The Finely Coordinated Action of SSB and NurA/HerA Complex Strictly Regulates the DNA End Resection Process in Saccharolobus solfataricus
Abstract
:1. Introduction
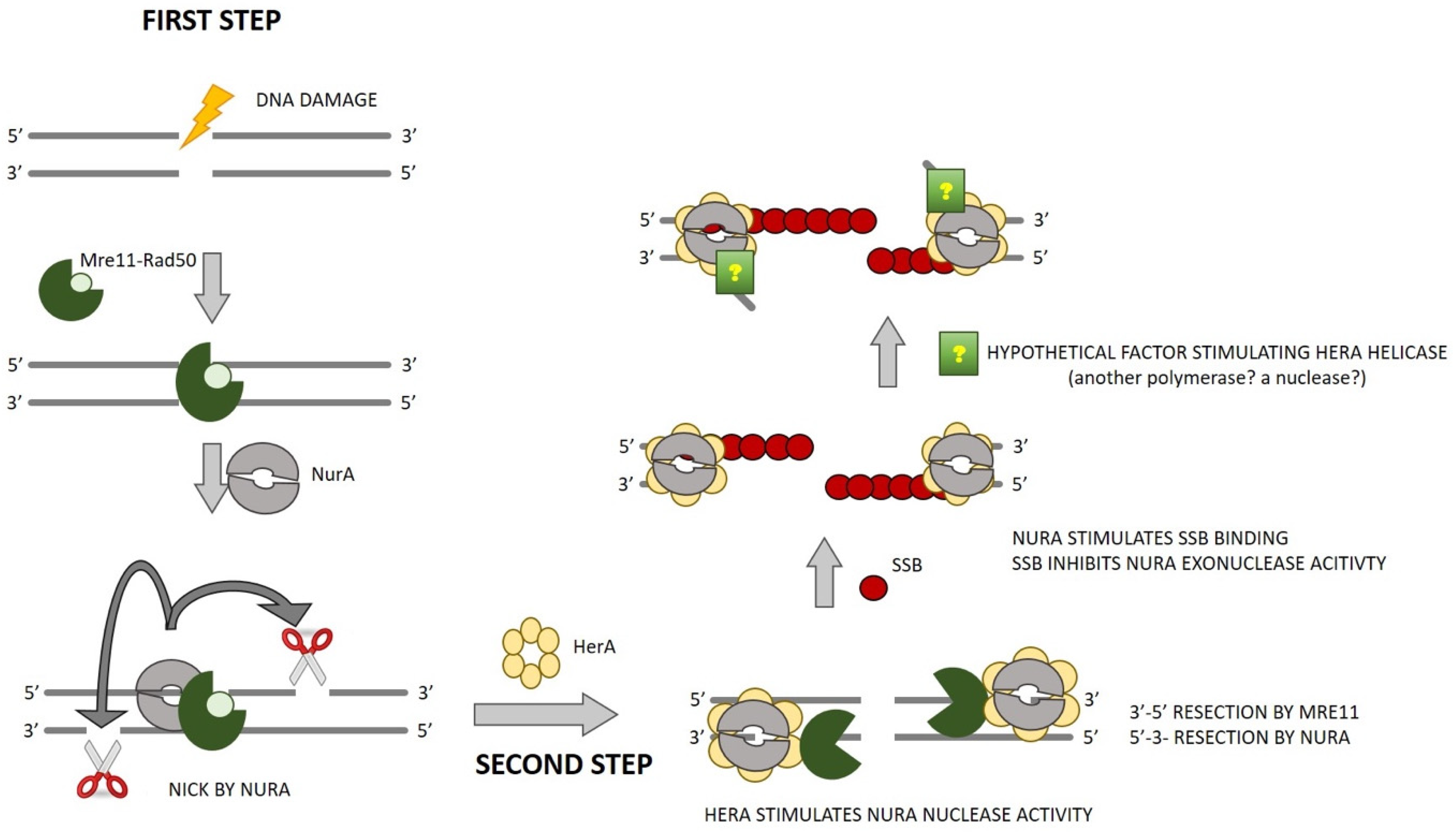
2. Results and Discussion
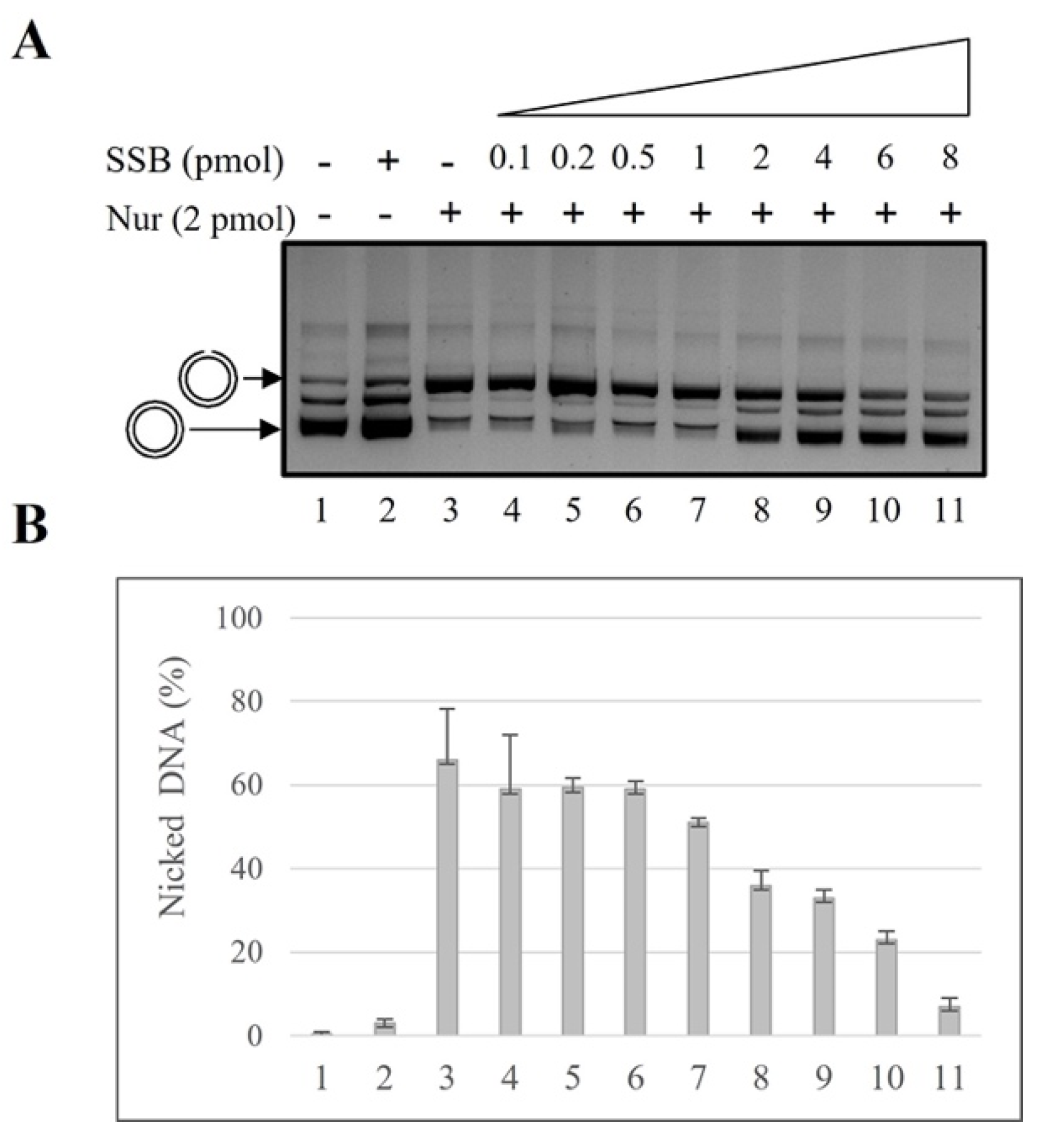
2.1. SSB Effect on NurA Endonuclease Activity
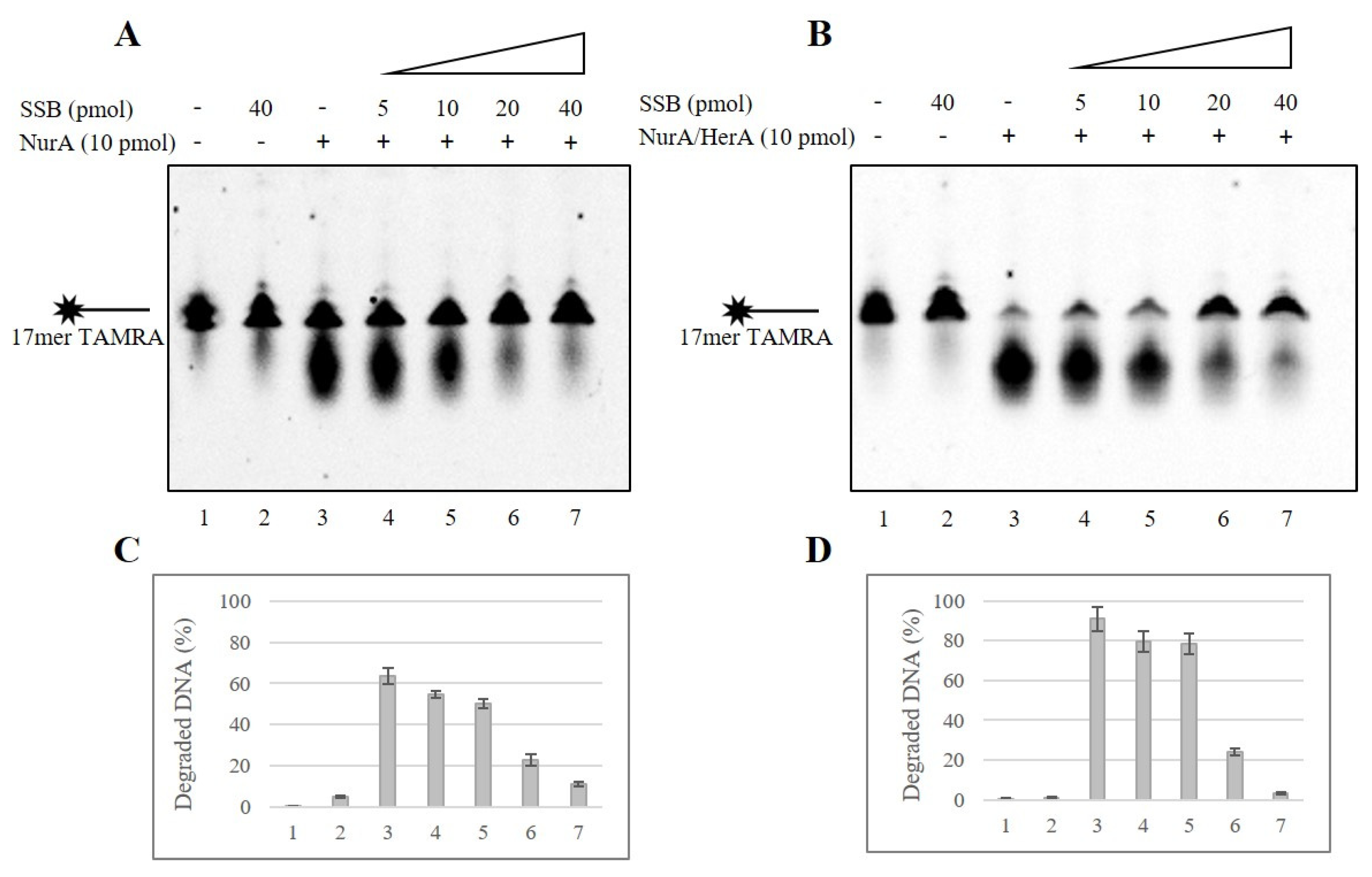
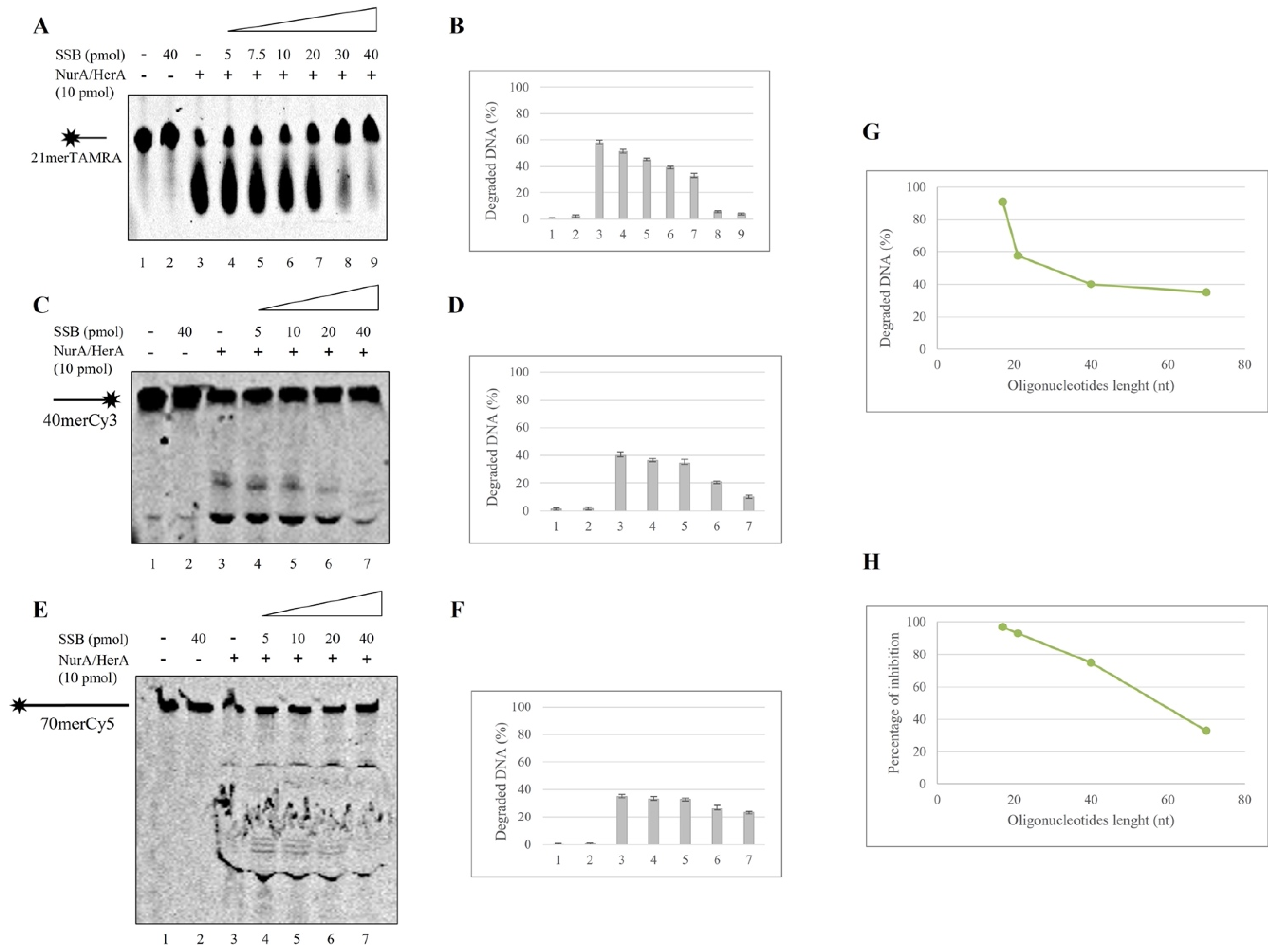
2.2. SSB Effect on NurA Exonuclease Activity on Different DNA Substrates
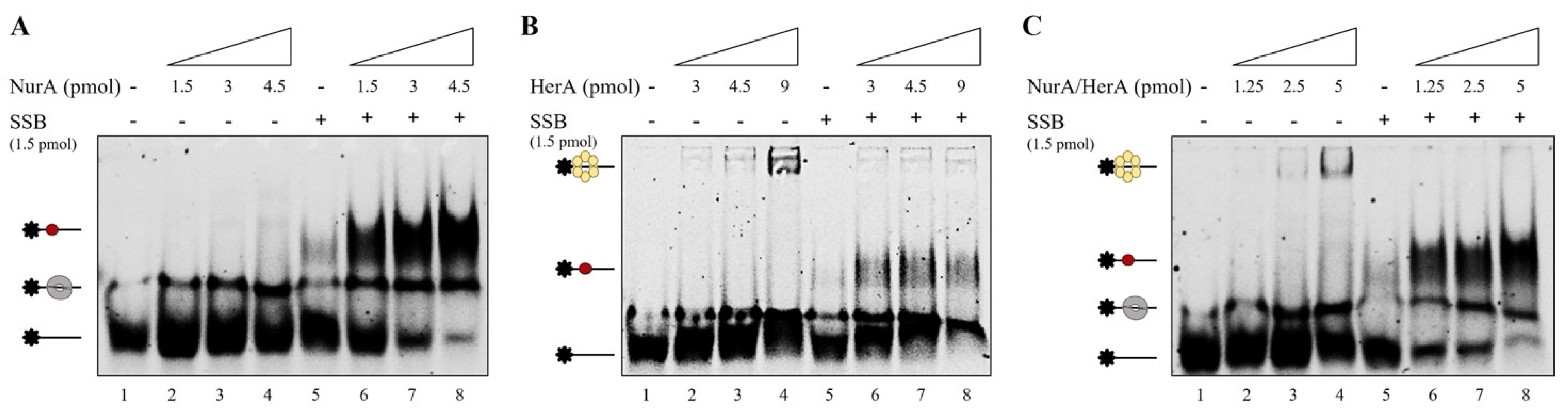
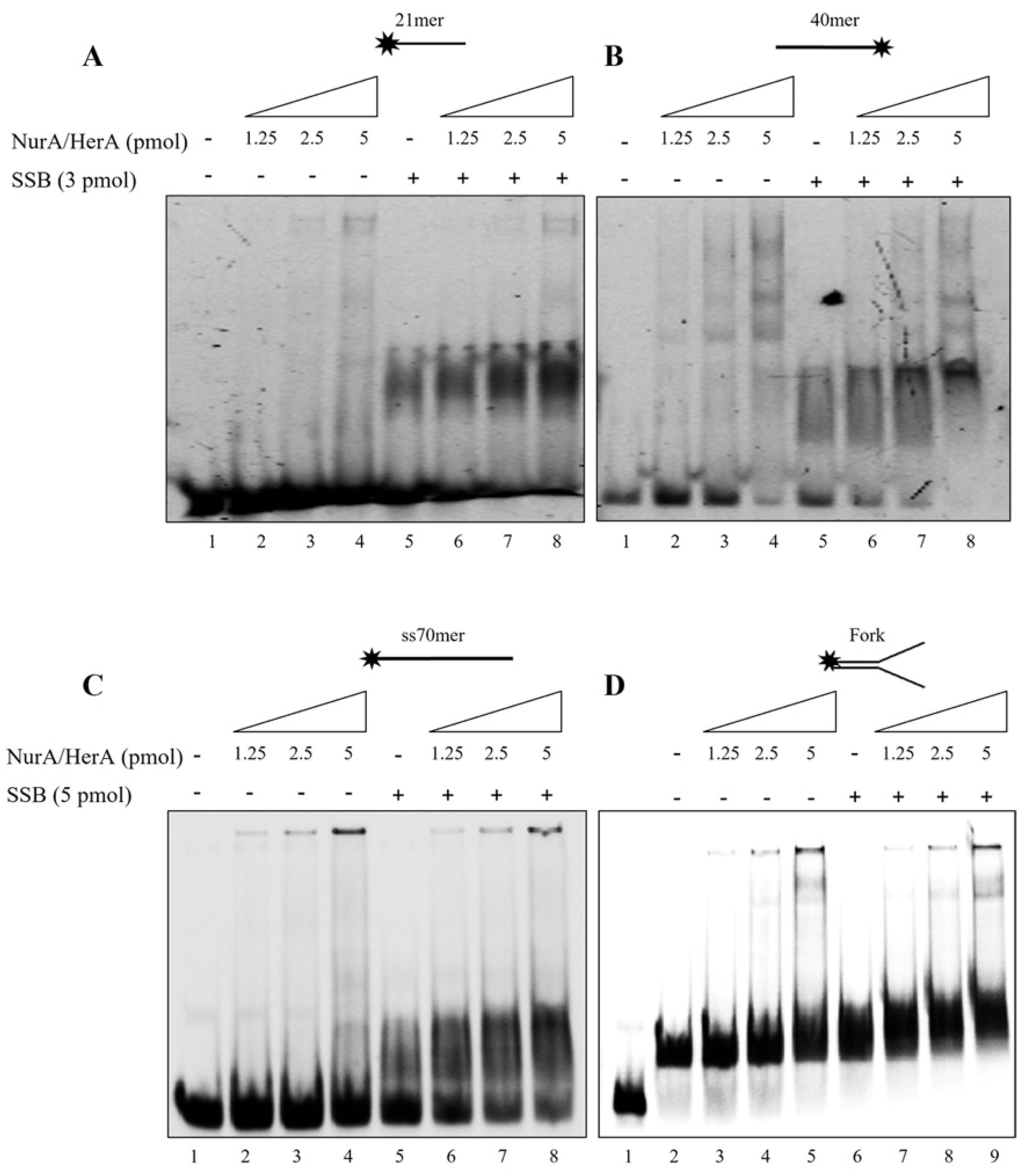
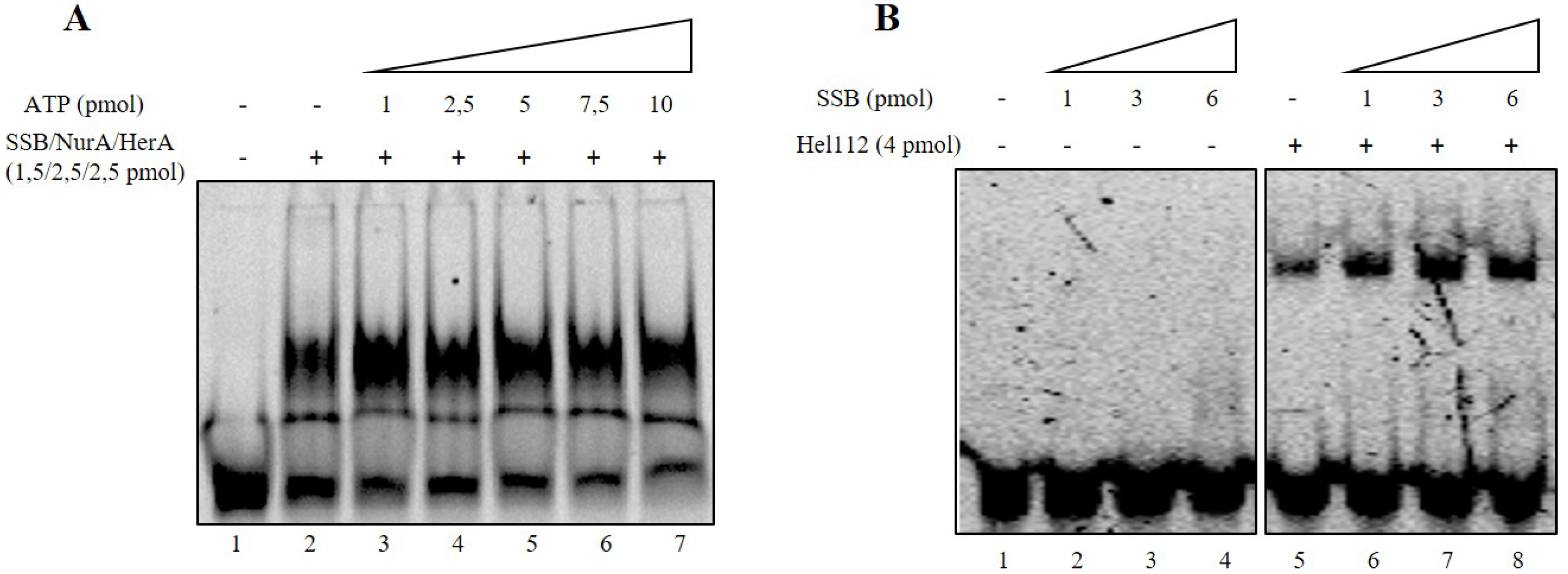
2.3. Mutual Influence of NurA, HerA and SSB on Their Binding onto the DNA
2.4. SSB Does Not Have Any Effect on HerA Helicase Activity
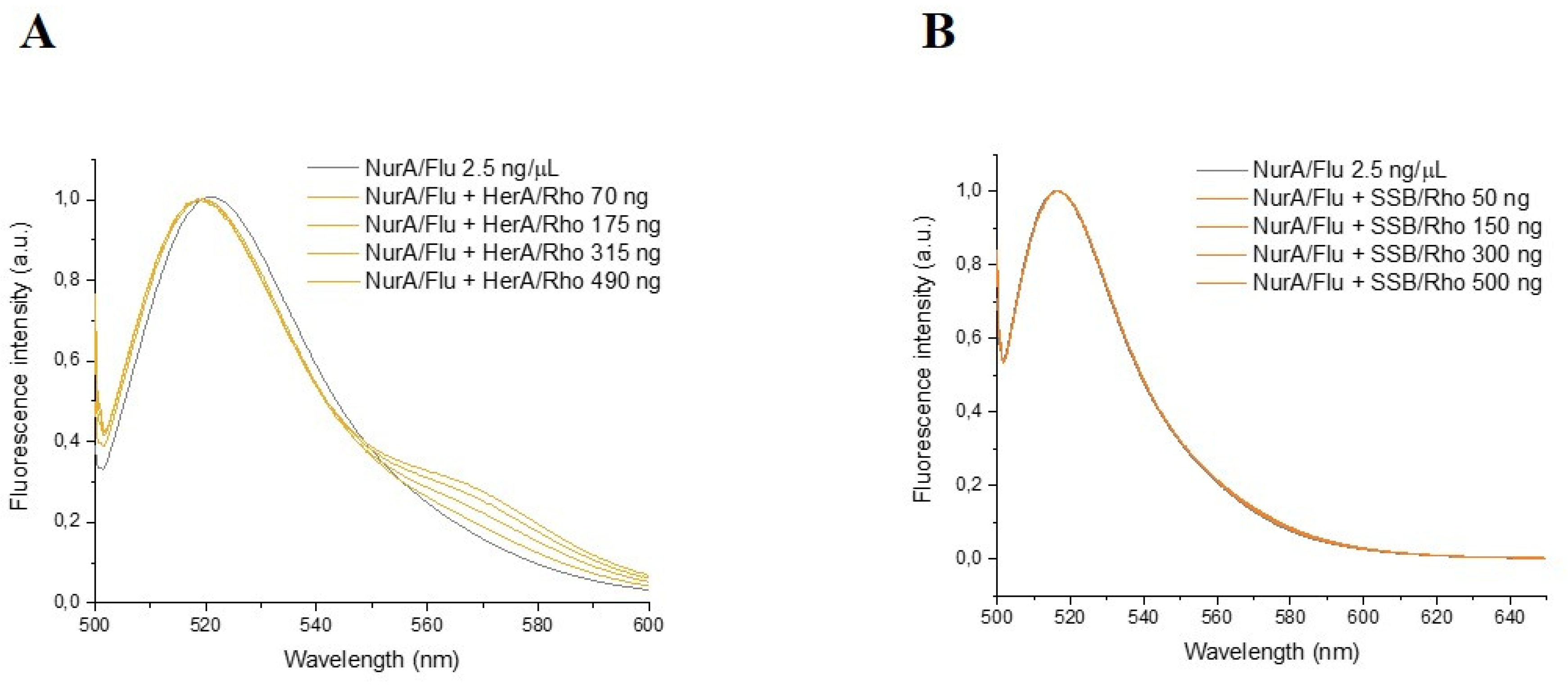
2.5. SSB Showed No Interaction with NurA
3. Materials and Methods
3.1. Chemicals
3.2. Expression and Purification of Recombinant Proteins
3.3. Substrate Preparation
| Name | Sequence |
|---|---|
| 17 merTAMRA | 5′-GTTTTCCCAGTCACGAC-3′ |
| 21 merTAMRA | 5′-GCTATCGTACATGATATCCTC-3′ |
| 40 merCy3 | 5′-GCCGTGATCACCAATGCAGATTGACGAACCTTTGCCCACGT-3′ |
| 70 lagFork | 5′-TTTTTTTTTTTTTTTTTTTTCACACTCACTTAAGCCGAATTCTTAGGGTTAGGGTTAACATCAAGTCACG-3′ |
| 70 leadCy5 | 5′-CGTGACTTGATGTTAACCCTAACCCTAAGAATTCGGCTTAAGTGAGTGTGAGGATATCATGTACGATAGC-3′ |
3.4. Endonuclease Assay
3.5. Exonuclease Assay
3.6. DNA Band-Shift Assays
3.7. DNA Helicase Assay
3.8. Absorption Spectroscopy
3.9. Labelling of NurA, HerA and SSB with Fluorescein and Rhodamine
3.10. Determination of the Degree of Labeling
3.11. Fluorescence Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, A.; Haber, J.E. Sources of DNA Double-Strand Breaks and Models of Recombinational DNA Repair. Cold Spring Harb. Perspect. Biol. 2014, 6, a016428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, J.R.; Taylor, M.R.; Boulton, S.J. Playing the End Game: DNA Double-Strand Break Repair Pathway Choice. Mol. Cell 2012, 47, 497–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandsma, I.; van Gent, D.C. Pathway choice in DNA double strand break repair: Observations of a balancing act. Genome Integr. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, K.; Rothstein, R. At Loose Ends: Resecting a Double-Strand Break. Cell 2009, 137, 807–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longhese, M.P.; Bonetti, D.; Manfrini, N.; Clerici, M. Mechanisms and regulation of DNA end resection. EMBO J. 2010, 29, 2864–2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimitou, E.P.; Symington, L.S. DNA end resection: Many nucleases make light work. DNA Repair 2009, 8, 983–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, H.; Chung, W.-H.; Zhu, Z.; Kwon, Y.; Zhao, W.; Chi, P.; Prakash, R.; Seong, C.; Liu, D.; Lu, L.; et al. Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae. Nature 2010, 467, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Lengsfeld, B.M.; Rattray, A.J.; Bhaskara, V.; Ghirlando, R.; Paull, T.T. Sae2 Is an Endonuclease that Processes Hairpin DNA Cooperatively with the Mre11/Rad50/Xrs2 Complex. Mol. Cell 2007, 28, 638–651. [Google Scholar] [CrossRef] [Green Version]
- Nicolette, M.L.; Lee, K.; Guo, Z.; Rani, M.; Chow, J.M.; Lee, S.E.; Paull, T.T. Mre11–Rad50–Xrs2 and Sae2 promote 5′ strand resection of DNA double-strand breaks. Nat. Struct. Mol. Biol. 2010, 17, 1478–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimitou, E.P.; Symington, L.S. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 2008, 455, 770–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cejka, P.; Cannavo, E.; Polaczek, P.; Masuda-Sasa, T.; Pokharel, S.; Campbell, J.L.; Kowalczykowski, S.C. DNA end resection by Dna2–Sgs1–RPA and its stimulation by Top3–Rmi1 and Mre11–Rad50–Xrs2. Nature 2010, 467, 112–116. [Google Scholar] [CrossRef]
- Broderick, R.; Nieminuszczy, J.; Baddock, H.T.; Deshpande, R.A.; Gileadi, O.; Paull, T.T.; McHugh, P.J.; Niedzwiedz, W. EXD2 promotes homologous recombination by facilitating DNA end resection. Nat. Cell Biol. 2016, 18, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Gravel, S.; Chapman, J.R.; Magill, C.; Jackson, S.P. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 2008, 22, 2767–2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Chung, W.-H.; Shim, E.Y.; Lee, S.E.; Ira, G. Sgs1 Helicase and Two Nucleases Dna2 and Exo1 Resect DNA Double-Strand Break Ends. Cell 2008, 134, 981–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Hua, Y.; Li, R.; Kong, D. Cdc24 Is Essential for Long-range End Resection in the Repair of Double-stranded DNA Breaks. J. Biol. Chem. 2016, 291, 24961–24973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannavo, E.; Cejka, P.; Kowalczykowski, S.C. Relationship of DNA degradation by Saccharomyces cerevisiae Exonuclease 1 and its stimulation by RPA and Mre11-Rad50-Xrs2 to DNA end resection. Proc. Natl. Acad. Sci. USA 2013, 110, E1661–E1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Pourmal, S.; Pavletich, N.P. Dna2 nuclease-helicase structure, mechanism and regulation by Rpa. eLife 2015, 4, e09832. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Bi, L.; Hou, X.-M.; Zhang, S.; Zhang, X.; Lu, Y.; Li, M.; Modesti, M.; Xi, X.-G.; Sun, B. Human RPA activates BLM’s bidirectional DNA unwinding from a nick. eLife 2020, 9, e54098. [Google Scholar] [CrossRef]
- Nimonkar, A.V.; Genschel, J.; Kinoshita, E.; Polaczek, P.; Campbell, J.L.; Wyman, C.; Modrich, P.; Kowalczykowski, S.C. BLM–DNA2–RPA–MRN and EXO1–BLM–RPA–MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 2011, 25, 350–362. [Google Scholar] [CrossRef] [Green Version]
- Constantinesco, F.; Forterre, P.; Elie, C. NurA, a novel 5′–3′ nuclease gene linked to rad50 and mre11 homologs of thermophilic Archaea. EMBO Rep. 2002, 3, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Constantinesco, F. A bipolar DNA helicase gene, herA, clusters with rad50, mre11 and nurA genes in thermophilic archaea. Nucleic Acids Res. 2004, 32, 1439–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaiser, A.; Constantinesco, F.; White, M.F.; Forterre, P.; Elie, C. The Mre11 protein interacts with both Rad50 and the HerA bipolar helicase and is recruited to DNA following gamma irradiation in the archaeon Sulfolobus acidocaldarius. BMC Mol. Biol. 2008, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wei, T.; Hou, G.; Zhang, C.; Liang, P.; Ni, J.; Sheng, D.; Shen, Y. Archaeal DNA helicase HerA interacts with Mre11 homologue and unwinds blunt-ended double-stranded DNA and recombination intermediates. DNA Repair 2008, 7, 380–391. [Google Scholar] [CrossRef]
- Rolfsmeier, M.L.; Laughery, M.F.; Haseltine, C.A. Repair of DNA Double-Strand Breaks following UV Damage in Three Sulfolobus solfataricus Strains. J. Bacteriol. 2010, 192, 4954–4962. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, B.B.; Paull, T.T. The P. furiosus mre11/rad50 complex promotes 5’ strand resection at a DNA double-strand break. Cell 2008, 135, 250–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.; Chen, X.; Xu, G.; Wang, L.; Xu, H.; Yang, S.; Zhao, Y.; Hua, Y. Biochemical and Functional Characterization of the NurA-HerA Complex from Deinococcus radiodurans. J. Bacteriol. 2015, 197, 2048–2061. [Google Scholar] [CrossRef] [Green Version]
- Blackwood, J.K.; Rzechorzek, N.J.; Abrams, A.S.; Maman, J.D.; Pellegrini, L.; Robinson, N.P. Structural and functional insights into DNA-end processing by the archaeal HerA helicase–NurA nuclease complex. Nucleic Acids Res. 2012, 40, 3183–3196. [Google Scholar] [CrossRef] [Green Version]
- De Falco, M.; Catalano, F.; Rossi, M.; Ciaramella, M.; De Felice, M. NurA Is Endowed with Endo- and Exonuclease Activities that Are Modulated by HerA: New Insight into Their Role in DNA-End Processing. PLoS ONE 2015, 10, e0142345. [Google Scholar] [CrossRef]
- Chédin, F.; Seitz, E.M.; Kowalczykowski, S.C. Novel homologs of replication protein A in archaea: Implications for the evolution of ssDNA-binding proteins. Trends Biochem. Sci. 1998, 23, 273–277. [Google Scholar] [CrossRef]
- Bernstein, D.A.; Eggington, J.M.; Killoran, M.P.; Misic, A.M.; Cox, M.M.; Keck, J.L. Crystal structure of the Deinococcus radiodurans single-stranded DNA-binding protein suggests a mechanism for coping with DNA damage. Proc. Natl. Acad. Sci. USA 2004, 101, 8575–8580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.; Keck, J.L. Structural basis of Escherichia coli single-stranded DNA-binding protein stimulation of exonuclease I. Proc. Natl. Acad. Sci. USA 2008, 105, 9169–9174. [Google Scholar] [CrossRef] [Green Version]
- Richard, D.J.; Bell, S.D.; White, M.F. Physical and functional interaction of the archaeal single-stranded DNA-binding protein SSB with RNA polymerase. Nucleic Acids Res. 2004, 32, 1065–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.V.; Varshney, U. Contrasting effects of single stranded DNA binding protein on the activity of uracil DNA glycosylase from Escherichia coli towards different DNA substrates. Nucleic Acids Res. 1997, 25, 2336–2343. [Google Scholar] [CrossRef] [Green Version]
- Shereda, R.D.; Bernstein, D.A.; Keck, J. A Central Role for SSB in Escherichia coli RecQ DNA Helicase Function. J. Biol. Chem. 2007, 282, 19247–19258. [Google Scholar] [CrossRef] [Green Version]
- He, Z.-G.; Richardson, C.C. Effect of Single-stranded DNA-binding Proteins on the Helicase and Primase Activities of the Bacteriophage T7 Gene 4 Protein. J. Biol. Chem. 2004, 279, 22190–22197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butland, G.; Alvarez, J.; Li, J.; Yang, W.; Yang, X.; Canadien, V.; Starostine, A.; Richards, D.; Beattie, B.; Krogan, N.; et al. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 2005, 433, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Han, E.S.; Cooper, D.L.; Persky, N.S.; Sutera, V.A., Jr.; Whitaker, R.D.; Montello, M.L.; Lovett, S.T. RecJ exonuclease: Substrates, products and interaction with SSB. Nucleic Acids Res. 2006, 34, 1084–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Rao, D.N. Orchestration of Haemophilus influenzae RecJ Exonuclease by Interaction with Single-Stranded DNA-Binding Protein. J. Mol. Biol. 2009, 385, 1375–1396. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, S.; Zhu, S.; Sheng, D.; Ni, J.; Shen, Y. Physical and functional interaction between archaeal single-stranded DNA-binding protein and the 5′–3′ nuclease NurA. Biochem. Biophys. Res. Commun. 2008, 367, 523–529. [Google Scholar] [CrossRef] [PubMed]
- De Falco, M.; Massa, F.; Rossi, M.; De Felice, M. The Sulfolobus solfataricus RecQ-like DNA helicase Hel112 inhibits the NurA/HerA complex exonuclease activity. Extremophiles 2018, 22, 581–589. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, L.; Liu, J.; Ni, J.; She, Q.; Shen, Y. Efficient 5′-3′ DNA end resection by HerA and NurA is essential for cell viability in the crenarchaeon Sulfolobus islandicus. BMC Mol. Biol. 2015, 16, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Felice, M.; Aria, V.; Esposito, L.; De Falco, M.; Pucci, B.; Rossi, M.; Pisani, F.M. A novel DNA helicase with strand-annealing activity from the crenarchaeon Sulfolobus solfataricus. Biochem. J. 2007, 408, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Valenti, A.; De Felice, M.; Perugino, G.; Bizard, A.; Nadal, M.; Rossi, M.; Ciaramella, M. Synergic and Opposing Activities of Thermophilic RecQ-like Helicase and Topoisomerase 3 Proteins in Holliday Junction Processing and Replication Fork Stabilization. J. Biol. Chem. 2012, 287, 30282–30295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzan, A.; Pfeiffer, G.; Hefferin, M.L.; Lang, C.E.; Carney, J.P.; Hopfner, K.P. MlaA, a hexameric ATPase linked to the Mre11 complex in archaeal genomes. EMBO Rep. 2004, 5, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.; Shin, S.; Uhm, H.; Hong, H.; Kirk, J.; Hyun, K.; Kulikowicz, T.; Kim, J.; Ahn, B.; Bohr, V.A.; et al. Multiple RPAs make WRN syndrome protein a superhelicase. Nucleic Acids Res. 2018, 46, 4689–4698. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, R.I.M. Identification and properties of the crenarchaeal single-stranded DNA binding protein from Sulfolobus solfataricus. Nucleic Acids Res. 2001, 29, 914–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Falco, M.; De Felice, M. Take a Break to Repair: A Dip in the World of Double-Strand Break Repair Mechanisms Pointing the Gaze on Archaea. Int. J. Mol. Sci. 2021, 22, 13296. [Google Scholar] [CrossRef]
- Yan, H.; Toczylowski, T.; McCane, J.; Chen, C.; Liao, S. Replication protein A promotes 5′-3′ end processing during homology-dependent DNA double-strand break repair. J. Cell Biol. 2011, 192, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Falco, M.; Porritiello, A.; Rota, F.; Scognamiglio, V.; Antonacci, A.; del Monaco, G.; De Felice, M. The Finely Coordinated Action of SSB and NurA/HerA Complex Strictly Regulates the DNA End Resection Process in Saccharolobus solfataricus. Int. J. Mol. Sci. 2022, 23, 2582. https://doi.org/10.3390/ijms23052582
De Falco M, Porritiello A, Rota F, Scognamiglio V, Antonacci A, del Monaco G, De Felice M. The Finely Coordinated Action of SSB and NurA/HerA Complex Strictly Regulates the DNA End Resection Process in Saccharolobus solfataricus. International Journal of Molecular Sciences. 2022; 23(5):2582. https://doi.org/10.3390/ijms23052582
Chicago/Turabian StyleDe Falco, Mariarosaria, Alessandra Porritiello, Federica Rota, Viviana Scognamiglio, Amina Antonacci, Giovanni del Monaco, and Mariarita De Felice. 2022. "The Finely Coordinated Action of SSB and NurA/HerA Complex Strictly Regulates the DNA End Resection Process in Saccharolobus solfataricus" International Journal of Molecular Sciences 23, no. 5: 2582. https://doi.org/10.3390/ijms23052582
APA StyleDe Falco, M., Porritiello, A., Rota, F., Scognamiglio, V., Antonacci, A., del Monaco, G., & De Felice, M. (2022). The Finely Coordinated Action of SSB and NurA/HerA Complex Strictly Regulates the DNA End Resection Process in Saccharolobus solfataricus. International Journal of Molecular Sciences, 23(5), 2582. https://doi.org/10.3390/ijms23052582










