Chemical Compounds Released from Specific Osteoinductive Bioactive Materials Stimulate Human Bone Marrow Mesenchymal Stem Cell Migration
Abstract
:1. Introduction
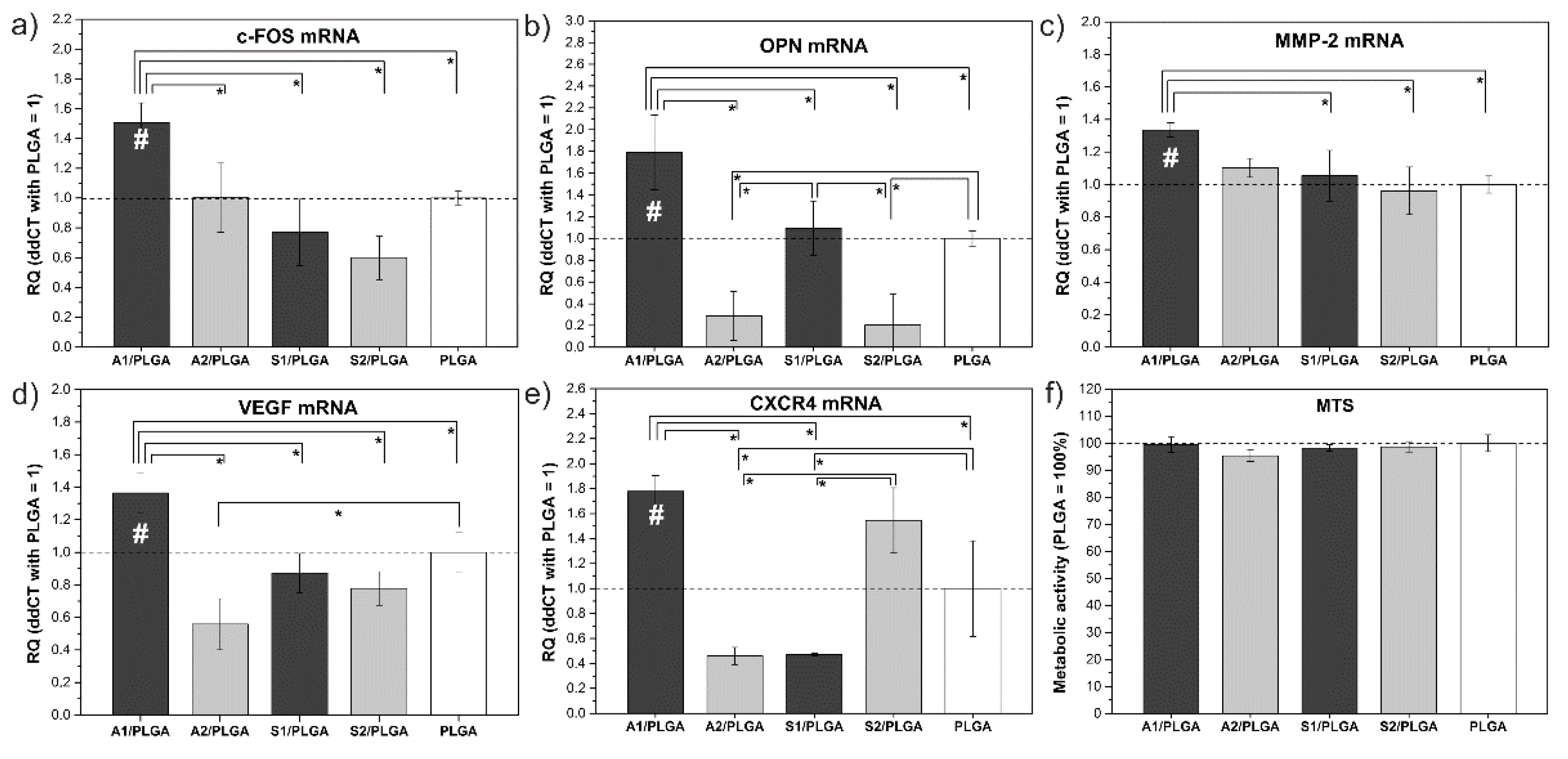
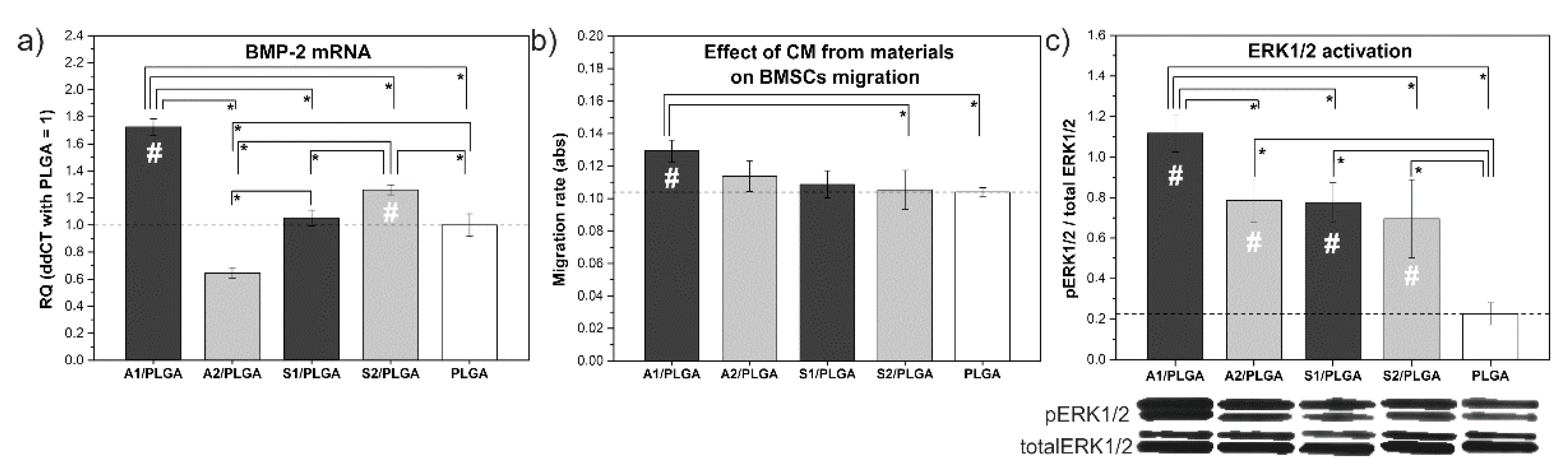
2. Results
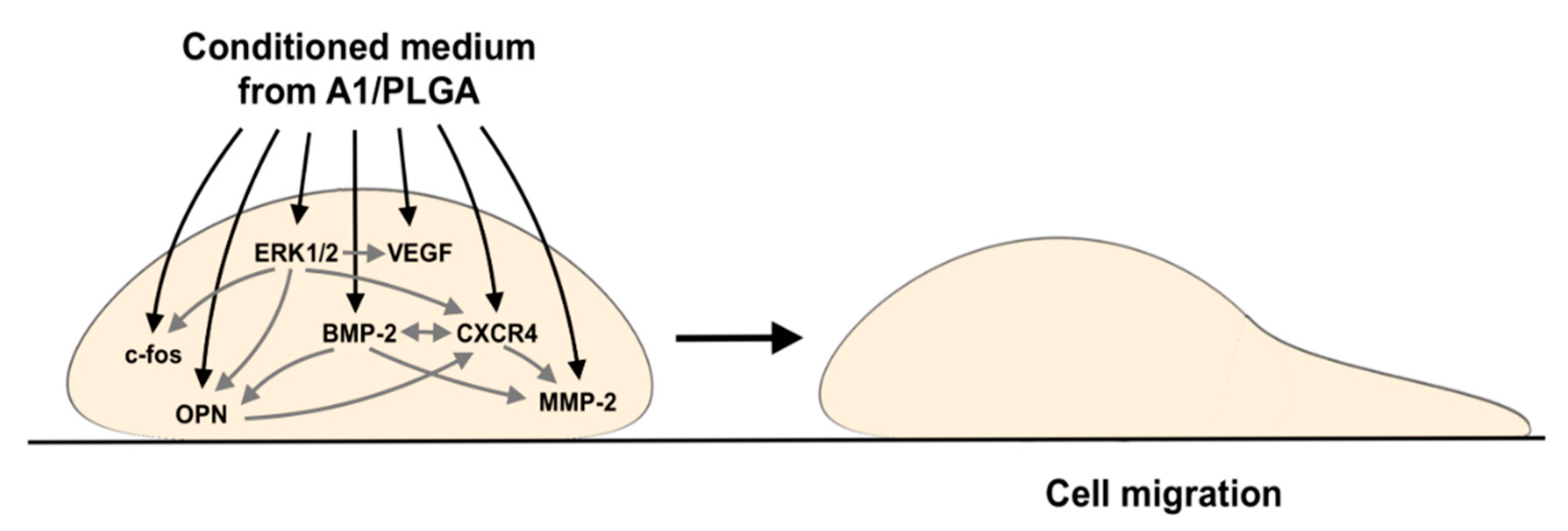
3. Discussion
4. Materials and Methods
4.1. Composite Components
4.2. Composite Sheets Fabrication
4.3. HBMSC Isolation and Culture Expansion
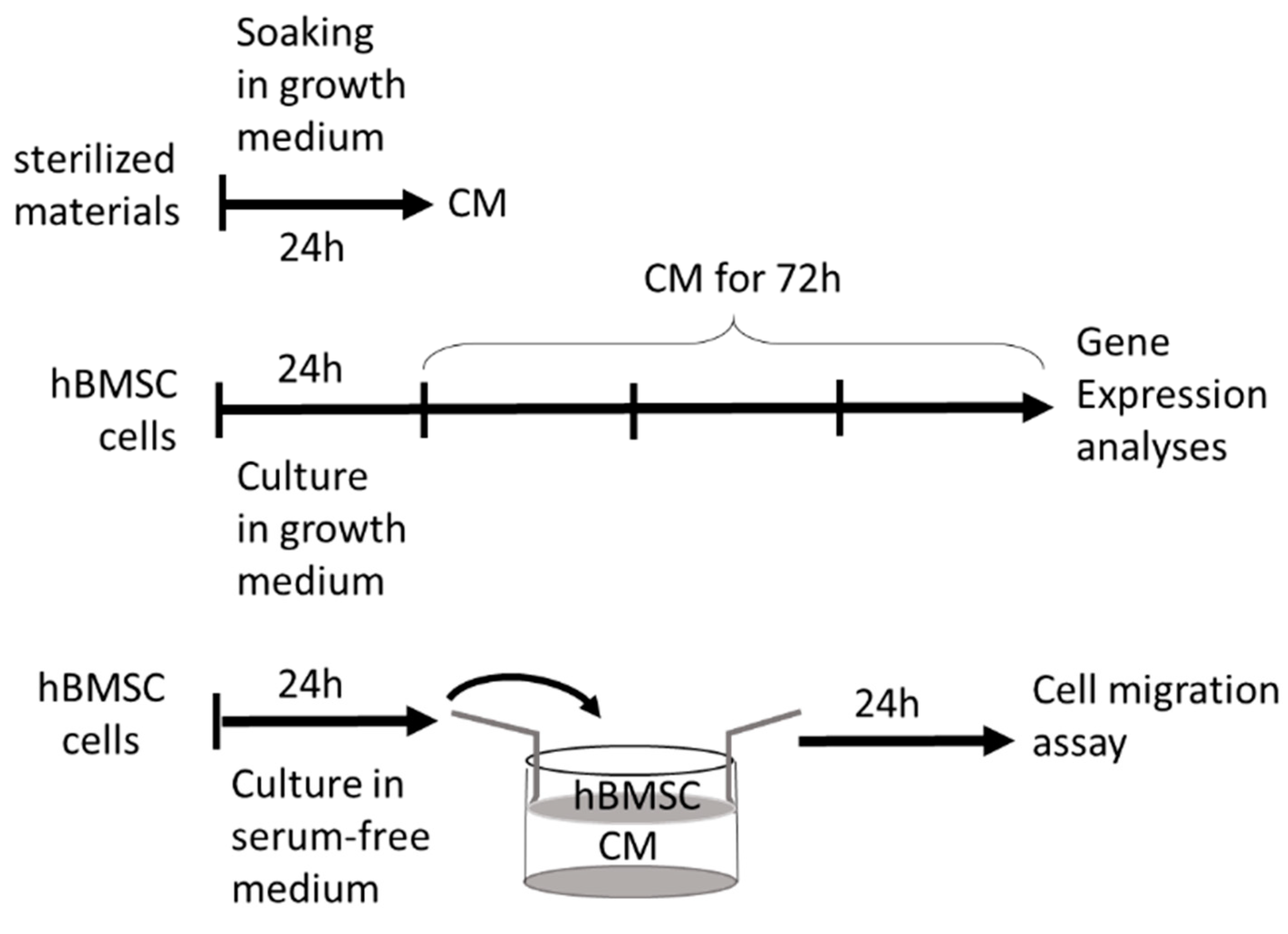
4.4. Cultures in the Presence of CMs Harvested from the Materials
4.5. Migration Studies
4.6. Metabolic Activity of Cells
4.7. Gene Expression Analyses
4.8. Western Blot Analyses
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, P.; Tian, Y.; Yang, C.; Ma, X.; Wang, X.; Pei, J.; Qian, A. Mesenchymal Stem Cell Migration during Bone Formation and Bone Diseases Therapy. Int. J. Mol. Sci. 2018, 19, 2343. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Xu, L.; Zwingenberger, S.; Gibon, E.; Goodman, S.B.; Li, G. Mesenchymal Stem Cells Homing to Improve Bone Healing. J. Orthop. Transl. 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Villalobo, A.; Berchtold, M.W. The Role of Calmodulin in Tumor Cell Migration, Invasiveness, and Metastasis. Int. J. Mol. Sci. 2020, 21, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Zheng, L.; Jiang, J.; Gui, J.; Zhang, L.; Huang, Y.; Chen, X.; Ji, J.; Fan, Y. Calcium Hydroxide-Induced Proliferation, Migration, Osteogenic Differentiation, and Mineralization via the Mitogen-Activated Protein Kinase Pathway in Human Dental Pulp Stem Cells. J. Endod. 2016, 42, 1355–1361. [Google Scholar] [CrossRef]
- Aquino-Martínez, R.; Angelo, A.P.; Pujol, F.V. Calcium-Containing Scaffolds Induce Bone Regeneration by Regulating Mesenchymal Stem Cell Differentiation and Migration. Stem Cell Res. Ther. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamula, E.; Kokoszka, J.; Cholewa-Kowalska, K.; Laczka, M.; Kantor, L.; Niedzwiedzki, L.; Reilly, G.C.; Filipowska, J.; Madej, W.; Kolodziejczyk, M.; et al. Degradation, Bioactivity, and Osteogenic Potential of Composites Made of PLGA and Two Different Sol–Gel Bioactive Glasses. Ann. Biomed. Eng. 2011, 39, 2114. [Google Scholar] [CrossRef] [Green Version]
- Łukowicz, K.; Zagrajczuk, B.; Nowak, A.; Niedźwiedzki, Ł.; Laczka, M.; Cholewa-Kowalska, K.; Osyczka, A.M. The Role of CaO/SiO2 Ratio and P2O5 Content in Gel-Derived Bioactive Glass-Polymer Composites in the Modulation of Their Bioactivity and Osteoinductivity in Human BMSCs. Mater. Sci. Eng. C 2020, 109, 110535. [Google Scholar] [CrossRef]
- Ghaffari-Nazari, H. The Known Molecules Involved in MSC Homing and Migration. Res. Artic. J. Stem Cell Res. Med. 2018, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [Green Version]
- Ju, L.; Zhou, Z.; Jiang, B.; Lou, Y.; Guo, X. Autocrine VEGF and IL-8 Promote Migration via Src/Vav2/Rac1/PAK1 Signaling in Human Umbilical Vein Endothelial Cells. Cell. Physiol. Biochem. Int. J. Exp. 2017, 41, 1346–1359. [Google Scholar] [CrossRef]
- Haque, N.; Fareez, I.M.; Fong, L.F.; Mandal, C.; Kasim, N.H.A.; Kacharaju, K.R.; Soesilawati, P. Role of the CXCR4-SDF1-HMGB1 Pathway in the Directional Migration of Cells and Regeneration of Affected Organs. World J. Stem Cells 2020, 12, 938. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Jiang, L.; Li, Z.; Lee, S.; Liu, C.; Wang, J.; Zhang, J. Recombinant Human BMP-2 Accelerates the Migration of Bone Marrow Mesenchymal Stem Cells via the CDC42/PAK1/LIMK1 Pathway in Vitro and in Vivo. Biomater. Sci. 2018, 7, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.N.; Hwang, H.S.; Oh, S.H.; Roshanzadeh, A.; Kim, J.W.; Song, J.H.; Kim, E.S.; Koh, J.T. Elevated Extracellular Calcium Ions Promote Proliferation and Migration of Mesenchymal Stem Cells via Increasing Osteopontin Expression. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.C.; Shi, X.L.; Ren, H.Z.; Yuan, X.W.; Ding, Y.T. Targeted Migration of Mesenchymal Stem Cells Modified with CXCR4 to Acute Failing Liver Improves Liver Regeneration. World J. Gastroenterol. 2014, 20, 14884–14894. [Google Scholar] [CrossRef]
- Bhakta, S.; Hong, P.; Koc, O. The Surface Adhesion Molecule CXCR4 Stimulates Mesenchymal Stem Cell Migration to Stromal Cell-Derived Factor-1 in Vitro but Does Not Decrease Apoptosis under Serum Deprivation. Cardiovasc. Eevascularization Med. Incl. Mol. Interv. 2006, 7, 19–24. [Google Scholar] [CrossRef]
- Jin, W.; Liang, X.; Brooks, A.; Futrega, K.; Liu, X.; Doran, M.R.; Simpson, M.J.; Roberts, M.S.; Wang, H. Modelling of the SDF-1/CXCR4 Regulated in Vivo Homing of Therapeutic Mesenchymal Stem/Stromal Cells in Mice. PeerJ 2018, 6, e6072. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-Ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Almalki, S.G.; Agrawal, D.K. Effects of Matrix Metalloproteinases on the Fate of Mesenchymal Stem Cells. Stem Cell Res. Ther. 2016, 7, 129. [Google Scholar] [CrossRef] [Green Version]
- Rashid, F.; Shiba, H.; Mizuno, N.; Mouri, Y.; Fujita, T.; Shinohara, H.; Ogawa, T.; Kawaguchi, H.; Kurihara, H. The Effect of Extracellular Calcium Ion on Gene Expression of Bone-Related Proteins in Human Pulp Cells. J. Endod. 2003, 29, 104–107. [Google Scholar] [CrossRef]
- Łukowicz, K.; Zagrajczuk, B.; Wieczorek, J.; Millan-Ciesielska, K.; Polkowska, I.; Cholewa-Kowalska, K.; Osyczka, A.M. Molecular Indicators of Biomaterials Osteoinductivity—Cell Migration, BMP Production and Signalling Turns a Key. Stem Cell Rev. Rep. 2021, 1–19. [Google Scholar] [CrossRef]
- Wu, Q.; Shao, H.; Darwin Eton, D.; Li, J.; Li, J.; Yang, B.; Webster, K.A.; Yu, H. Extracellular Calcium Increases CXCR4 Expression on Bone Marrow-Derived Cells and Enhances pro-Angiogenesis Therapy. J. Cell. Mol. Med. 2009, 13, 3764–3773. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Detsch, R.; Esteban-Tejeda, L.; Grünewald, A.; Moya, J.S.; Boccaccini, A.R. Influence of Dissolution Products of a Novel Ca-Enriched Silicate Bioactive Glass-Ceramic on VEGF Release from Bone Marrow Stromal Cells. Biomed. Glasses 2017, 3, 104–110. [Google Scholar] [CrossRef]
- Barradas, A.M.C.; Fernandes, H.A.M.; Groen, N.; Chai, Y.C.; Schrooten, J.; van de Peppel, J.; van Leeuwen, J.P.T.M.; van Blitterswijk, C.A.; de Boer, J. A Calcium-Induced Signaling Cascade Leading to Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stromal Cells. Biomaterials 2012, 33, 3205–3215. [Google Scholar] [CrossRef]
- Tezvergil-Mutluay, A.; Agee, K.A.; Hoshika, T.; Carrilho, M.; Breschi, L.; Tjäderhane, L.; Nishitani, Y.; Carvalho, R.M.; Looney, S.; Tay, F.R.; et al. The Requirement of Zinc and Calcium Ions for Functional MMP Activity in Demineralized Dentin Matrices. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2010, 26, 1059–1067. [Google Scholar] [CrossRef] [Green Version]
- Dziadek, M.; Zagrajczuk, B.; Menaszek, E.; Cholewa-Kowalska, K. A New Insight into in Vitro Behaviour of Poly(ε-Caprolactone)/Bioactive Glass Composites in Biologically Related Fluids. J. Mater. Sci. 2018, 53, 3939–3958. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.N.; Lin, J.H.; Cheng, Y.R.; Zhang, L.; Huang, J.; Wu, Z.Y.; Wang, F.S.; Xu, S.G.; Lin, W.P.; Lan, W.B.; et al. FIM-A, a Phosphorus-Containing Sirolimus, Inhibits the Angiogenesis and Proliferation of Osteosarcomas. Oncol. Res. 2013, 20, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Veerendhar, A.; Reich, R.; Breuer, E. Phosphorus Based Inhibitors of Matrix Metalloproteinases. Comptes Rendus Chim. 2010, 13, 1191–1202. [Google Scholar] [CrossRef]
- Han, P.; Wu, C.; Xiao, Y. The Effect of Silicate Ions on Proliferation, Osteogenic Differentiation and Cell Signalling Pathways (WNT and SHH) of Bone Marrow Stromal Cells. Biomater. Sci. 2013, 1, 379–392. [Google Scholar] [CrossRef]
- Cho, Y.J.; Seo, M.S.; Kim, J.K.; Lim, Y.; Chae, G.; Ha, K.S.; Lee, K.H. Silica-Induced Generation of Reactive Oxygen Species in Rat2 Fibroblast: Role in Activation of Mitogen-Activated Protein Kinase. Biochem. Biophys. Res. Commun. 1999, 262, 708–712. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Jin, Z.; Jiang, H.; Wen, J. Silica-Induced TNF-α and TGF-Β1 Expression in RAW264.7 Cells Are Dependent on Src-ERK/AP-1 Pathways. Toxicol. Mech. Methods 2008, 19, 51–58. [Google Scholar] [CrossRef]
- Kim, K.J.; Joe, Y.A.; Kim, M.K.; Lee, S.J.; Ryu, Y.H.; Cho, D.W.; Rhie, J.W. Silica Nanoparticles Increase Human Adipose Tissue-Derived Stem Cell Proliferation through ERK1/2 Activation. Int. J. Nanomed. 2015, 10, 2261–2272. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, Y.; Luo, M.; Mäkilä, E.; Day, B.W.; Voelcker, N.H.; Tong, W.Y. Effectiveness of Porous Silicon Nanoparticle Treatment at Inhibiting the Migration of a Heterogeneous Glioma Cell Population. J. Nanobiotechnology 2021, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; de Santis Puzzonia, M.; Ricci, R.; Aureli, F.; Guarguaglini, G.; Cubadda, F.; Leyns, L.; Cundari, E.; Kirsch-Volders, M. Amorphous Silica Nanoparticles Alter Microtubule Dynamics and Cell Migration. Nanotoxicology 2015, 9, 729–736. [Google Scholar] [CrossRef]
- Tanimura, S.; Takeda, K. ERK Signalling as a Regulator of Cell Motility. J. Biochem. 2017, 162, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, C.; Luo, Q.; Qin, J.; Shi, Y.; Yang, L.; Ju, B.; Song, G. Osteopontin Promotes Mesenchymal Stem Cell Migration and Lessens Cell Stiffness via Integrin Β1, FAK, and ERK Pathways. Cell Biochem. Biophys. 2013, 65, 455–462. [Google Scholar] [CrossRef]
- Busillo, J.M.; Benovic, J.L. Regulation of CXCR4 Signaling. Biochim. Et Biophys. Acta 2007, 1768, 952–963. [Google Scholar] [CrossRef] [Green Version]
- Fish, J.E.; Gutierrez, M.C.; Dang, L.T.; Khyzha, N.; Chen, Z.; Veitch, S.; Cheng, H.S.; Khor, M.; Antounians, L.; Njock, M.S.; et al. Dynamic Regulation of VEGF-Inducible Genes by an ERK/ERG/P300 Transcriptional Network. Development 2017, 144, 2428–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deyama, Y.; Matsumoto, A.; Yoshimura, Y.; Suzuki, K. The Effect of Calcium Ion on the C-FOS MRNA Expression in the Clonal Osteoblastic MC3T3-E1 Cells Derived from Mouse Calvaria. Oral Ther. Pharmacol. 1995, 14, 132–137. [Google Scholar]
- Li, Z.; Wang, W.; Xu, H.; Ning, Y.; Fang, W.; Liao, W.; Zou, J.; Yang, Y.; Shao, N. Effects of Altered CXCL12/CXCR4 Axis on BMP2/Smad/Runx2/Osterix Axis and Osteogenic Gene Expressions during Osteogenic Differentiation of MSCs. Am. J. Transl. Res. 2017, 9, 1693. [Google Scholar]
- Dobrzynski, P.; Kasperczyk, J.; Janeczek, H.; Bero, M. Synthesis of Biodegradable Copolymers with the Use of Low Toxic Zirconium Compounds. 1. Copolymerization of Glycolide with l-Lactide Initiated by Zr(Acac)4. Macromolecules 2001, 34, 5090–5098. [Google Scholar] [CrossRef]
- Zagrajczuk, B.; Dziadek, M.; Olejniczak, Z.; Cholewa-Kowalska, K.; Laczka, M. Structural and Chemical Investigation of the Gel-Derived Bioactive Materials from the SiO2–CaO and SiO2-CaO-P2O5 Systems. Ceram. Int. 2017, 43, 12742–12754. [Google Scholar] [CrossRef]
| Material | Ions Concentration [mM/dm3] | ||
|---|---|---|---|
| Calcium Ions (Ca2+) | Phosphate Ions (PO43−) | Silicate Ions (SiO44−) | |
| A1/PLGA | 10.835 ± 0.047 | 0.637 ± 0.016 | 1.372 ± 0.030 |
| A2/PLGA | 8.404 ± 0.075 | 0.707± 0.013 | 1.835 ± 0.093 |
| S1/PLGA | 5.510 ± 0.025 | 1.180± 0.034 | 0.631 ± 0.006 |
| S2/PLGA | 2.465 ± 0.049 | 1.164 ± 0.030 | 1.205 ± 0.018 |
| PLGA | 0.674 ± 0.020 | 1.366 ± 0.046 | 0.002 ± 3.746 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukowicz, K.; Zagrajczuk, B.; Truchan, K.; Niedzwiedzki, Ł.; Cholewa-Kowalska, K.; Osyczka, A.M. Chemical Compounds Released from Specific Osteoinductive Bioactive Materials Stimulate Human Bone Marrow Mesenchymal Stem Cell Migration. Int. J. Mol. Sci. 2022, 23, 2598. https://doi.org/10.3390/ijms23052598
Łukowicz K, Zagrajczuk B, Truchan K, Niedzwiedzki Ł, Cholewa-Kowalska K, Osyczka AM. Chemical Compounds Released from Specific Osteoinductive Bioactive Materials Stimulate Human Bone Marrow Mesenchymal Stem Cell Migration. International Journal of Molecular Sciences. 2022; 23(5):2598. https://doi.org/10.3390/ijms23052598
Chicago/Turabian StyleŁukowicz, Krzysztof, Barbara Zagrajczuk, Karolina Truchan, Łukasz Niedzwiedzki, Katarzyna Cholewa-Kowalska, and Anna M. Osyczka. 2022. "Chemical Compounds Released from Specific Osteoinductive Bioactive Materials Stimulate Human Bone Marrow Mesenchymal Stem Cell Migration" International Journal of Molecular Sciences 23, no. 5: 2598. https://doi.org/10.3390/ijms23052598






