Treatment of Chronic Kidney Disease with Extracellular Vesicles from Mesenchymal Stem Cells and CD133+ Expanded Cells: A Comparative Preclinical Analysis
Abstract
:1. Introduction
2. Results
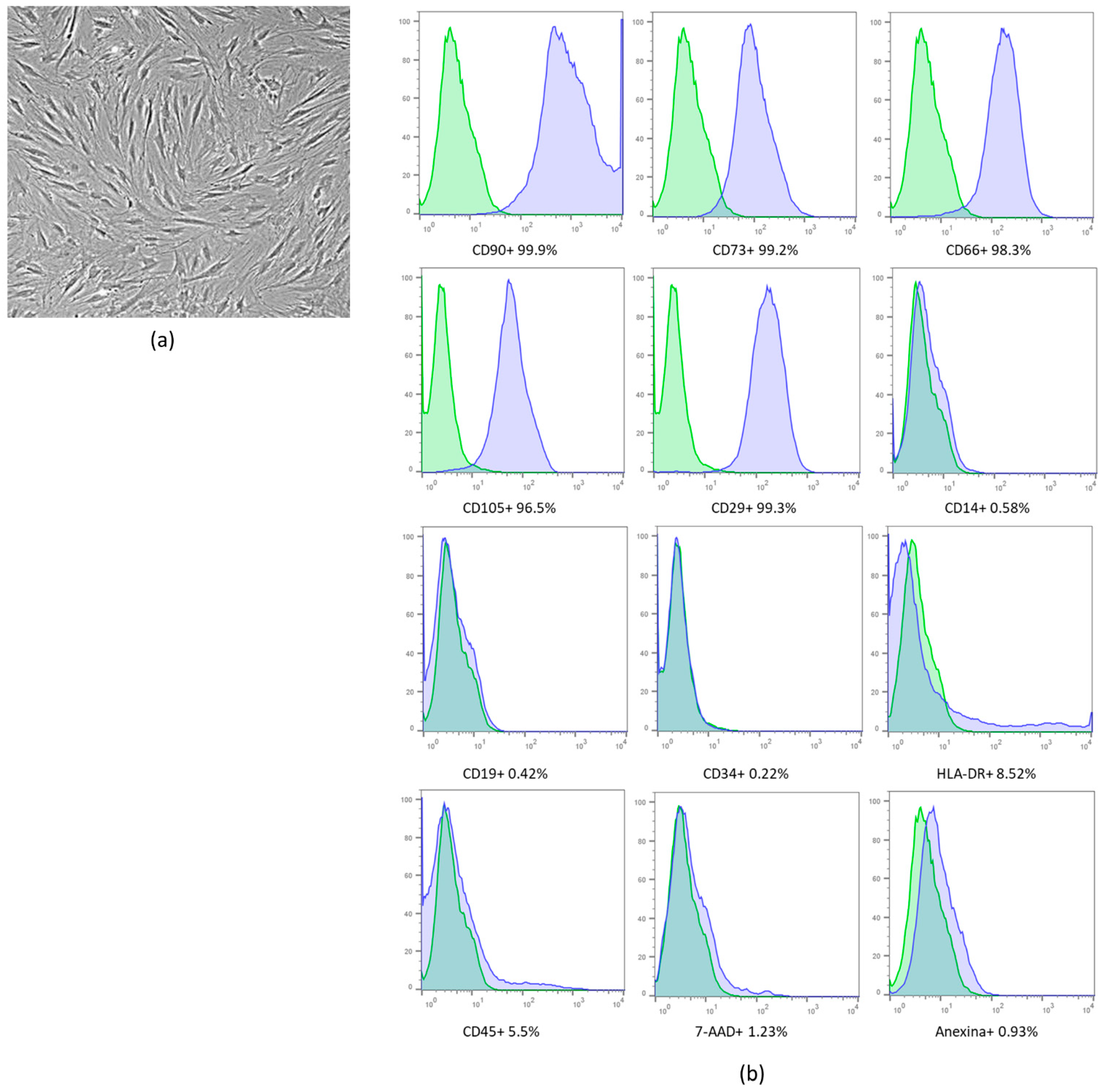
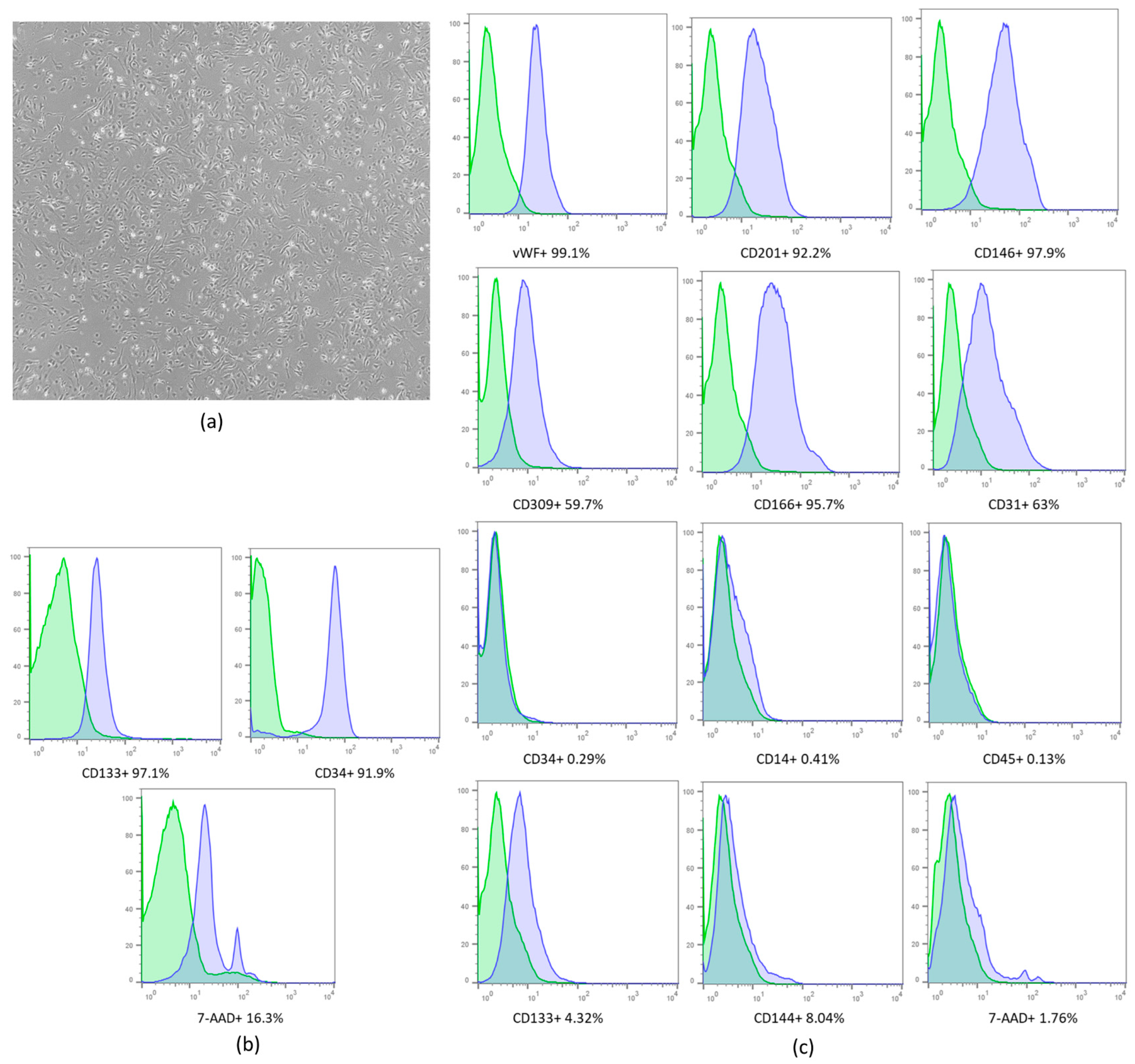
2.1. Cell Expansion and Characterization
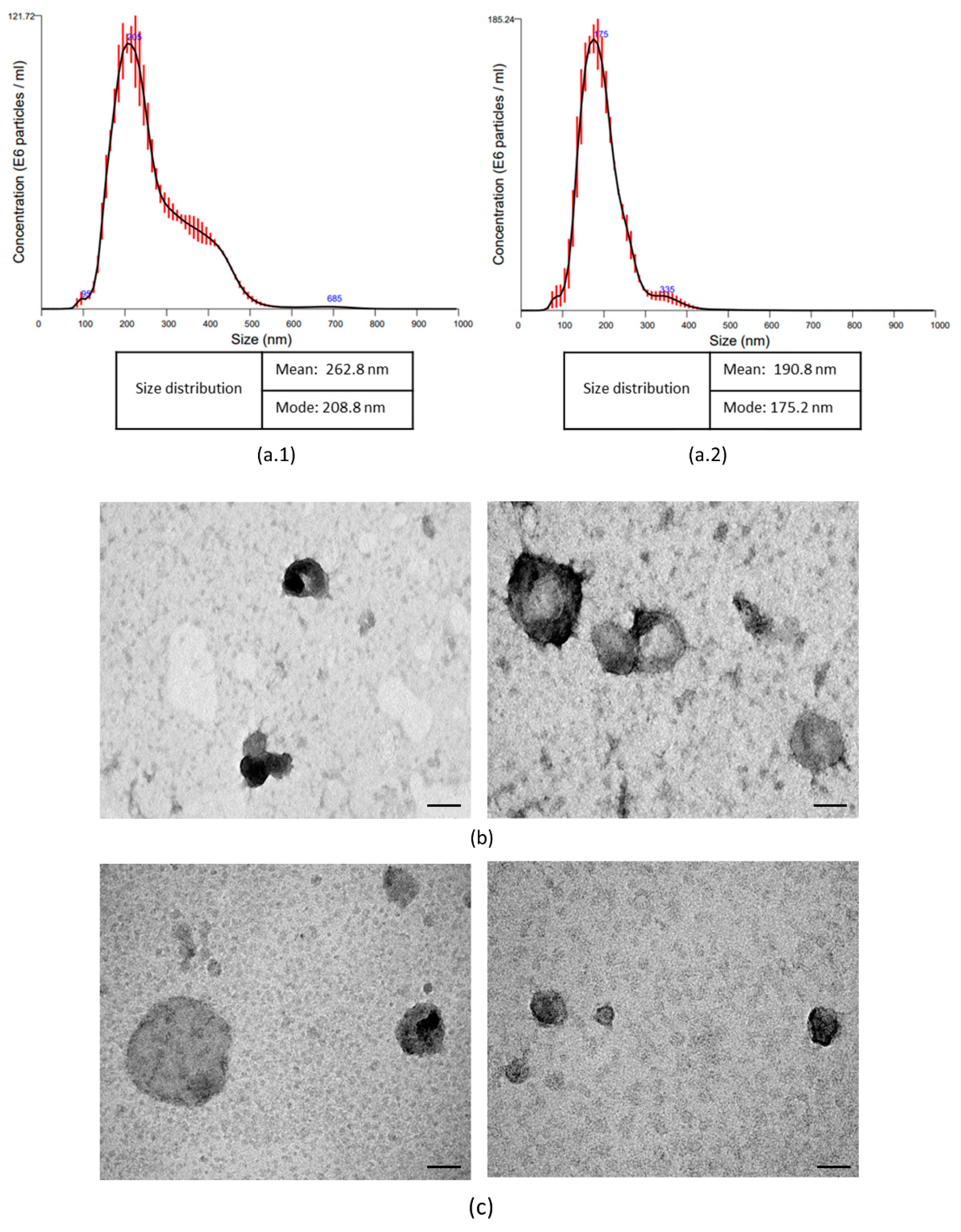
2.2. Isolation and Characterization Extracellular Vesicles
2.3. The Animal Model Showed the Characteristics of CKD
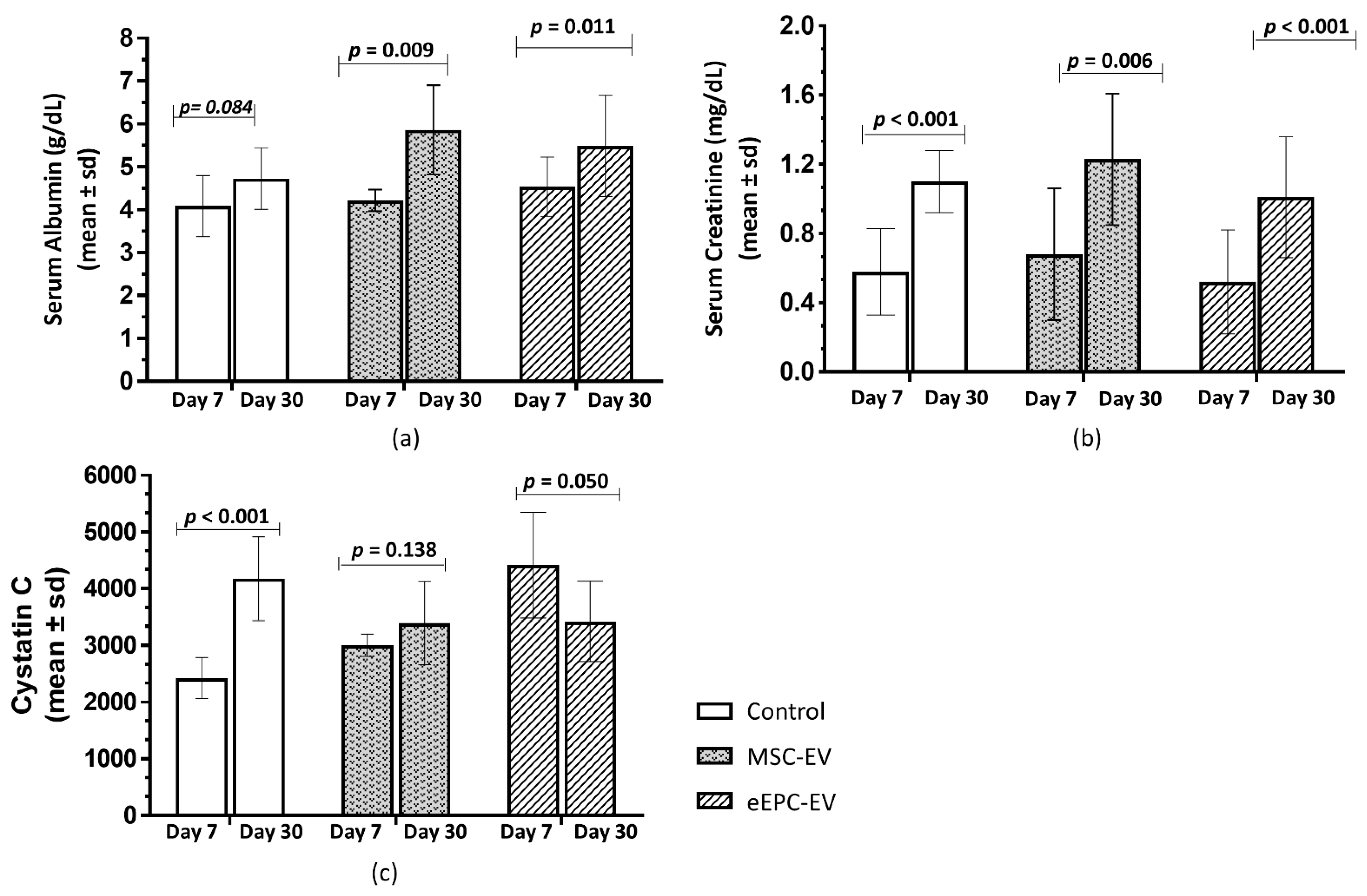
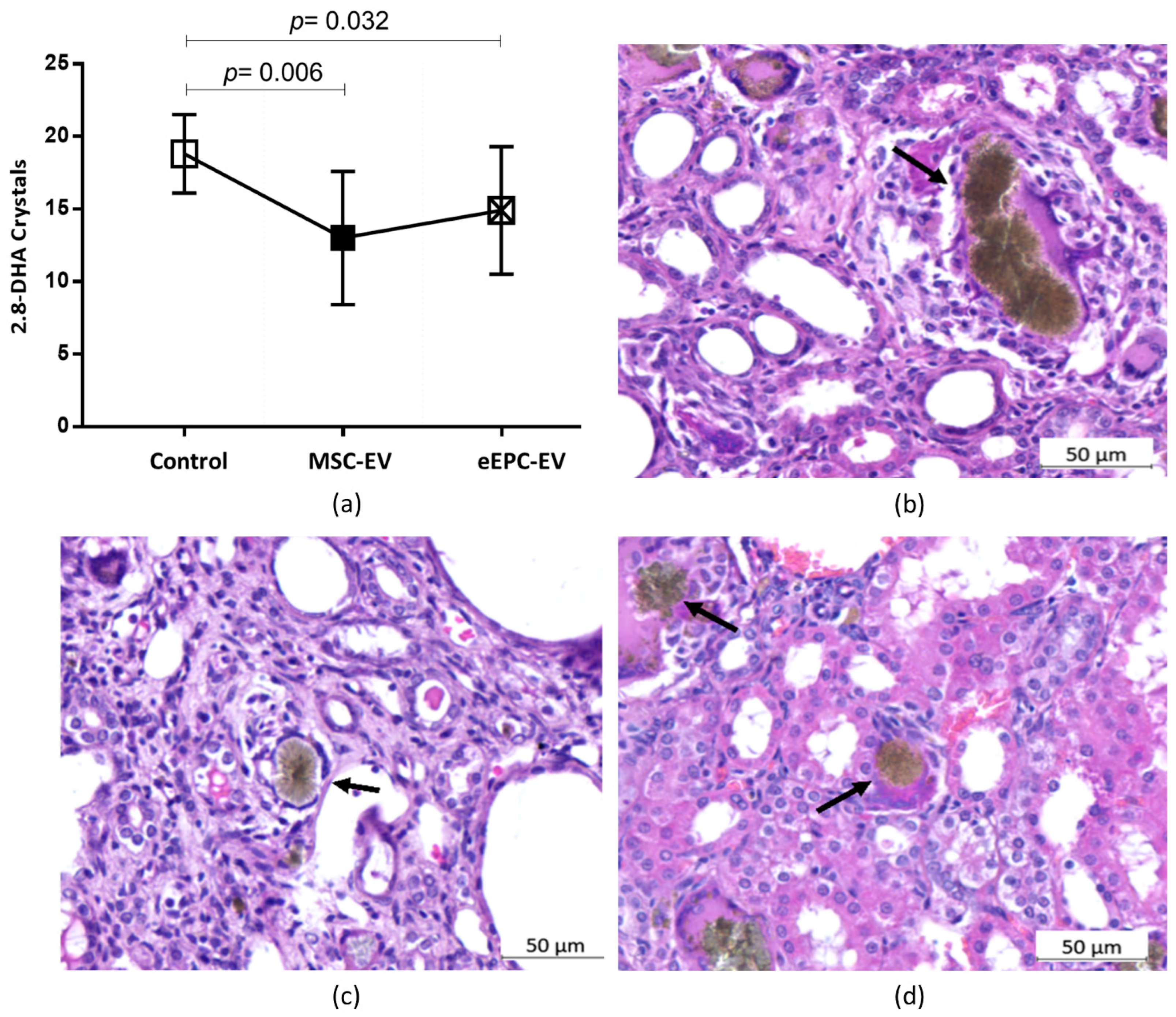
2.4. Treatment with EVs and Biochemical Analyses
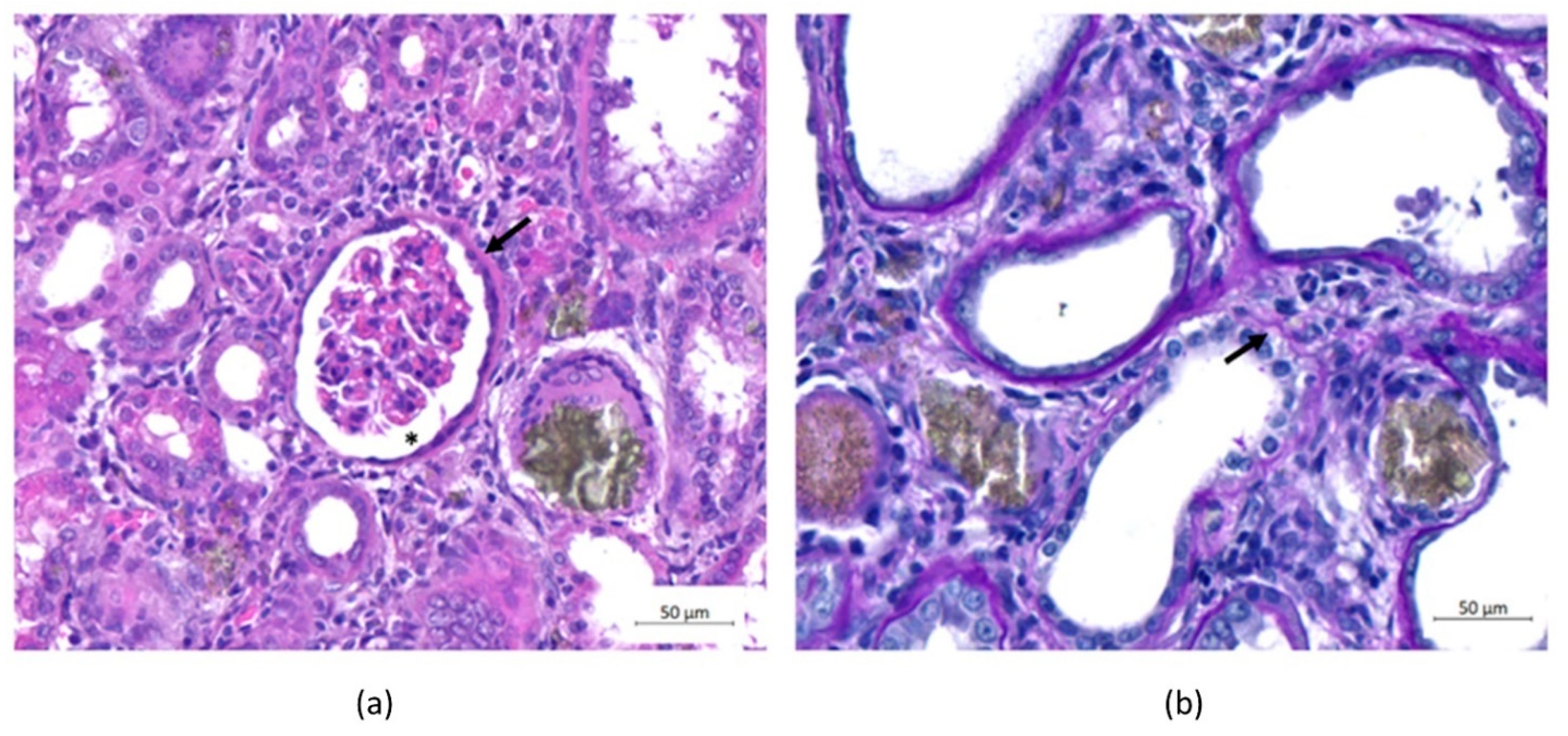
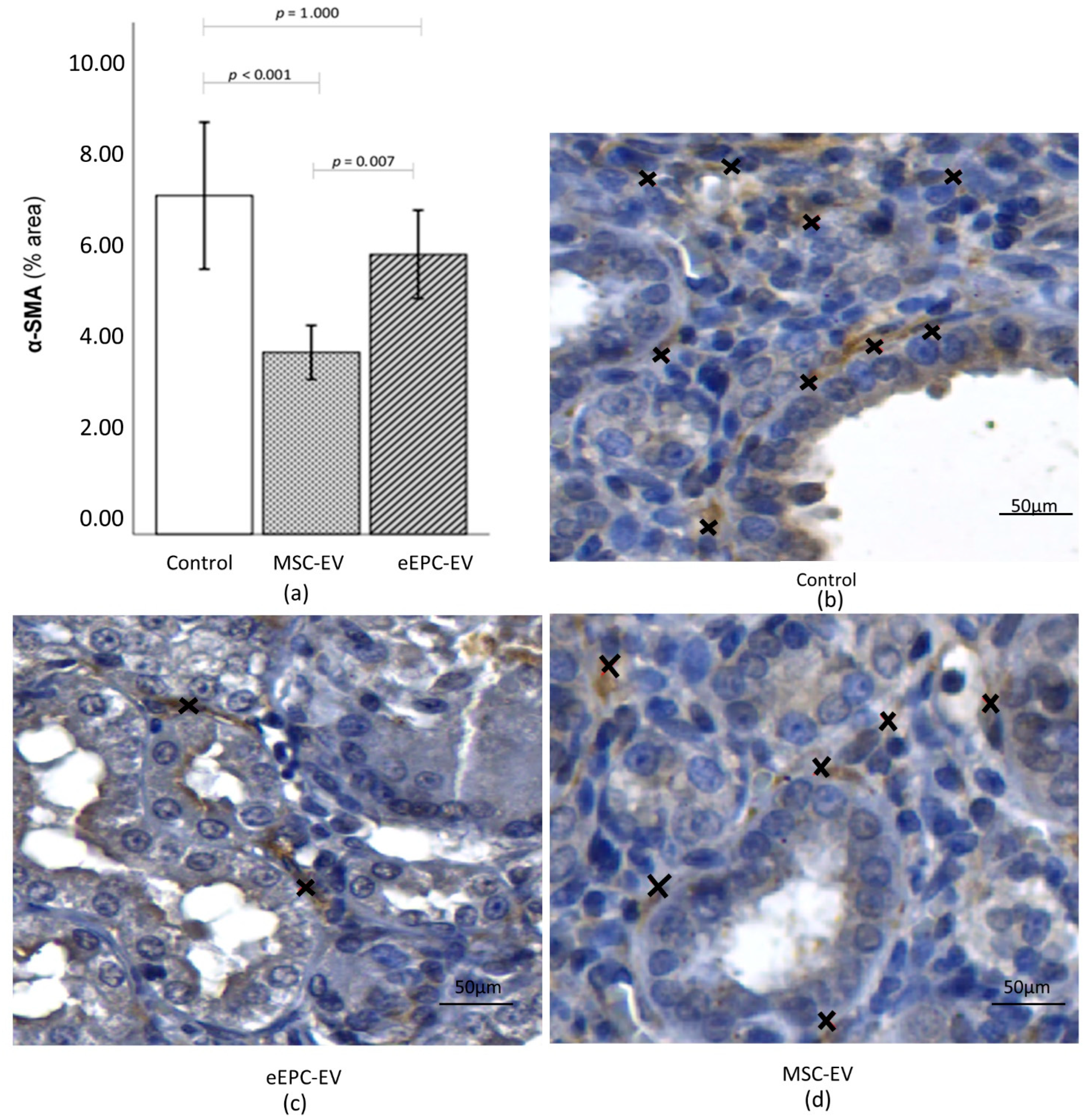
2.5. Histological Analysis Showed the Induction of CKD and Improvements in the Disease after EV Treatment
3. Discussion
4. Materials and Methods
4.1. Isolation and Expansion of CD133+ Cells
4.2. Isolation and Expansion of MSCs
4.3. Isolation of Extracellular Vesicles
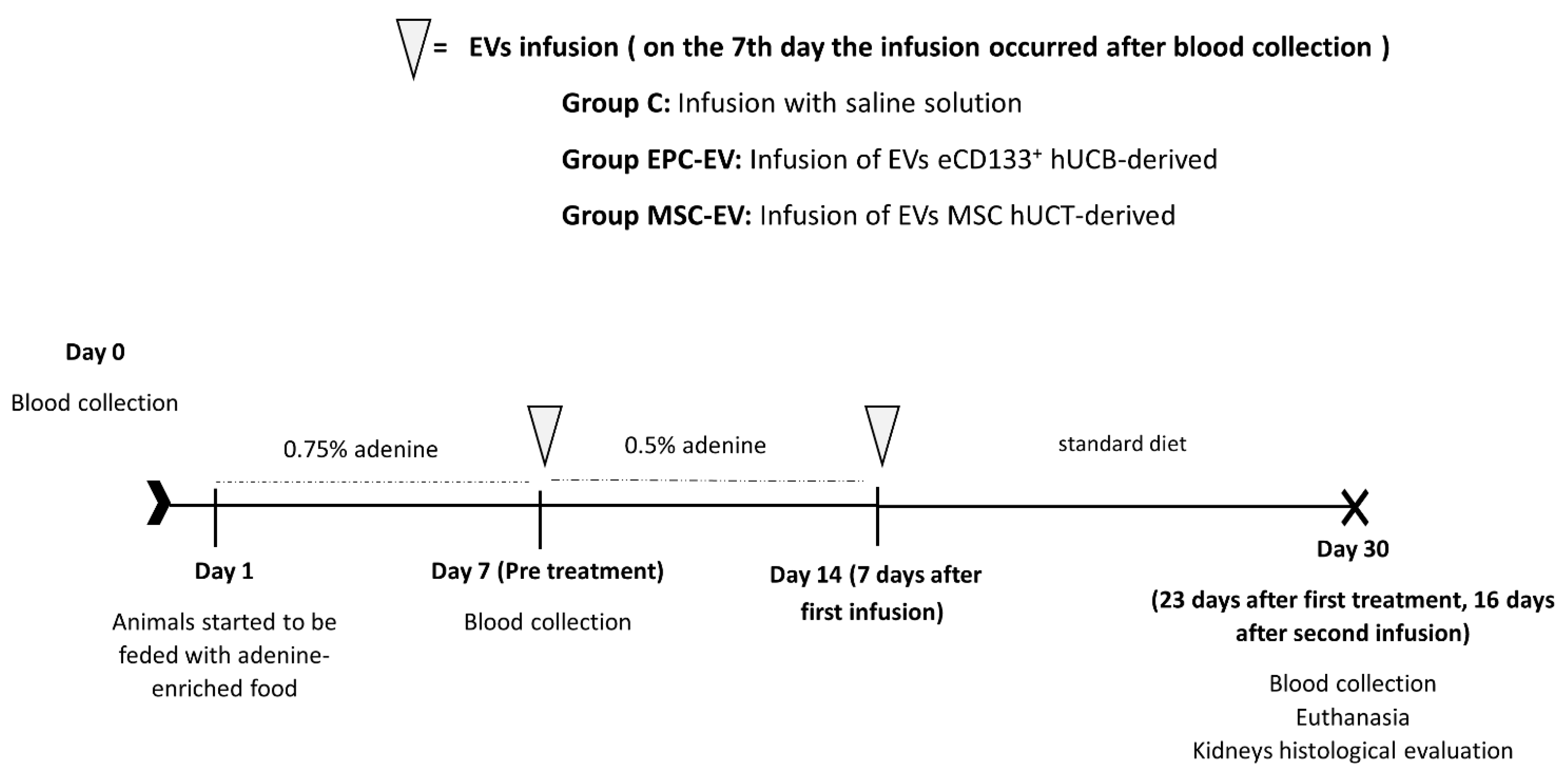
4.4. Experimental Model of CKD & EV Infusions
4.5. Biochemical Analysis
4.6. Histopathological Analysis
4.7. Immunohistochemistry
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The Global Burden of Kidney Disease and the Sustainable Development Goals. Bull. World Health Organ. 2018, 96, 414–422C. [Google Scholar] [CrossRef] [PubMed]
- Filipska, A.; Bohdan, B.; Wieczorek, P.P.; Hudz, N. Chronic Kidney Disease and Dialysis Therapy: Incidence and Prevalence in the World. Pharmacia 2021, 68, 463–470. [Google Scholar] [CrossRef]
- Li, P.K.T.; Garcia-Garcia, G.; Lui, S.F.; Andreoli, S.; Fung, W.W.S.; Hradsky, A.; Kumaraswami, L.; Liakopoulos, V.; Rakhimova, Z.; Saadi, G.; et al. Kidney Health for Everyone Everywhere—From Prevention to Detection and Equitable Access to Care. Nefrologia 2020, 40, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Kalluri, R. Possible Mechanisms of Kidney Repair. Fibrogenes. Tissue Repair 2009, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Peired, A.J.; Sisti, A.; Romagnani, P. Mesenchymal Stem Cell-Based Therapy for Kidney Disease: A Review of Clinical Evidence. Stem Cells Int. 2016, 2016, 4798639. [Google Scholar] [CrossRef] [Green Version]
- Karpman, D.; Ståhl, A.L.; Arvidsson, I. Extracellular Vesicles in Renal Disease. Nat. Rev. Nephrol. 2017, 13, 545–562. [Google Scholar] [CrossRef]
- Ozkok, A.; Yildiz, A. Endothelial Progenitor Cells and Kidney Diseases. Kidney Blood Press. Res. 2018, 43, 701–718. [Google Scholar] [CrossRef]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; Van Der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of Mesenchymal Stem/Stromal Cell Function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef] [Green Version]
- Bunnell, B.A.; Betancourt, A.M.; Sullivan, D.E.; Barry, F.P.; Murphy, J.M. Mesenchymal Stem Cells: Clinical Applications and Biological Characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar]
- Altabas, V.; Altabas, K.; Kirigin, L. Endothelial Progenitor Cells (EPCs) in Ageing and Age-Related Diseases: How Currently Available Treatment Modalities Affect EPC Biology, Atherosclerosis, and Cardiovascular Outcomes. Mech. Ageing Dev. 2016, 159, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles Derived from Human Adult Mesenchymal Stem Cells Protect against Ischaemia-Reperfusion-Induced Acute and Chronic Kidney Injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef] [Green Version]
- Cantaluppi, V.; Gatti, S.; Medica, D.; Figliolini, F.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Endothelial Progenitor Cells Protect the Kidney from Ischemia-Reperfusion Injury by MicroRNA-Dependent Reprogramming of Resident Renal Cells. Kidney Int. 2012, 82, 412–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular Vesicles Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in a Rat Myocardial Infarction Model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.; Sun, S.; Yu, M.; Wang, C.; Pei, X.; Zhu, B.; Wu, J.; Zhao, W. Bone Marrow Stem Cells-Derived Microvesicles Protect against Renal Injury in the Mouse Remnant Kidney Model. Nephrology 2012, 17, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Oura, H. Takako of Dietary Purine in Rats, Faculty of Medicine, Toyama Medical and Pharmaceutical University, Were as Follows: Hypoxaiithine, Ular, the Intake of Adenine Exhibited Previously Published Data from Our Laboratory on Measuring the Level of Uric A. J. Nutr. Sci. Vitaminol. 1982, 28, 519–526. [Google Scholar]
- Angulski, A.B.B.; Capriglione, L.G.; Batista, M.; Marcon, B.H.; Senegaglia, A.C.; Stimamiglio, M.A.; Correa, A. The Protein Content of Extracellular Vesicles Derived from Expanded Human Umbilical Cord Blood-Derived CD133+ and Human Bone Marrow-Derived Mesenchymal Stem Cells Partially Explains Why Both Sources Are Advantageous for Regenerative Medicine. Stem Cell Rev. Rep. 2017, 13, 244–257. [Google Scholar] [CrossRef]
- Claramunt, D.; Gil-Peña, H.; Fuente, R.; Hernández-Frías, O.; Santos, F. Animal Models of Pediatric Chronic Kidney Disease. Is Adenine Intake an Appropriate Model? Nefrologia 2015, 35, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Engle, S.J.; Stockelmant, M.G.; Chen, J.U.; Boivint, G.; Yum, M.; Daviest, P.M.; Yingt, M.O.Y.I.N.; Sahota, A.; Simmondst, H.A.; Stambrookt, P.J.; et al. Develop 2,8-Dihydroxyadenine. Genetics 1996, 93, 5307–5312. [Google Scholar]
- Tögel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered Mesenchymal Stem Cells Protect against Ischemic Acute Renal Failure through Differentiation-Independent Mechanisms. Am. J. Physiol.-Ren. Physiol. 2005, 289, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.Q.; Deng, M.X.; He, J.; Zeng, Q.X.; Wen, W.; Wong, D.S.H.; Tse, H.F.; Xu, G.; Lian, Q.; Shi, J.; et al. Human Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Allergic Airway Inflammation in Mice. Stem Cells 2012, 30, 2692–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suss, P.H.; Capriglione, L.G.A.; Barchiki, F.; Miyague, L.; Jackowski, D.; Fracaro, L.; Schittini, A.V.; Senegaglia, A.C.; Rebelatto, C.L.K.; Olandoski, M.; et al. Direct Intracardiac Injection of Umbilical Cord-Derived Stromal Cells and Umbilical Cord Blood-Derived Endothelial Cells for the Treatment of Ischemic Cardiomyopathy. Exp. Biol. Med. 2015, 240, 969–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, A.; Ottoboni, G.S.; Senegaglia, A.C.; Capriglione, L.G.A.; Miyague, N.I.; Leite, L.M.B.; Jamur, V.R.; Rebelatto, C.L.K.; Olandoski, M.; Brofman, P.R.S. Expanded CD133+ Cells from Human Umbilical Cord Blood Improved Heart Function in Rats after Severe Myocardial Infarction. Stem Cells Int. 2018, 2018, 5412478. [Google Scholar] [CrossRef] [Green Version]
- Boldrini-Leite, L.M.; Michelotto, P.V.; de Moura, S.A.B.; Capriglione, L.G.A.; Barussi, F.C.M.; Fragoso, F.Y.I.; Senegaglia, A.C.; Brofman, P.R.S. Lung Tissue Damage Associated with Allergic Asthma in BALB/c Mice Could Be Controlled with a Single Injection of Mesenchymal Stem Cells from Human Bone Marrow up to 14 d After Transplantation. Cell Transplant. 2020, 29, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Mesenchymal Stem Cells Enhance Survival in a Lethal Model of Acute Kidney Injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Sokic, S.; Schardt, J.S.; Raiker, R.S.; Lin, J.W.; Jay, S.M. Emerging Roles for Extracellular Vesicles in Tissue Engineering and Regenerative Medicine. Tissue Eng.-Part B Rev. 2015, 21, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Meng, H.; Wan, W.; Xie, M.; Wen, C. Application Potential of Stem/Progenitor Cell-Derived Extracellular Vesicles in Renal Diseases. Stem Cell Res. Ther. 2019, 10, 8. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Tigges, J.; Toxavidis, V.; Ericsson, M.; Distel, R.J.; Ivanov, A.R.; Skog, J.; Kuo, W.P. Alternative Methods for Characterization of Extracellular Vesicles. Front. Physiol. 2012, 3, 354. [Google Scholar] [CrossRef] [Green Version]
- Van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.H.; Ziada, A.; Al Husseni, I.; Beegam, S.; Nemmar, A. Motor and Behavioral Changes in Rats with Adenine-Induced Chronic Renal Failure: Influence of Acacia Gum Treatment. Exp. Biol. Med. 2011, 236, 107–112. [Google Scholar] [CrossRef]
- Al Suleimani, Y.; Al, M.; Ramkumar, A.; Almahruqi, A. Influence of treatment with gum acacia on renal vascular responses in a rat model of chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 498–506. [Google Scholar] [PubMed]
- Diwan, V.; Brown, L.; Gobe, G.C. Adenine-Induced Chronic Kidney Disease in Rats. Nephrology 2018, 23, 5–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.M.; Chena, H.R.; Zha, X.Q.; Lu, C.Q.; Pan, L.H.; Luo, J.P. Renoprotective Effect of Chinese Chive Polysaccharides in Adenine-Induced Chronic Renal Failure. Int. J. Biol. Macromol. 2018, 106, 988–993. [Google Scholar] [CrossRef]
- Lou, X.; Jin, J.; Gong, J.; Zhao, L.; Li, Y.; He, Q. Comparison of the Effects of Indobufen and Warfarin in a Rat Model of Adenine-Induced Chronic Kidney Disease. Med. Sci. Monit. 2019, 25, 3566–3572. [Google Scholar] [CrossRef]
- Friedman, A.N.; Fadem, S.Z. Reassessment of Albumin as a Nutritional Marker in Kidney Disease. J. Am. Soc. Nephrol. 2010, 21, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Valdés, J.J.; García-Bañuelos, J.; Salazar-Montes, A.; García-Benavides, L.; Rosales-Dominguez, A.; Armendáriz-Borunda, J.; Sandoval-Rodríguez, A. Human Adipose Derived Stem Cells Regress Fibrosis in a Chronic Renal Fibrotic Model Induced by Adenine. PLoS ONE 2017, 12, e0187907. [Google Scholar] [CrossRef] [Green Version]
- Do Carmo, W.B.; Castro, B.B.A.; Rodrigues, C.A.; Custódio, M.R.; Sanders-Pinheiro, H. Chitosan-Fe (III) Complex as a Phosphate Chelator in Uraemic Rats: A Novel Treatment Option. Basic Clin. Pharmacol. Toxicol. 2018, 122, 120–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, B.H.; Al Za’abi, M.; Adham, S.A.; Al Suleimani, Y.; Karaca, T.; Manoj, P.; Al Kalbani, J.; Yasin, J.; Nemmar, A. The Effect of Sildenafil on Rats with Adenine—Induced Chronic Kidney Disease. Biomed. Pharmacother. 2018, 108, 391–402. [Google Scholar] [CrossRef]
- Thakur, R.; Sharma, A.; Lingaraju, M.C.; Begum, J.; Kumar, D.; Mathesh, K.; Kumar, P.; Singh, T.U.; Kumar, D. Ameliorative Effect of Ursolic Acid on Renal Fibrosis in Adenine-Induced Chronic Kidney Disease in Rats. Biomed. Pharmacother. 2018, 101, 972–980. [Google Scholar] [CrossRef]
- Dos Santos, N.S.J.; Draibe, S.A.; Kamimura, M.A.; Cuppari, L. Albumina Sérica Como Marcador Nutricional de Pacientes Em Hemodiálise. Rev. Nutr. 2004, 17, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Tynkevich, E.; Flamant, M.; Haymann, J.P.; Metzger, M.; Thervet, E.; Boffa, J.J.; Vrtovsnik, F.; Houillier, P.; Froissart, M.; Stengel, B.N.D. Decrease in Urinary Creatinine Excretion in Early Stage Chronic Kidney Disease. PLoS ONE 2014, 9, e111949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabe, C.; Borges, R.L.; de Almeida, D.C.; Fanelli, C.; Barlette, G.P.; Machado, F.G.; Arias, S.C.A.; Malheiros, D.M.A.C.; Camara, N.O.S.; Zatz, R.; et al. NF-ΚB Activation Mediates Crystal Translocation and Interstitial Inflammation in Adenine Overload Nephropathy. Am. J. Physiol.-Ren. Physiol. 2013, 305, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing Kidney Function—Measured and Estimated Glomerular Filtration Rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peralta, C.A.; Shlipak, M.G.; Judd, S.; Cushman, M.; McClellan, W.; Zakai, N.A.; Safford, M.M.; Zhang, X.; Muntner, P.; Warnock, D. Detection of Chronic Kidney Disease With Creatinine, Cystatin C, and Urine Albumin-to-Creatinine Ratio and Association With Progression to End-Stage Renal Disease and Mortality. JAMA 2011, 305, 1545–1552. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of Acute Kidney Injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463–493. [Google Scholar] [CrossRef] [Green Version]
- Shemesh, O.; Golbetz, H.; Kriss, J.P.; Myers, B.D. Limitations of Creatinine as a Filtration Marker in Glomerulopathic Patients. Kidney Int. 1985, 28, 830–838. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.X.; Sun, L.; Nicholas, S.B.; Lu, Y.; K, S.S.; Hua, R. Comparison of Bias and Accuracy Using Cystatin C and Creatinine in CKD-EPI Equations for GFR Estimation. Eur. J. Intern. Med. 2020, 80, 29–34. [Google Scholar] [CrossRef]
- Murty, M.S.N.; Sharma, U.K.; Pandey, V.B.; Kankare, S.B. Serum Cystatin C as a Marker of Renal Function in Detection of Early Acute Kidney Injury. Indian J. Nephrol. 2013, 23, 180–183. [Google Scholar] [CrossRef]
- Benoit, S.W.; Ciccia, E.A.; Devarajan, P. Cystatin C as a Biomarker of Chronic Kidney Disease: Latest Developments. Expert Rev. Mol. Diagn. 2020, 20, 1019–1026. [Google Scholar] [CrossRef]
- Gharaibeh, K.A.; Hamadah, A.M.; El-Zoghby, Z.M.; Lieske, J.C.; Larson, T.S.; Leung, N. Cystatin C Predicts Renal Recovery Earlier Than Creatinine Among Patients With Acute Kidney Injury. Kidney Int. Rep. 2018, 3, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Diwan, V.; Mistry, A.; Gobe, G.; Brown, L. Adenine-Induced Chronic Kidney and Cardiovascular Damage in Rats. J. Pharmacol. Toxicol. Methods 2013, 68, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Törmänen, S.; Pörsti, I.; Lakkisto, P.; Tikkanen, I.; Niemelä, O.; Paavonen, T.; Mustonen, J.; Eräranta, A. Endothelin A Receptor Blocker and Calcimimetic in the Adenine Rat Model of Chronic Renal Insufficiency. BMC Nephrol. 2017, 18, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puthumana, J.; Thiessen-Philbrook, H.; Xu, L.; Coca, S.G.; Garg, A.X.; Himmelfarb, J.; Bhatraju, P.K.; Ikizler, T.A.; Siew, E.D.; Ware, L.B.; et al. Biomarkers of Inflammation and Repair in Kidney Disease Progression. J. Clin. Investig. 2021, 131, e139927. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, R.L.; Forbes, M.S.; Thornhill, B.A. Ureteral Obstruction as a Model of Renal Interstitial Fibrosis and Obstructive Nephropathy. Kidney Int. 2009, 75, 1145–1152. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Nikolic-paterson, D.J.; Lan, H.Y. Inflammatory Processes in Renal Fibrosis. Nat. Publ. Gr. 2014, 10, 493–503. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Györfi, A.H.; Ramanujam, M.; Whitfield, M.L.; Königshoff, M.; Lafyatis, R. Shared and Distinct Mechanisms of Fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Djudjaj, S.; Boor, P. Cellular and Molecular Mechanisms of Kidney Fibrosis. Mol. Asp. Med. 2019, 65, 16–36. [Google Scholar] [CrossRef]
- Panizo, S.; Martínez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martín-Carro, B.; Fernández-Martín, J.L.; Naves-Díaz, M.; Carrillo-López, N.; Cannata-Andía, J.B. Fibrosis in Chronic Kidney Disease: Pathogenesis and Consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef]
- Meran, S.; Steadman, R. Fibroblasts and Myofibroblasts in Renal Fibrosis. Int. J. Exp. Pathol. 2011, 92, 158–167. [Google Scholar] [CrossRef]
- Rodrigues, P.C.; Da Costa Miguel, M.C.; De Aquino, S.N.; Fonseca, F.P.; Dos Santos Silva, A.R.; Leme, A.F.P.; Coletta, R.D. Stromal Myofibroblasts in Potentially Malignant and Malignant Lesions of the Oral Cavity. Oncol. Lett. 2015, 9, 667–670. [Google Scholar] [CrossRef]
- Orimo, A.; Sato, M.; Nogi, Y.; Inoue, S.; Muramatsu, M.; Tomioka, Y.; Oigawa, S.; Hata, T.; Shimizu, Y.; Kamata, K.; et al. Cancer-Associated Myofibroblasts Possess Various Factors to Promote Endometrial Tumor Progression. Clin. Cancer Res. 2001, 7, 3097–3105. [Google Scholar]
- Koyama, Y.; Brenner, D.A. Liver Inflammation and Fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lepreux, S.; Desmoulière, A. Human Liver Myofibroblasts during Development and Diseases with a Focus on Portal (Myo)Fibroblasts. Front. Physiol. 2015, 6, 173. [Google Scholar] [CrossRef] [PubMed]
- Dees, C.; Chakraborty, D.; Distler, J.H.W. Cellular and Molecular Mechanisms in Fibrosis. Exp. Dermatol. 2021, 30, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Tan, R.J.; Liu, Y. Myofibroblast in Kidney Fibrosis: Origin, Activation, and Regulation; Springer: Singapore, 2019; Volume 1165. [Google Scholar] [CrossRef]
- Isaka, Y. Targeting TGF-β Signaling in Kidney Fibrosis. Int. J. Mol. Sci. 2018, 19, 2532. [Google Scholar] [CrossRef] [Green Version]
- Eardley, K.S.; Kubal, C.; Zehnder, D.; Quinkler, M.; Lepenies, J.; Savage, C.O.; Howie, A.J.; Kaur, K.; Cooper, M.S.; Adu, D.; et al. The Role of Capillary Density, Macrophage Infiltration and Interstitial Scarring in the Pathogenesis of Human Chronic Kidney Disease. Kidney Int. 2008, 74, 495–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, C.E.; Capcha, J.M.C.; De Bragança, A.C.; Sanches, T.R.; Gouveia, P.Q.; De Oliveira, P.A.F.; Malheiros, D.M.A.C.; Volpini, R.A.; Santinho, M.A.R.; Santana, B.A.A.; et al. Human Umbilical Cord-Derived Mesenchymal Stromal Cells Protect against Premature Renal Senescence Resulting from Oxidative Stress in Rats with Acute Kidney Injury. Stem Cell Res. Ther. 2017, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, T.; Cheng, J.; Zhong, L.; Zhao, X.F.; Zhu, J.; Zhu, Y.J.; Liu, G.H. The Alleviation of Acute and Chronic Kidney Injury by Human Wharton’s Jelly-Derived Mesenchymal Stromal Cells Triggered by Ischemia-Reperfusion Injury via an Endocrine Mechanism. Cytotherapy 2012, 14, 1215–1227. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Miao, S.; Chen, J.; Du, T.; Zhong, L.; Ju, G.; Liu, G.; Zhu, Y. The Anti-Oxidative Role of Micro-Vesicles Derived from Human Wharton-Jelly Mesenchymal Stromal Cells through NOX2/Gp91(Phox) Suppression in Alleviating Renal Ischemia-Reperfusion Injury in Rats. PLoS ONE 2014, 9, e92129. [Google Scholar] [CrossRef]
- Wu, X.; Yan, T.; Wang, Z.; Wu, X.; Cao, G.; Zhang, C.; Tian, X.; Wang, J. Micro-Vesicles Derived from Human Wharton’s Jelly Mesenchymal Stromal Cells Mitigate Renal Ischemia-Reperfusion Injury in Rats after Cardiac Death Renal Transplantation. J. Cell. Biochem. 2018, 119, 1879–1888. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.; Lu, M.; Zhu, Y. Microvesicles Derived from Human Wharton’s Jelly Mesenchymal Stromal Cells Ameliorate Renal Ischemia-Reperfusion Injury in Rats by Suppressing CX3CL1. Stem Cell Res. Ther. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, J.L.; Gorin, Y. Myofibroblast Differentiation During Fibrosis: Role of NAD(P)H Oxidases. Kidney Int. 2011, 79, 944–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, M.B.; Bussolati, B.; Bruno, S.; Fonsato, V.; Romanazzi, G.M.; Camussi, G. Mesenchymal Stem Cells Contribute to the Renal Repair of Acute Tubular Epithelial Injury. Int. J. Mol. Med. 2004, 14, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Tögel, F.; Ittrich, H.; Clayton, F.; Nolte-Ernsting, C.; Zander, A.R.; Westenfelder, C. Administered Mesenchymal Stem Cells Enhance Recovery from Ischemia/Reperfusion-Induced Acute Renal Failure in Rats. Kidney Int. 2005, 68, 1613–1617. [Google Scholar] [CrossRef] [Green Version]
- Morigi, M.; Introna, M.; Imberti, B.; Corna, D.; Abbate, M.; Rota, C.; Rottoli, D.; Benigni, A.; Perico, N.; Zoja, C.; et al. Human Bone Marrow Mesenchymal Stem Cells Accelerate Recovery of Acute Renal Injury and Prolong Survival in Mice. Stem Cells 2008, 26, 2075–2082. [Google Scholar] [CrossRef]
- Chade, A.R.; Zhu, X.; Lavi, R.; Krier, J.D.; Pislaru, S.; Simari, R.D.; Napoli, C.; Lerman, A.; Lerman, L.O. Endothelial Progenitor Cells Restore Renal Function in Chronic Experimental Renovascular Disease. Circulation 2009, 119, 547–557. [Google Scholar] [CrossRef]
- Eirin, A.; Lerman, L.O. Mesenchymal Stem Cell Treatment for Chronic Renal Failure. Stem Cell Res. Ther. 2014, 5, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Semedo, P.; Correa-Costa, M.; Cenedeze, M.A.; Malheiros, D.M.A.C.; Dos Reis, M.A.; Shimizu, M.H.; Seguro, A.C.; Pacheco-Silva, A.; Ĉamara, N.O.S. Mesenchymal Stem Cells Attenuate Renal Fibrosis through Immune Modulation and Remodeling Properties in a Rat Remnant Kidney Model. Stem Cells 2009, 27, 3063–3073. [Google Scholar] [CrossRef]
- Tögel, F.; Weiss, K.; Yang, Y.; Hu, Z.; Zhang, P.; Westenfelder, C. Vasculotropic, Paracrine Actions of Infused Mesenchymal Stem Cells Are Important to the Recovery from Acute Kidney Injury. Am. J. Physiol.-Ren. Physiol. 2007, 292, F1626–F1635. [Google Scholar] [CrossRef] [Green Version]
- De Jong, O.G.; van Balkom, B.W.M.; Schiffelers, R.M.; Bouten, C.V.C.; Verhaar, M.C. Extracellular Vesicles: Potential Roles in Regenerative Medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef] [Green Version]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.N.; Essa, W.; Adel, H. Umbilical Cord Mesenchymal Stem Cells Derived Extracellular Vesicles Can Safely Ameliorate the Progression of Chronic Kidney Diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, M.P.; Ang, D.S.; Pall, A.; Struthers, A.D. Oxidative Stress in Renal Dysfunction: Mechanisms, Clinical Sequelae and Therapeutic Options. J. Hum. Hypertens. 2010, 24, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Swiercz, J.M.; Bañón-Rodríguez, I.; Matković, I.; Federico, G.; Sun, T.; Franz, T.; Brakebusch, C.H.; Kumanogoh, A.; Friedel, R.H.; et al. Semaphorin-Plexin Signaling Controls Mitotic Spindle Orientation during Epithelial Morphogenesis and Repair. Dev. Cell 2015, 33, 299–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bøyum, A. Isolation of Mononuclear Cells and Granulocytes from Human Blood. Scand. J. Clin. Lab. Investig. 1968, 21, 77–89. [Google Scholar]
- Senegaglia, A.C.; Barboza, L.A.; Dallagiovanna, B.; Aita, C.A.M.; Hansen, P.; Rebelatto, C.L.K.; Aguiar, A.M.; Miyague, N.I.; Barchiki, F.; Correa, A.; et al. Better at Improving Cardiac Function? Exp. Biol. Med. 2010, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Rebelatto, C.K.; Aguiar, A.M.; Moretão, M.P.; Senegaglia, A.C.; Hansen, P.; Barchiki, F.; Oliveira, J.; Martins, J.; Kuligovski, C.; Mansur, F.; et al. Dissimilar Differentiation of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, and Adipose Tissue. Exp. Biol. Med. 2008, 233, 901–913. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]

| Serum Markers | Evaluation Time | n | Mean | p * |
|---|---|---|---|---|
| SERUM ALBUMIN (g/dL) | Preinduction (Day 0) | 27 | 4.51 ± 0.28 | 0.061 |
| Day 7 | 27 | 4.29 ± 0.63 | ||
| Dif (Day 7–Day 0) | 27 | −0.22 ± 0.57 | ||
| SERUM CREATININE (mg/dL) | Preinduction (Day 0) | 27 | 0.38 ± 0.09 | 0.001 |
| Day 7 | 27 | 0.58 ± 0.30 | ||
| Dif (Day 7–Day 0) | 27 | 0.20 ± 0.28 | ||
| CYSTATIN C | Preinduction (Day 0) | 26 | 2498 ± 254 | 0.001 |
| Day 7 | 26 | 3269 ± 1054 | ||
| Dif (Day 7–Day 0) | 26 | 770.9 ± 1040.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyasaki, D.M.; Senegaglia, A.C.; de Moura, S.A.B.; Leitolis, A.; Capriglione, L.G.A.; Fracaro, L.; Boldrini Leite, L.M.; Utumi, P.H.; Fragoso, F.Y.I.; Meyer, F.; et al. Treatment of Chronic Kidney Disease with Extracellular Vesicles from Mesenchymal Stem Cells and CD133+ Expanded Cells: A Comparative Preclinical Analysis. Int. J. Mol. Sci. 2022, 23, 2521. https://doi.org/10.3390/ijms23052521
Miyasaki DM, Senegaglia AC, de Moura SAB, Leitolis A, Capriglione LGA, Fracaro L, Boldrini Leite LM, Utumi PH, Fragoso FYI, Meyer F, et al. Treatment of Chronic Kidney Disease with Extracellular Vesicles from Mesenchymal Stem Cells and CD133+ Expanded Cells: A Comparative Preclinical Analysis. International Journal of Molecular Sciences. 2022; 23(5):2521. https://doi.org/10.3390/ijms23052521
Chicago/Turabian StyleMiyasaki, Dayane Mayumi, Alexandra Cristina Senegaglia, Sérgio Adriane Bezerra de Moura, Amanda Leitolis, Luiz Guilherme Achcar Capriglione, Letícia Fracaro, Lidiane Maria Boldrini Leite, Paulo Henrique Utumi, Felipe Yukio Ishikawa Fragoso, Fernando Meyer, and et al. 2022. "Treatment of Chronic Kidney Disease with Extracellular Vesicles from Mesenchymal Stem Cells and CD133+ Expanded Cells: A Comparative Preclinical Analysis" International Journal of Molecular Sciences 23, no. 5: 2521. https://doi.org/10.3390/ijms23052521






