Effect of Irradiation on Structural Changes of Levan
Abstract
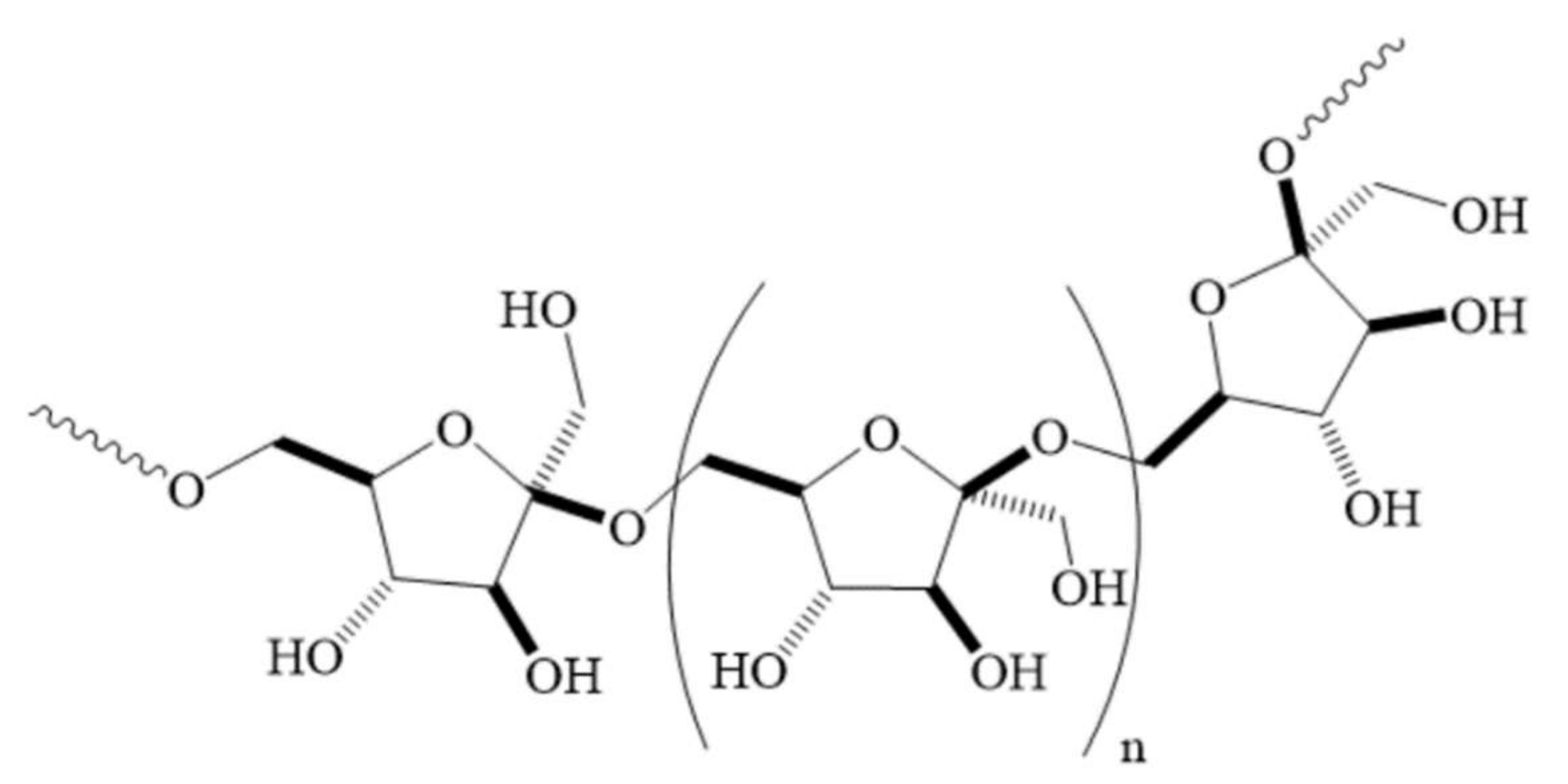
:1. Introduction
2. Results and Discussion
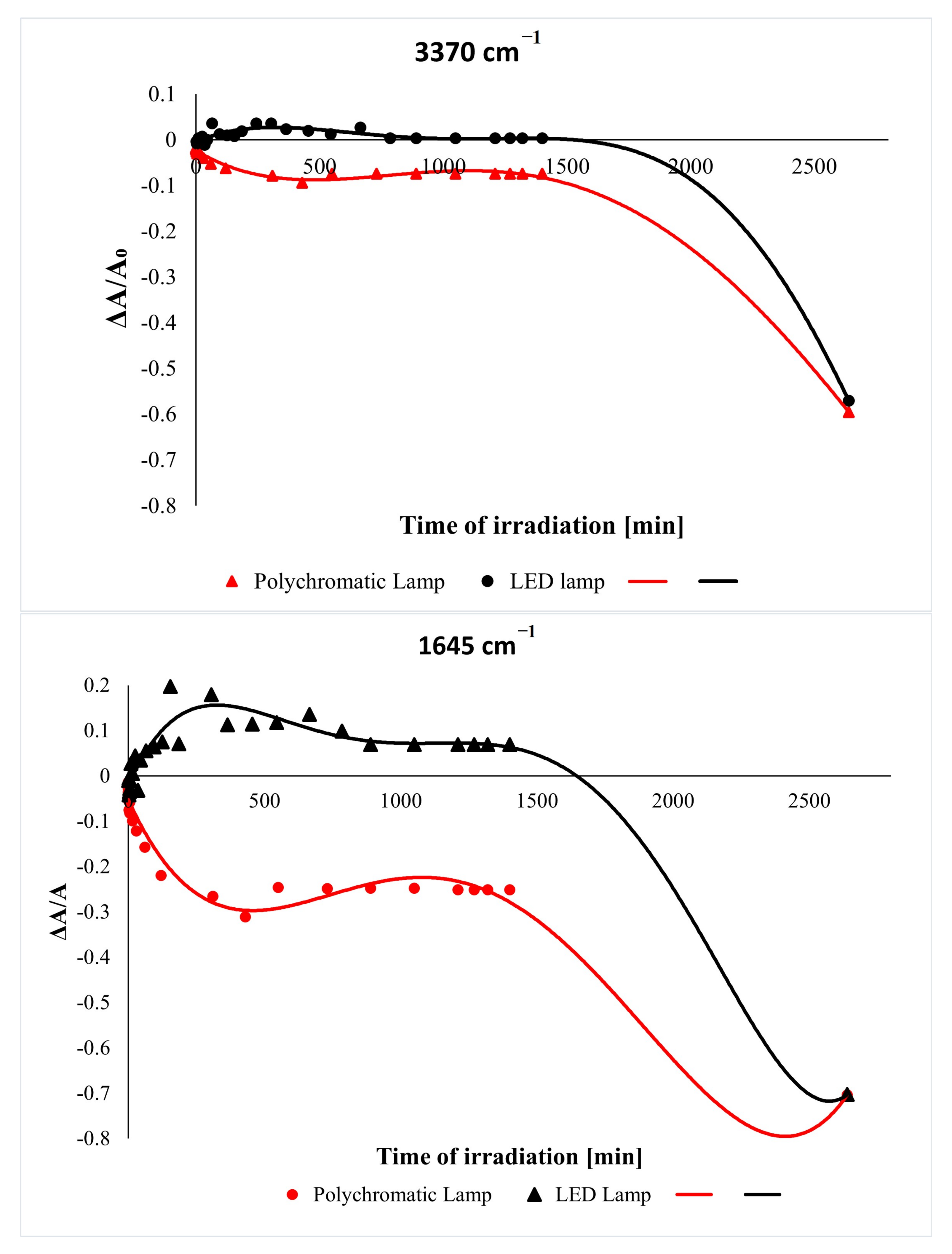
2.1. Effect of UV Irradiation
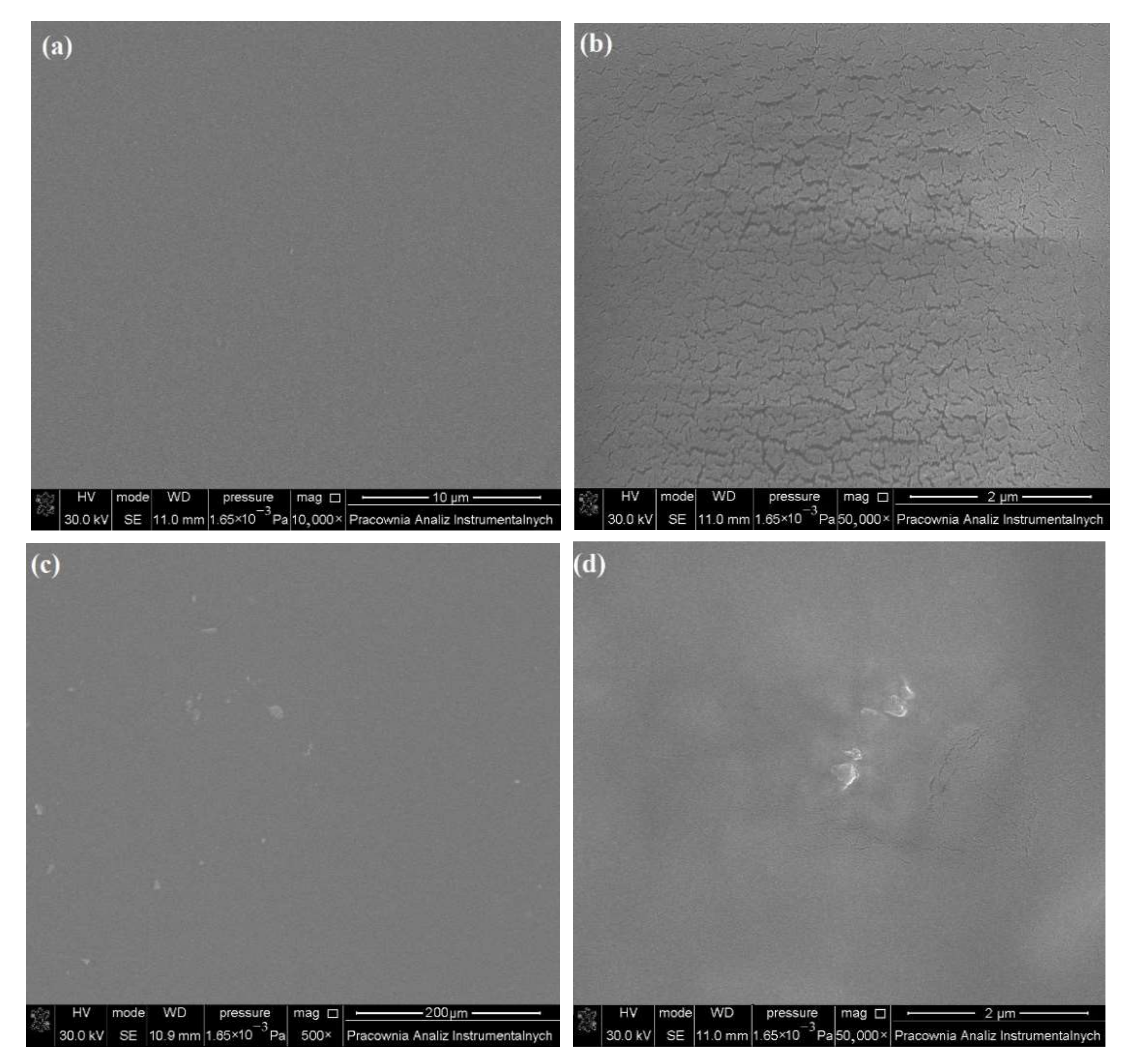
2.2. SEM Images of Levan Films
2.3. AFM Images of Levan Films
2.4. Contact Angle Measurement
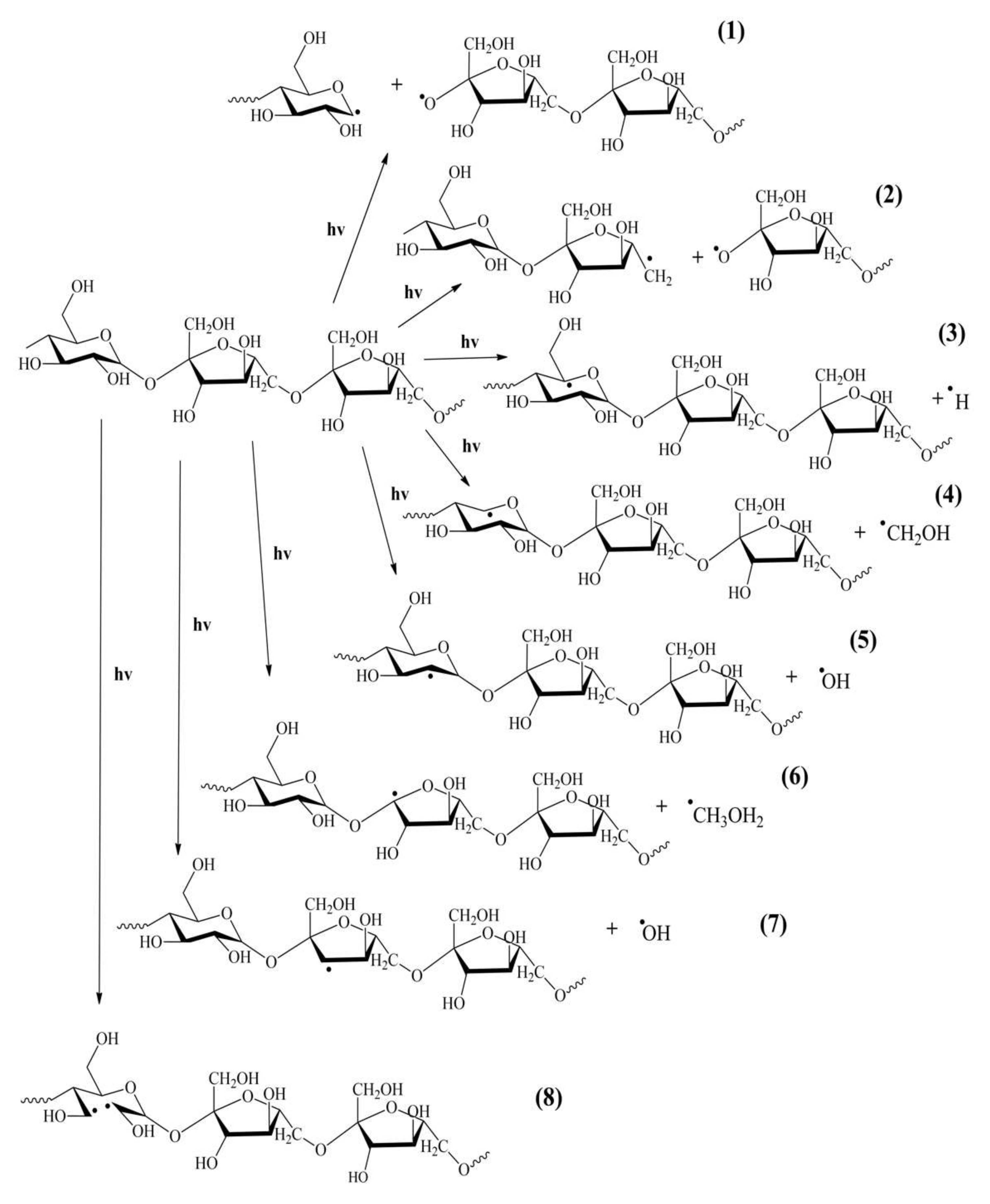
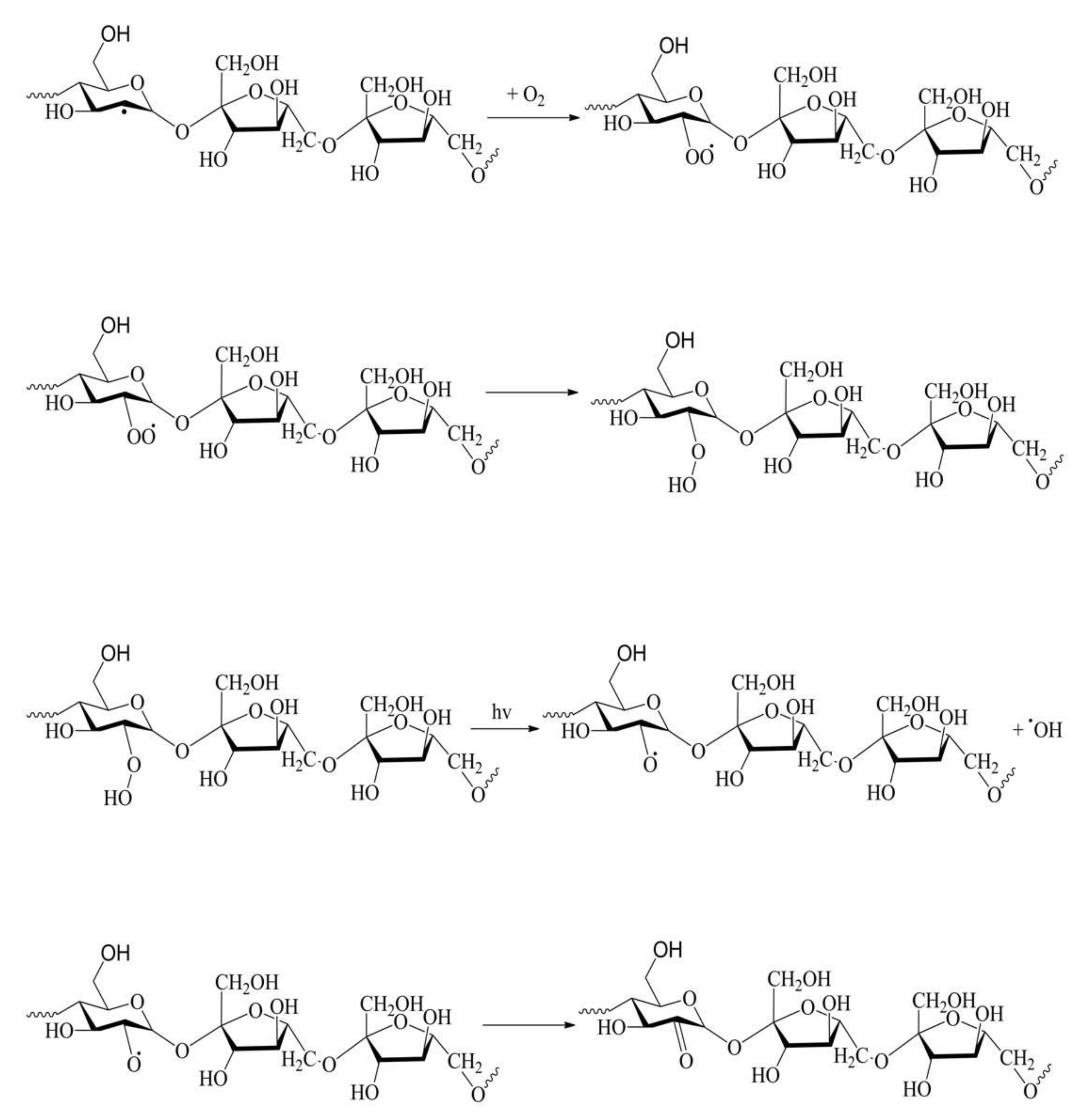
2.5. Discussion of Photodegradation Mechanism
3. Materials and Methods
3.1. Materials and Sample Preparation
3.2. Methods
3.2.1. Photostability Studies
3.2.2. Characterization of Levan Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srikanth, R.; Reddy, C.H.S.; Siddartha, G.; Ramaiah, M.J.; Uppuluri, K.B. Review on production, characterization and applications of microbial levan. Carbohydr. Polym. 2015, 120, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Moussa, T.A.A.; Al-Qaysi, S.A.S.; Thabit, Z.A.; Kadhem, S.B. Microbial levan from Brachybacterium phenoliresistens: Characterization and enhancement of production. Process Biochem. 2017, 57, 9–15. [Google Scholar] [CrossRef]
- Arvidson, S.A.; Rinehart, B.T.; Gadala-Maria, F. Concentration regimes of solutions of levan polysaccharide from Bacillus sp. Carbohydr. Polym. 2006, 65, 144–149. [Google Scholar] [CrossRef]
- Dahech, I.; Belghith, K.S.; Hamden, K.; Feki, A.; Belghith, H.; Mejdoub, H. Oral administration of levan polysaccharide reduces the alloxan-induced oxidative stress in rats. Int. J. Biol. Macromol. 2011, 49, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Öner, E.T.; Hernández, L.; Combie, J. Review of Levan polysaccharide: From a century of past experiences to future prospects. Biotechnol. Adv. 2016, 34, 827–844. [Google Scholar] [CrossRef]
- Porras-Domínguez, J.R.; Ávila-Fernández, A.; Miranda-Molina, A.; Rodríguez-Alegría, M.E.; Munguía, A.L. Bacillus subtilis 168 levansucrase (SacB) activity affects average levan molecular weight. Carbohydr. Polym. 2015, 132, 338–344. [Google Scholar] [CrossRef]
- De Vuyst, L.; De Vin, F.; Vaningelgem, F.; Degeest, B. Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int. Dairy J. 2001, 11, 687–707. [Google Scholar] [CrossRef]
- Clarke, M.A.; Roberts, E.J.; Garegg, P.J. New compounds from microbiological products of sucrose. Carbohydr. Polym. 1997, 34, 425. [Google Scholar] [CrossRef]
- Chelminiak-Dudkiewicz, D.; Rybczynski, P.; Smolarkiewicz-Wyczachowski, A.; Mlynarczyk, D.T.; Wegrzynowska-Drzymalska, K.; Ilnicka, A.; Goslinski, T.; Marszałł, M.P.; Ziegler-Borowska, M. Photosensitizing potential of tailored magnetite hybrid nanoparticles functionalized with levan and zinc (II) phthalocyanine. Appl. Surf. Sci. 2020, 524, 146602. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.M.; Gamal-Eldeen, A.M.; Helmy, W.A.; Esawy, M.A. Antitumor and antioxidant activities of levan and its derivative from the isolate Bacillus subtilis NRC1aza. Carbohydr. Polym. 2012, 89, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Belghith, K.S.; Dahech, I.; Belghith, H.; Mejdoub, H. Microbial production of levansucrase for synthesis of fructooligosaccharides and levan. Int. J. Biol. Macromol. 2012, 50, 451–458. [Google Scholar] [CrossRef]
- Jathore, N.R.; Bule, M.V.; Tilay, A.V.; Annapure, U.S. Microbial levan from Pseudomonas fluorescens: Characterization and medium optimization for enhanced production. Food Sci. Biotechnol. 2012, 21, 1045–1053. [Google Scholar] [CrossRef]
- Chen, X.; Gao, H.; Ploehn, H.J. Montmorillonite–levan nanocomposites with improved thermal and mechanical properties. Carbohydr. Polym. 2014, 101, 565–573. [Google Scholar] [CrossRef]
- Yasuda, N.; Wang, Y.; Tsukegi, T.; Shirai, Y.; Nishida, H. Quantitative evaluation of photodegradation and racemization of poly(l-lactic acid) under UV-C irradiation. Polym. Degrad. Stab. 2010, 95, 1238–1243. [Google Scholar] [CrossRef]
- Wasikiewicz, J.M.; Yoshii, F.; Nagasawa, N.; Wach, R.A.; Mitomo, H. Degradation of chitosan and sodium alginate by gamma radiation, sonochemical and ultraviolet methods. Radiat. Phys. Chem. 2005, 73, 287–295. [Google Scholar] [CrossRef]
- Yue, W.; He, R.; Yao, P.; Wei, Y. Ultraviolet radiation-induced accelerated degradation of chitosan by ozone treatment. Carbohydr. Polym. 2009, 77, 639–642. [Google Scholar] [CrossRef]
- Bertolini, A.C.; Mestres, C.; Raffi, J.; Buléon, A.; Lerner, D.; Colonna, P. Photodegradation of Cassava and Corn Starches. J. Agric. Food Chem. 2001, 49, 675–682. [Google Scholar] [CrossRef]
- Asha, S.; Sangappa, Y.; Ganesh, S. Tuning the Refractive Index and Optical Band Gap of Silk Fibroin Films by Electron Irradiation. J. Spectrosc. 2015, 4, 879296. [Google Scholar] [CrossRef] [Green Version]
- La Mantia, F.P.; Morreale, M.; Botta, L.; Mistretta, M.C.; Ceraulo, M.; Scaffaro, R. Degradation of polymer blends: A brief review. Polym. Degrad. Stab. 2017, 145, 79–92. [Google Scholar] [CrossRef]
- Chełminiak-Dudkiewicz, D.; Ziegler-Borowska, M.; Stolarska, M.; Sobotta, Ł.; Falkowski, M.; Mielcarek, J.; Goslinski, T.; Kowalonek, J.; Węgrzynowska-Drzymalska, K.; Kaczmarek, H. The chitosan—Porphyrazine hybrid materials and their photochemical properties. J. Photochem. Photobiol. B: Biol. 2018, 181, 1–13. [Google Scholar] [CrossRef]
- Luste, S.; Sillanpää, M. UVC irradiation–based water treatment. In Advanced Water Treatment; Chapter 3; Sillanpää, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 95–128. [Google Scholar]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Medium optimization and structural characterization of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2010, 79, 206–213. [Google Scholar] [CrossRef]
- Wu, D.; Shu, Q.; Wang, Z.; Xia, Y. Effect of gamma irradiation on starch viscosity and physicochemical properties of different rice. Radiat. Phys. Chem. 2002, 65, 79–86. [Google Scholar] [CrossRef]
- Rus, A.Z.M.; Hassan, N.N.M. Thermal Degradation and Damping Characteristic of UV Irradiated Biopolymer. Int. J. Polym. Sci. 2015, 2015, 615284. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Wegrzynowska-Drzymalska, K.; Chelminiak-Dudkiewicz, D.; Kowalonek, J.; Kaczmarek, H. Photochemical Reactions in Dialdehyde Starch. Molecules 2018, 23, 3358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratanakamnuan, U.; Aht-Ong, D. Photobiodegradation of low-density polyethylene/banana starch films. J. Appl. Polym. Sci. 2006, 100, 2725–2736. [Google Scholar] [CrossRef]
- Jayanthi Kalaivani, G.; Suja, S.K. TiO2 (rutile) embedded inulin—A versatile bio-nanocomposite for photocatalytic degradation of methylene blue. Carbohydr. Polym. 2016, 143, 51–60. [Google Scholar] [CrossRef]
- Elvidge, C.D.; Keith, D.M.; Tuttle, B.T.; Baugh, K.E. Spectral Identification of Lighting Type and Character. Sensors 2010, 10, 3961–3988. [Google Scholar] [CrossRef]
- Technical Application Guide. PrevaLED® Core Z6 LED Modules. Available online: www.osram.com/prevaled-core (accessed on 16 July 2020).


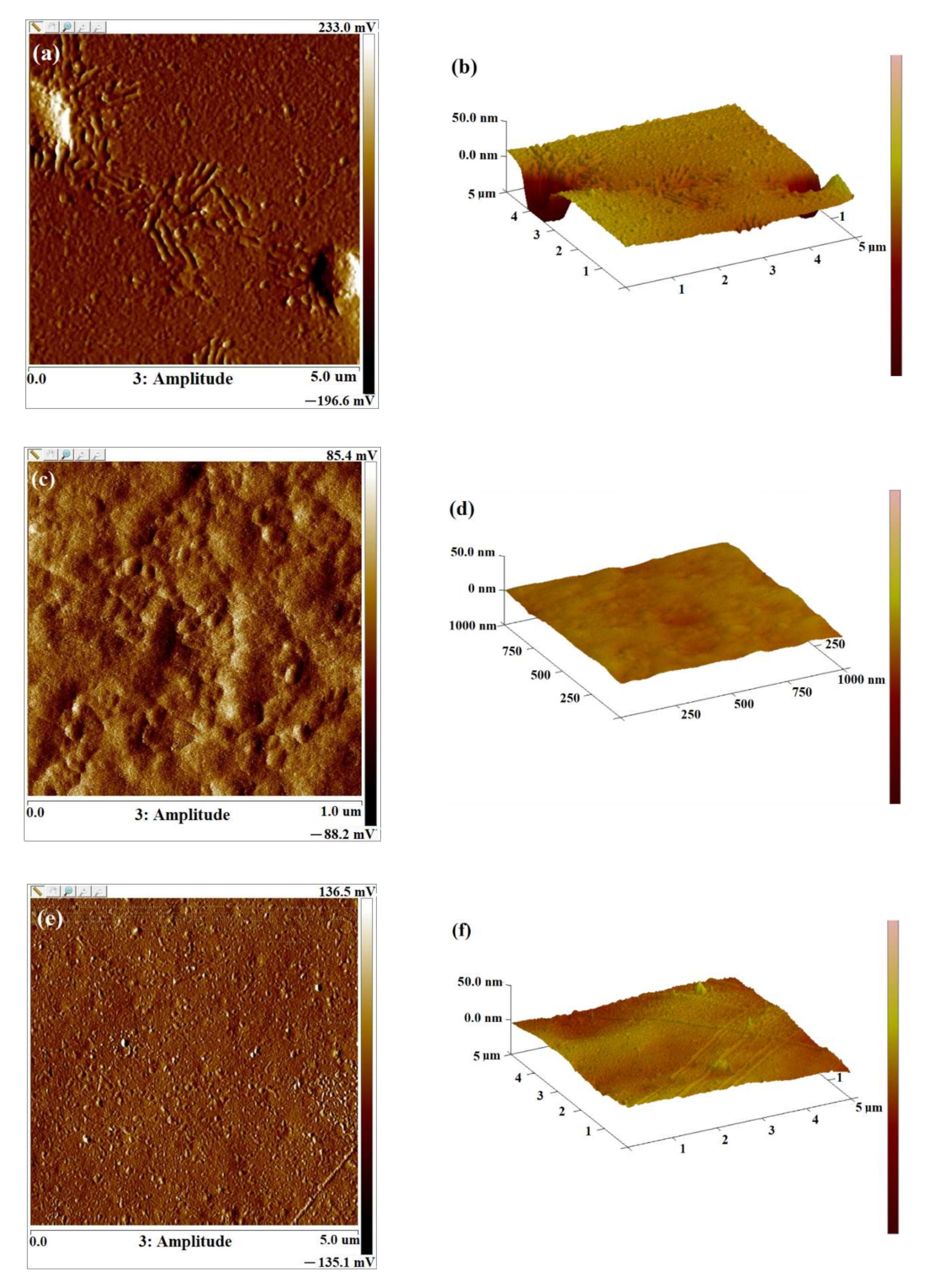
| Time of Irradiation [h] | Roughness Parameters [nm] | |||||
|---|---|---|---|---|---|---|
| LED Lamp | PCh Lamp | |||||
| Rq | Ra | Rmax | Rq | Ra | Rmax | |
| 0 | 10.00 | 4.90 | 101.0 | 10.00 | 4.90 | 101.0 |
| 13 | 5.45 | 4.41 | 57.7 | 4.19 | 3.31 | 34.4 |
| 44 | 3.63 | 2.97 | 37.6 | 1.95 | 1.47 | 14.7 |
| Time of Irradiation [h] | LED Lamp | PCh Lamp | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average Contact Angle [θ, °] | Surface Free Energy [mJ/m2] | Average Contact Angle [θ, °] | Surface Free Energy [mJ/m2] | |||||||
| Glycerin | Diiodomethane | γs | γsd | γsp | Glycerin | Diiodomethane | γs | γsd | γsp | |
| 0 | 73.4 | 71.1 | 45.33 | 28.61 | 16.72 | 73.4 | 71.1 | 45.33 | 28.61 | 16.72 |
| 13 | 67.3 | 66.5 | 37.21 | 21.90 | 15.31 | 65.6 | 63.9 | 33.76 | 19.23 | 14.53 |
| 44 | 55.7 | 53.8 | 26.35 | 15.46 | 10.89 | 53.1 | 52.2 | 21.78 | 12.44 | 9.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelminiak-Dudkiewicz, D.; Smolarkiewicz-Wyczachowski, A.; Wegrzynowska-Drzymalska, K.; Ziegler-Borowska, M. Effect of Irradiation on Structural Changes of Levan. Int. J. Mol. Sci. 2022, 23, 2463. https://doi.org/10.3390/ijms23052463
Chelminiak-Dudkiewicz D, Smolarkiewicz-Wyczachowski A, Wegrzynowska-Drzymalska K, Ziegler-Borowska M. Effect of Irradiation on Structural Changes of Levan. International Journal of Molecular Sciences. 2022; 23(5):2463. https://doi.org/10.3390/ijms23052463
Chicago/Turabian StyleChelminiak-Dudkiewicz, Dorota, Aleksander Smolarkiewicz-Wyczachowski, Katarzyna Wegrzynowska-Drzymalska, and Marta Ziegler-Borowska. 2022. "Effect of Irradiation on Structural Changes of Levan" International Journal of Molecular Sciences 23, no. 5: 2463. https://doi.org/10.3390/ijms23052463
APA StyleChelminiak-Dudkiewicz, D., Smolarkiewicz-Wyczachowski, A., Wegrzynowska-Drzymalska, K., & Ziegler-Borowska, M. (2022). Effect of Irradiation on Structural Changes of Levan. International Journal of Molecular Sciences, 23(5), 2463. https://doi.org/10.3390/ijms23052463






