–5100. [Google Scholar] [CrossRef]
- Janicka, M.; Sztanke, M.; Sztanke, K. Reversed-phase liquid chromatography with octadecylsilyl, immobilized artificial membrane and cholesterol columns in correlation studies with in silico biological descriptors of newly synthesized antiproliferative and analgesic active compounds. J. Chromatogr. A 2013, 1318, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Janicka, M.; Sztanke, M.; Sztanke, K. Predicting the blood-brain barrier permeability of new drug-like compounds via HPLC with various stationary phases. Molecules 2020, 25, 487. [Google Scholar] [CrossRef] [Green Version]
- Sztanke, M.; Tuzimski, T.; Janicka, M.; Sztanke, K. Structure-retention behaviour of biologically active fused 1,2,4-triazinones—Correlation with in silico molecular properties. Eur. J. Pharm. Sci. 2015, 68, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Dong, Q.; Li, C.; Chen, J.; Ma, X.; Huang, Y.; Song, D.; Ji, C.; Lei, Y. Electrochemical sensor for detecting pain reliever/fever reducer drug acetaminophen based on electrospun CeBiOx nanofibers modified screen-printed electrode. Sens. Actuators B 2018, 256, 143–150. [Google Scholar] [CrossRef]
- Seguro, I.; Pacheco, J.G.; Delerue-Matos, C. Low cost, easy to prepare and disposable electrochemical molecularly imprinted sensor for diclofenac detection. Sensors 2021, 21, 1975. [Google Scholar] [CrossRef]
- Jahani, P.M.; Mohammadi, S.Z.; Khodabakhshzadeh, A.; Asl, M.S.; Jang, H.W.; Shokouhimehr, M.; Zhang, K.; Van Le, Q.; Peng, W. Simultaneous voltammetric detection of acetaminophen and tramadol using molybdenum tungsten disulfide-modified graphite screen-printed electrode. Int. J. Electrochem. Sci. 2020, 15, 9024–9036. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Zhang, J.; Zhang, H.; Li, Y. Simultaneous voltammetric determination of acetaminophen and isoniazid using MXene modified screen-printed electrode. Biosens. Bioelectron. 2019, 130, 315–321. [Google Scholar] [CrossRef]
- Baezzat, M.R.; Tavakkoli, N.; Zamani, H. Construction of a new electrochemical sensor based on MoS2 nanosheets modified graphite screen printed electrode for simultaneous determination of diclofenac and morphine. Anal. Bioanal. Chem. Res. 2022, 9, 153–162. [Google Scholar]
- Zhang, C.; Cao, Z.; Zhang, G.; Yan, Y.; Yang, X.; Chang, J.; Song, Y.; Jia, Y.; Pan, P.; Mi, W.; et al. An electrochemical sensor based on plasma-treated zinc oxide nanoflowers for the simultaneous detection of dopamine and diclofenac sodium. Microchem. J. 2020, 158, 105237J. [Google Scholar] [CrossRef]
- Amin, S.; Soomro, M.T.; Memon, N.; Solangi, A.R.; Uddin, S.; Qureshi, T.; Behzad, A.R. Disposable screen printed graphite electrode for the direct determination of ibuprofen in surface water. Environ. Nanotechnol. Monit. Manag. 2014, 1–2, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Serrano, N.; Castilla, O.; Ariño, C.; Diaz-Cruz, M.S.; Diaz-Cruz, J.M. Commercial screen-printed electrodes based on carbon nanomaterials for a fast and cost-effective voltammetric determination of paracetamol, ibuprofen and caffeine in water samples. Sensors 2019, 19, 4039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.-L.; He, Y.; Qin, D.; Chang, A.; Huang, A.; Xie, X.-J.; Zhang, Y. Fabrication, characterization and performance evaluation of screen-printed carbon electrodes: Determination of acetaminophen in Tylenol. Chin. J. Anal. Chem. 2021, 49, 21187–21196. [Google Scholar] [CrossRef]
- Sima, V.; Cristea, C.; Bodoki, E.; Duţu, G.; Săndulescu, R. Screen-printed electrodes modified with HRP-zirconium alkoxide film for the development of a biosensor for acetaminophen detection. Cent. Eur. J. Chem. 2010, 8, 1034–1040. [Google Scholar]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Machado, S.A.S.; Oliveira, O.N., Jr. Simultaneous, ultrasensitive detection of hydroquinone, paracetamol and estradiol for quality control of tap water with a simple electrochemical method. J. Electroanal. Chem. 2019, 848, 113319. [Google Scholar] [CrossRef]
- Kruanetr, S.; Prabhu, R.; Pollard, P.; Fernandez, C. Pharmaceutical electrochemistry: The electrochemical detection of aspirin utilising screen printed Graphene electrodes as sensors platforms. Surf. Eng. Appl. Electrochem. 2015, 51, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Stefano, J.S.; Montes, R.H.O.; Richter, E.M.; Munoz, R.A.A. Flow-injection analysis with multiple-pulse amperometry for simultaneous determination of paracetamol and naproxen using a homemade flow cell for screen-printed electrodes. J. Braz. Chem. Soc. 2014, 25, 484–491. [Google Scholar] [CrossRef]
- Kondori, T.; Tajik, S.; Akbarzadeh, T.N.; Beitollahi, H.; Graiff, C.; Jang, H.W.; Shokouhimehr, M. Synthesis and characterization of bipyridine cobalt(II) complex modified graphite screen printed electrode: An electrochemical sensor for simultaneous detection of acetaminophen and naproxen. RSC Adv. 2021, 11, 3049–3057. [Google Scholar] [CrossRef]
- Bagherinasab, Z.; Beitollahi, H.; Yousefi, M.; Bagherzadeh, M.; Hekmati, M. Rapid sol gel synthesis of BaFe12O19 nanoparticles: An excellent catalytic application in the electrochemical detection of tramadol in the presence of acetaminophen. Microchem. J. 2020, 156, 104803. [Google Scholar] [CrossRef]
- Jahani, P.M.; Mohammadi, S.Z.; Khodabakhshzadeh, A.; Cha, J.W.; Asl, M.S.; Jang, H.W.; Shokouhimehr, M.; Zhang, K.; Van Le, Q.; Peng, W. Simultaneous voltammetric detection of morphine and diclofenac using graphene nanoribbon modified screen-printed electrode. Int. J. Electrochem. Sci. 2020, 15, 9037–9048. [Google Scholar] [CrossRef]
- Yaghoubian, H.; Tajik, S.; Baitollahi, H.; Sarhadi, H.; Sheikhshoaie. Fe2MoO4 magnetic nanocomposite modified screen printed graphite electrode as a voltammetric sensor for simultaneous determination of nalbuphine and diclofenac. J. Mater. Sci. Mater. Electron. 2021, 32, 17311–17323. [Google Scholar] [CrossRef]
- Jahromi, Z.; Mirzael, E.; Savardashtaki, A.; Afzali, M.; Afzali, Z. A rapid and selective electrochemical sensor based on electrospun carbon nanofibers for tramadol detection. Microchem. J. 2020, 157, 104942. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Bejinaru, A.A.; Boev, M.; Apetrei, C.; Buzia, O.D. Determination of ibuprofen based on screen-printed electrodes modified with carbon nanofibers. Farmacia 2017, 65, 790–795. [Google Scholar]
- Bounegru, A.V.; Apetrei, C. Voltamperometric sensors and biosensors based on carbon nanomaterials used for detecting of caffeic acid-a review. Int. J. Mol. Sci. 2020, 21, 9275. [Google Scholar] [CrossRef] [PubMed]
- Sipa, K.; Brycht, M.; Leniart, A.; Skrzypek, S. The application of carbon nanomaterials as electrode surface modifiers for the voltammetric sensing of nitroxinil—A comparative studies. J. Electroanal. Chem. 2019, 848, 113294. [Google Scholar] [CrossRef]
- Kaewket, K.; Karuwan, C.; Sonsupap, S.; Maensiri, S.; Ngamchuea, K. Anti-fouling effects of carbon nanofiber in electrochemical sensing of phenolic compounds. J. Electrochem. Soc. 2021, 168, 067501. [Google Scholar] [CrossRef]
- Sasal, A.; Tyszczuk-Rotko, K.; Wójciak, M.; Sowa, I. First electrochemical sensor (screen-printed carbon electrode modified with carboxyl functionalized multiwalled carbon nanotubes) for ultratrace determination of diclofenac. Materials 2020, 13, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasal, A.; Tyszczuk-Rotko, K.; Wójciak, M.; Sowa, I.; Kuryło, M. Simultaneous analysis of paracetamol and diclofenac using MWCNTs-COOH modified screen-printed carbon electrode and pulsed potential accumulation. Materials 2020, 13, 3091. [Google Scholar] [CrossRef] [PubMed]
- Tyszczuk-Rotko, K.; Kozak, J.; Sztanke, M.; Sztanke, K.; Sadok, I. A screen-printed sensor coupled with flow system for quantitative determination of a novel promising anticancer agent candidate. Sensors 2020, 20, 5217. [Google Scholar] [CrossRef]
- Ludvik, J.; Zuman, P. Electrochemical proof of the single bond character of the N–N bonds in some 1,2,4-triazines. Indian J. Chem. 2003, 42A, 847–848. [Google Scholar]
- Stępniowska, A.; Sztanke, M.; Tuzimski, T.; Korolczuk, M.; Sztanke, K. A simple stripping voltammetric method for the determination of a new anticancer prodrug in serum. Biosens. Bioelectron. 2017, 94, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, J.; Riedl, F.; Liska, F.; Zuman, P. Electrochemical reduction of metamitron. J. Electroanal. Chem. 1998, 457, 177–190. [Google Scholar] [CrossRef]
- Gosser, D.K. Cyclic Voltammetry, Simulation and Analysis of Reaction Mechanisms; Wiley VCH: New York, NY, USA, 1993. [Google Scholar]
- Mocak, J.; Bond, A.M.; Mitchell, S.; Scollary, G. A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: Application to voltammetric and stripping techniques. Pure Appl. Chem. 1997, 69, 297–328. [Google Scholar] [CrossRef]

| 4-Cl-PIMT Concentration [nM] ± SD (n = 3) | |||||
|---|---|---|---|---|---|
| Added | Found SWAdSV | Found UHPLC-ESI-MS | Recovery * SWAdSV [%] | Recovery ** UHPLC-ESI-MS [%] | Relative Error *** [%] |
| 2.0 20.0 | 2.10 ± 0.072 20.13 ± 0.55 | 2.32 ± 0.34 19.7 ± 0.30 | 105.0 100.7 | 116.0 98.5 | 9.48 2.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozak, J.; Tyszczuk-Rotko, K.; Sadok, I.; Sztanke, K.; Sztanke, M. Application of a Screen-Printed Sensor Modified with Carbon Nanofibers for the Voltammetric Analysis of an Anticancer Disubstituted Fused Triazinone. Int. J. Mol. Sci. 2022, 23, 2429. https://doi.org/10.3390/ijms23052429
Kozak J, Tyszczuk-Rotko K, Sadok I, Sztanke K, Sztanke M. Application of a Screen-Printed Sensor Modified with Carbon Nanofibers for the Voltammetric Analysis of an Anticancer Disubstituted Fused Triazinone. International Journal of Molecular Sciences. 2022; 23(5):2429. https://doi.org/10.3390/ijms23052429
Chicago/Turabian StyleKozak, Jędrzej, Katarzyna Tyszczuk-Rotko, Ilona Sadok, Krzysztof Sztanke, and Małgorzata Sztanke. 2022. "Application of a Screen-Printed Sensor Modified with Carbon Nanofibers for the Voltammetric Analysis of an Anticancer Disubstituted Fused Triazinone" International Journal of Molecular Sciences 23, no. 5: 2429. https://doi.org/10.3390/ijms23052429
APA StyleKozak, J., Tyszczuk-Rotko, K., Sadok, I., Sztanke, K., & Sztanke, M. (2022). Application of a Screen-Printed Sensor Modified with Carbon Nanofibers for the Voltammetric Analysis of an Anticancer Disubstituted Fused Triazinone. International Journal of Molecular Sciences, 23(5), 2429. https://doi.org/10.3390/ijms23052429







