Microalgal Biorefinery Concepts’ Developments for Biofuel and Bioproducts: Current Perspective and Bottlenecks
Abstract
:1. Introduction
2. Biorefinery
2.1. Hypothesis
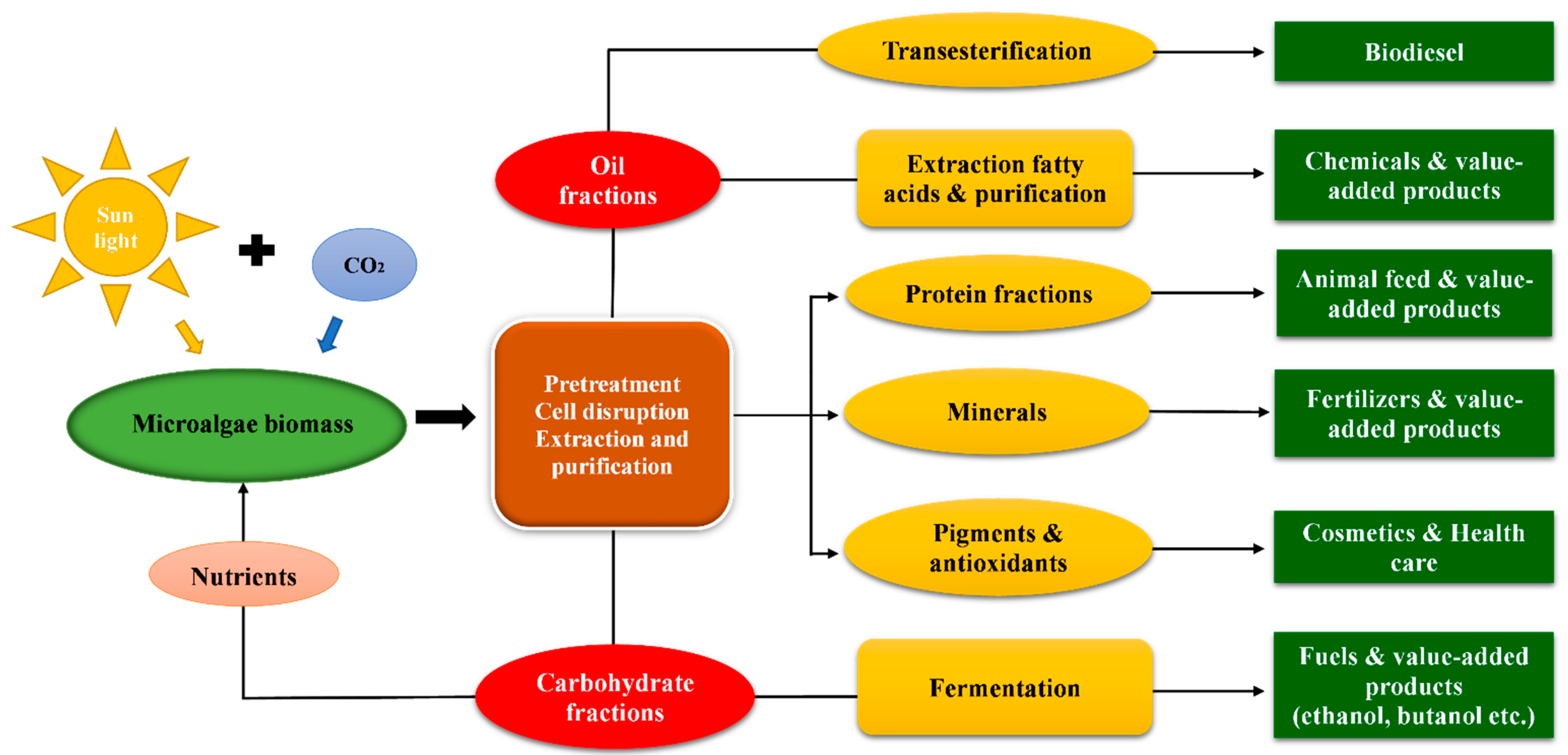
2.2. Microalgal Biorefinery
2.3. Transesterification
2.4. Photosynthetic Microbial Fuel Cells
2.5. Biochemical Conversion
2.5.1. Biogas Production
2.5.2. Bioethanol Production
2.5.3. Biohydrogen Production
2.6. Thermochemical Conversion
2.7. Bioplastics
2.8. Potentials of Microalgae for Biorefinery
3. Valuable Biochemical Compositions Available in Microalgae
3.1. Lipids
3.2. PUFA (Polyunsaturated Fatty Acids)
3.3. Carbohydrates
3.4. Proteins
3.5. Pigments
3.6. Microelements
4. Clinically Important Compounds
4.1. Anticancer Agents
4.2. Antiviral Compounds
4.3. Anti-Inflammatory Products
5. Ecological Valuable Compounds
6. Wastewater Treatment by Microalgae
7. Technological and Economic Analysis
8. Commercial Microalgal Products Available in the Market
9. Bottlenecks and Future Perspectives
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andrade, D.S.; Amaral, H.F.; Gavilanes, F.Z.; Morioka, L.R.I.; Nassar, J.M.; de Melo, J.M.; Silva, H.R.; Telles, T.S. Microalgae: Cultivation, Biotechnological, Environmental, and Agricultural Applications. In Advances in the Domain of Environmental Biotechnology; Maddela, N.R., Cruzatty, L.C.G., Chakraborty, S., Eds.; Springer: Singapore, 2021; pp. 635–701. [Google Scholar]
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.-H.; Show, P.L. Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour. Technol. 2020, 304, 122997. [Google Scholar] [CrossRef]
- Verma, R.; Kumari, K.V.L.K.; Srivastava, A.; Kumar, A. Photoautotrophic, mixotrophic, and heterotrophic culture media optimization for enhanced microalgae production. J. Environ. Chem. Eng. 2020, 8, 104149. [Google Scholar] [CrossRef]
- Baicha, Z.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Hernández-Fernández, F.J.; de los Ríos, A.P.; Labjar, N.; Lotfi, E.; Elmahi, M. A critical review on microalgae as an alternative source for bioenergy production: A promising low cost substrate for microbial fuel cells. Fuel Process. Technol. 2016, 154, 104–116. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Chang, J.-S.; Ling, T.C.; Juan, J.C. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol. 2015, 184, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Vonshak, A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Feng, S.; Hu, L.; Zhang, Q.; Zhang, F.; Du, J.; Liang, G.; Li, A.; Song, G.; Liu, Y. CRISPR/Cas technology promotes the various application of Dunaliella salina system. Appl. Microbiol. Biotechnol. 2020, 104, 8621–8630. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Incharoensakdi, A. Microalgae as feedstock for biodiesel production under ultrasound treatment—A review. Bioresour. Technol. 2018, 250, 877–887. [Google Scholar] [CrossRef]
- Juan, J.C.; Kartika, D.A.; Wu, T.Y.; Hin, T.-Y.Y. Biodiesel production from jatropha oil by catalytic and non-catalytic approaches: An overview. Bioresour. Technol. 2011, 102, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Suresh, S.; Incharoensakdi, A. Chlamydomonas sp. as dynamic biorefinery feedstock for the production of methyl ester and ɛ-polylysine. Bioresour. Technol. 2019, 272, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.A.B.; Chew, K.W.; Chia, W.Y.; Mubashir, M.; Sankaran, R.; Lam, M.K.; Lim, J.W.; Ho, Y.-C.; Show, P.L. Green bioprocessing of protein from Chlorella vulgaris microalgae towards circular bioeconomy. Bioresour. Technol. 2021, 333, 125197. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Singhania, R.R.; Sim, S.J.; Dong, C.D. Recent advancements in mixotrophic bioprocessing for production of high value microalgal products. Bioresour. Technol. 2021, 320, 124421. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Incharoensakdi, A. Higher efficiency of microalgal biorefinery is achieved with integrated than one-way method. Fuel 2021, 300, 120988. [Google Scholar] [CrossRef]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Incharoensakdi, A. Enhancement of total lipid yield by nitrogen, carbon, and iron supplementation in isolated microalgae. J. Phycol. 2017, 53, 855–868. [Google Scholar] [CrossRef]
- Aravantinou, A.F.; Manariotis, I.D. Effect of operating conditions on Chlorococcum sp. growth and lipid production. J. Environ. Chem. Eng. 2016, 4, 1217–1223. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Lopes, E.; Mérida, L.R.; Queiroz, M.I.; Zepka, L.Q. Microalgal biorefineries. In Biomass Production and Uses; Jacob-Lopes, E., Zepka, L.Q., Eds.; InTech: Rijeka, Croatia, 2015; pp. 81–106. [Google Scholar]
- Daneshvar, E.; Sik Ok, Y.; Tavakoli, S.; Sarkar, B.; Shaheen, S.M.; Hong, H.; Luo, Y.; Rinklebe, J.; Song, H.; Bhatnagar, A. Insights into upstream processing of microalgae: A review. Bioresour. Technol. 2021, 329, 124870. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Mariano, A.B.; Vargas, J.V.C. A review on microalgae, a versatile source for sustainable energy and materials. Int. J. Energy Res. 2011, 35, 291–311. [Google Scholar] [CrossRef]
- Ali, S.K.; Saleh, A.M. Spirulina-an overview. Int. J. Pharm. Pharm. Sci. 2012, 4, 9–15. [Google Scholar]
- Zabed, H.; Boyce, A.N.; Sahu, J.N.; Faruq, G. Evaluation of the quality of dried distiller’s grains with solubles for normal and high sugary corn genotypes during dry–grind ethanol production. J. Clean. Prod. 2017, 142, 4282–4293. [Google Scholar] [CrossRef]
- Voltolina, D.; Sánchez-Saavedra, M.d.P.; Torres-Rodríguez, L.M. Outdoor mass microalgae production in Bahia Kino, Sonora, NW Mexico. Aquac. Eng. 2008, 38, 93–96. [Google Scholar] [CrossRef]
- Demirbas, M.F. Biofuels from algae for sustainable development. Appl. Energy 2011, 88, 3473–3480. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B.; Skill, S.C.; Lea-Langton, A.; Balasundaram, B.; Hall, C.; Riley, R.; Llewellyn, C.A. Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res. 2012, 1, 70–76. [Google Scholar] [CrossRef]
- Ajeej, A.; Thanikal, J.V.; Narayanan, C.M.; Senthil Kumar, R. An overview of bio augmentation of methane by anaerobic co-digestion of municipal sludge along with microalgae and waste paper. Renew. Sustain. Energy Rev. 2015, 50, 270–276. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Torres, A.; Muñoz, R.; Bartacek, J.; Jeison, D. Mesophilic and thermophilic anaerobic digestion of lipid-extracted microalgae N. gaditana for methane production. Renew. Energy 2017, 105, 539–546. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Muthukumar, K. Isolation of Thermo-stable and Solvent-Tolerant Bacillus sp. Lipase for the Production of Biodiesel. Appl. Biochem. Biotechnol. 2012, 166, 1095–1111. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Direct transesterification of Botryococcus sp. catalysed by immobilized lipase: Ultrasound treatment can reduce reaction time with high yield of methyl ester. Fuel 2017, 191, 363–370. [Google Scholar] [CrossRef]
- Uggetti, E.; Puigagut, J. Photosynthetic membrane-less microbial fuel cells to enhance microalgal biomass concentration. Bioresour. Technol. 2016, 218, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Neves, C.; Sebastião, D.; Nobre, B.P.; Matos, C.T. Effect of light on the production of bioelectricity and added-value microalgae biomass in a Photosynthetic Alga Microbial Fuel Cell. Bioresour. Technol. 2014, 154, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, R.K.; Ramadoss, G.; Jain, A.K.; Dhiman, R.K.; Bhatia, S.K.; Bhatt, A.K. Conversion of Waste Biomass into Gaseous Fuel: Present Status and Challenges in India. BioEnergy Res. 2020, 13, 1046–1068. [Google Scholar] [CrossRef]
- Ramadoss, G.; Muthukumar, K. Ultrasound assisted metal chloride treatment of sugarcane bagasse for bioethanol production. Renew. Energy 2016, 99, 1092–1102. [Google Scholar] [CrossRef]
- Uziel, M. Solar Energy Fixation and Conversion with Algal Bacterial Systems; University of California: Berkeley, CA, USA, 1978. [Google Scholar]
- Samson, R.; Leduy, A. Biogas production from anaerobic digestion of Spirulina maxima algal biomass. Biotechnol. Bioeng. 1982, 24, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-W.; Brune, D.E. Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour. Technol. 2007, 98, 130–134. [Google Scholar] [CrossRef]
- Ras, M.; Lardon, L.; Bruno, S.; Bernet, N.; Steyer, J.-P. Experimental study on a coupled process of production and anaerobic digestion of Chlorella vulgaris. Bioresour. Technol. 2011, 102, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhang, Z.; Luo, Y.; Qiao, W.; Xiao, M.; Zhang, M. Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source. Bioresour. Technol. 2012, 114, 281–286. [Google Scholar] [CrossRef]
- Kaewpintong, K.; Shotipruk, A.; Powtongsook, S.; Pavasant, P. Photoautotrophic high-density cultivation of vegetative cells of Haematococcus pluvialis in airlift bioreactor. Bioresour. Technol. 2007, 98, 288–295. [Google Scholar] [CrossRef]
- Golueke, C.G.; Oswald, W.J.; Gotaas, H.B. Anaerobic digestion of algae. Appl. Microbiol. 1957, 5, 47. [Google Scholar] [CrossRef]
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 2011, 102, 10163–10172. [Google Scholar] [CrossRef] [PubMed]
- Klassen, V.; Blifernez-Klassen, O.; Wibberg, D.; Winkler, A.; Kalinowski, J.; Posten, C.; Kruse, O. Highly efficient methane generation from untreated microalgae biomass. Biotechnol. Biofuels 2017, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from microalgae: Technologies, challenges and opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Thorin, E.; Olsson, J.; Schwede, S.; Nehrenheim, E. Co-digestion of sewage sludge and microalgae—Biogas production investigations. Appl. Energy 2018, 227, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2006, 69, 627–642. [Google Scholar] [CrossRef]
- Choi, S.P.; Nguyen, M.T.; Sim, S.J. Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol. 2010, 101, 5330–5336. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, Y.; Wu, X.; Gong, X.; Wang, Q. Hydrolysis of Chlorella biomass for fermentable sugars in the presence of HCl and MgCl2. Bioresour. Technol. 2011, 102, 10158–10161. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Zheng, Y.; Labavitch, J.M.; VanderGheynst, J.S. Virus infection of Chlorella variabilis and enzymatic saccharification of algal biomass for bioethanol production. Bioresour. Technol. 2013, 137, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Oh, Y.; Kim, D.; Kwon, D.; Lee, C.; Lee, J. Converting Carbohydrates Extracted from Marine Algae into Ethanol Using Various Ethanolic Escherichia coli Strains. Appl. Biochem. Biotechnol. 2011, 164, 878–888. [Google Scholar] [CrossRef]
- Ho, S.-H.; Huang, S.-W.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef]
- Harun, R.; Jason, W.S.Y.; Cherrington, T.; Danquah, M.K. Exploring alkaline pre-treatment of microalgal biomass for bioethanol production. Appl. Energy 2011, 88, 3464–3467. [Google Scholar] [CrossRef]
- Lee, O.K.; Kim, A.L.; Seong, D.H.; Lee, C.G.; Jung, Y.T.; Lee, J.W.; Lee, E.Y. Chemo-enzymatic saccharification and bioethanol fermentation of lipid-extracted residual biomass of the microalga, Dunaliella tertiolecta. Bioresour. Technol. 2013, 132, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Oh, C.H.; Bae, H.-J. Comparison of red microalgae (Porphyridium cruentum) culture conditions for bioethanol production. Bioresour. Technol. 2017, 233, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Kumar, P.; Mehariya, S.; Purohit, H.J.; Lee, J.-K.; Kalia, V.C. Enhancement in hydrogen production by co-cultures of Bacillus and Enterobacter. Int. J. Hydrogen Energy 2014, 39, 14663–14668. [Google Scholar] [CrossRef]
- Oey, M.; Sawyer, A.L.; Ross, I.L.; Hankamer, B. Challenges and opportunities for hydrogen production from microalgae. Plant Biotechnol. J. 2016, 14, 1487–1499. [Google Scholar] [CrossRef] [Green Version]
- Medisetty, V.M.; Kumar, R.; Ahmadi, M.H.; Vo, D.-V.N.; Ochoa, A.; Solanki, R. Overview on the current status of hydrogen energy research and development in India. Chem. Eng. Technol. 2020, 43, 613–624. [Google Scholar] [CrossRef]
- Ortigueira, J.; Pinto, T.; Gouveia, L.; Moura, P. Production and storage of biohydrogen during sequential batch fermentation of Spirogyra hydrolyzate by Clostridium butyricum. Energy 2015, 88, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yin, Y. Fermentative hydrogen production using various biomass-based materials as feedstock. Renew. Sustain. Energy Rev. 2018, 92, 284–306. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Kondo, A.; Chang, J.-S. Recent insights into biohydrogen production by microalgae—From biophotolysis to dark fermentation. Bioresour. Technol. 2017, 227, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Markl, E.; Grünbichler, H.; Lackner, M. PHB-bio based and biodegradable replacement for PP: A review. Nov. Tech. Nutr. Food Sci. 2018, 2, 206–209. [Google Scholar] [CrossRef]
- Monshupanee, T.; Nimdach, P.; Incharoensakdi, A. Two-stage (photoautotrophy and heterotrophy) cultivation enables efficient production of bioplastic poly-3-hydroxybutyrate in auto-sedimenting cyanobacterium. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva Vaz, B.; Moreira, J.B.; de Morais, M.G.; Costa, J.A.V. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar]
- Asiri, F.; Chu, K.-H. A Novel Recirculating Aquaculture System for Sustainable Aquaculture: Enabling Wastewater Reuse and Conversion of Waste-to-Immune-Stimulating Fish Feed. ACS Sustain. Chem. Eng. 2020, 8, 18094–18105. [Google Scholar] [CrossRef]
- Nishioka, M.; Nakai, K.; Miyake, M.; Asada, Y.; Taya, M. Production of poly-β-hydroxybutyrate by thermophilic cyanobacterium, Synechococcus sp. MA19, under phosphate-limited conditions. Biotechnol. Lett. 2001, 23, 1095–1099. [Google Scholar] [CrossRef]
- Panda, B.; Jain, P.; Sharma, L.; Mallick, N. Optimization of cultural and nutritional conditions for accumulation of poly-β-hydroxybutyrate in Synechocystis sp. PCC 6803. Bioresour. Technol. 2006, 97, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Sathish, A.; Stanley, J. Production Of Biofuel And Bioplastic From Chlorella Pyrenoidosa. Mater. Today Proc. 2018, 5, 16774–16781. [Google Scholar] [CrossRef]
- Kato, N. Production of crude bioplastic-beads with microalgae: Proof-of-concept. Bioresour. Technol. Rep. 2019, 6, 81–84. [Google Scholar] [CrossRef]
- Poltronieri, P.; Mezzolla, V.; D’Urso, O.F. PHB Production in Biofermentors Assisted through Biosensor Applications. In Proceedings of the 3rd International Electronic Conference on Sensors and Applications, Online, 15–30 November 2016; p. 4. [Google Scholar]
- Panda, B.; Mallick, N. Enhanced poly-β-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett. Appl. Microbiol. 2007, 44, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Carpine, R.; Olivieri, G.; Hellingwerf, K.; Pollio, A.; Marzocchella, A. The cyanobacterial route to produce poly-β-hydroxybutyrate. Chem. Eng. Trans. 2015, 43, 289–294. [Google Scholar]
- Wang, H.-M.D.; Chen, C.-C.; Huynh, P.; Chang, J.-S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.; Bandarra, N.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Incharoensakdi, A. Utilization of microalgae feedstock for concomitant production of bioethanol and biodiesel. Fuel 2018, 217, 458–466. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Chen, G.; Zhang, J.; Wang, C.; Liu, B. Extraction and purification of eicosapentaenoic acid and docosahexaenoic acid from microalgae: A critical review. Algal Res. 2019, 43, 101619. [Google Scholar] [CrossRef]
- Kwak, H.S.; Kim, J.Y.H.; Woo, H.M.; Jin, E.; Min, B.K.; Sim, S.J. Synergistic effect of multiple stress conditions for improving microalgal lipid production. Algal Res. 2016, 19, 215–224. [Google Scholar] [CrossRef]
- Yeh, K.-L.; Chang, J.-S. Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresour. Technol. 2012, 105, 120–127. [Google Scholar] [CrossRef]
- Hernández, D.; Solana, M.; Riaño, B.; García-González, M.C.; Bertucco, A. Biofuels from microalgae: Lipid extraction and methane production from the residual biomass in a biorefinery approach. Bioresour. Technol. 2014, 170, 370–378. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chang, J.-S.; Lai, Y.-Y.; Chen, C.-N.N. Achieving high lipid productivity of a thermotolerant microalga Desmodesmus sp. F2 by optimizing environmental factors and nutrient conditions. Bioresour. Technol. 2014, 156, 108–116. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.-D.; Zhao, X.-Y.; Liu, X.; Dong, T.; Wu, F.-A. From microalgae oil to produce novel structured triacylglycerols enriched with unsaturated fatty acids. Bioresour. Technol. 2015, 184, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chen, Y.-C.; Huang, H.-C.; Ho, S.-H.; Chang, J.-S. Enhancing the production of eicosapentaenoic acid (EPA) from Nannochloropsis oceanica CY2 using innovative photobioreactors with optimal light source arrangements. Bioresour. Technol. 2015, 191, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, H.-W.; Yang, S.-C.; Chen, C.-H.; Jesisca; Chang, J. -S. Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresour. Technol. 2015, 184, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Incharoensakdi, A. Plant hormone induced enrichment of Chlorella sp. omega-3 fatty acids. Biotechnol. Biofuels 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, M.; Han, J.-I. Ultrasound-assisted in-situ transesterification of wet Aurantiochytrium sp. KRS 101 using potassium carbonate. Bioresour. Technol. 2018, 261, 117–121. [Google Scholar] [CrossRef]
- Yen, H.-W.; Hu, I.C.; Chen, C.-Y.; Ho, S.-H.; Lee, D.-J.; Chang, J.-S. Microalgae-based biorefinery—From biofuels to natural products. Bioresour. Technol. 2013, 135, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, S.; Izumi, Y.; Matsuda, F.; Hasunuma, T.; Chang, J.-S.; Kondo, A. Synergistic enhancement of glycogen production in Arthrospira platensis by optimization of light intensity and nitrate supply. Bioresour. Technol. 2012, 108, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Karemore, A.; Sen, R. Downstream processing of microalgal feedstock for lipid and carbohydrate in a biorefinery concept: A holistic approach for biofuel applications. RSC Adv. 2016, 6, 29486–29496. [Google Scholar] [CrossRef]
- Spiden, E.M.; Yap, B.H.; Hill, D.R.; Kentish, S.E.; Scales, P.J.; Martin, G.J. Quantitative evaluation of the ease of rupture of industrially promising microalgae by high pressure homogenization. Bioresour. Technol. 2013, 140, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; De Francesco, F.; Aguinaga, A.; Parameswaran, P.; Rittmann, B.E. Improving lipid recovery from Scenedesmus wet biomass by surfactant-assisted disruption. Green Chem. 2016, 18, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Unterlander, N.; Champagne, P.; Plaxton, W.C. Lyophilization pretreatment facilitates extraction of soluble proteins and active enzymes from the oil-accumulating microalga Chlorella vulgaris. Algal Res. 2017, 25, 439–444. [Google Scholar] [CrossRef]
- Gerde, J.A.; Wang, T.; Yao, L.; Jung, S.; Johnson, L.A.; Lamsal, B. Optimizing protein isolation from defatted and non-defatted Nannochloropsis microalgae biomass. Algal Res. 2013, 2, 145–153. [Google Scholar] [CrossRef]
- Ursu, A.-V.; Marcati, A.; Sayd, T.; Sante-Lhoutellier, V.; Djelveh, G.; Michaud, P. Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Mujumdar, A.S. Handbook of Industrial Drying; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Sattayasai, N. Protein purification. Chem. Biol. 2012, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Bjornsson, W.J.; MacDougall, K.M.; Melanson, J.E.; O’Leary, S.J.B.; McGinn, P.J. Pilot-scale supercritical carbon dioxide extractions for the recovery of triacylglycerols from microalgae: A practical tool for algal biofuels research. J. Appl. Phycol. 2012, 24, 547–555. [Google Scholar] [CrossRef]
- Buchmann, L.; Mathys, A. Perspective on Pulsed Electric Field Treatment in the Bio-based Industry. Front. Bioeng. Biotechnol. 2019, 7, 265. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, P.; Zhang, G.; Peng, M. Biomass and pigments production in photosynthetic bacteria wastewater treatment: Effects of photoperiod. Bioresour. Technol. 2015, 190, 196–200. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Raghavarao, K.S.M.S. Recovery of chlorophylls from spent biomass of Arthrospira platensis obtained after extraction of phycobiliproteins. Bioresour. Technol. 2019, 271, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Parniakov, O.; Apicella, E.; Koubaa, M.; Barba, F.J.; Grimi, N.; Lebovka, N.; Pataro, G.; Ferrari, G.; Vorobiev, E. Ultrasound-assisted green solvent extraction of high-added value compounds from microalgae Nannochloropsis spp. Bioresour. Technol. 2015, 198, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Sovová, H.; Stateva, R.P. New developments in the modelling of carotenoids extraction from microalgae with supercritical CO2. J. Supercrit. Fluids 2019, 148, 93–103. [Google Scholar] [CrossRef]
- Singh, D.; Puri, M.; Wilkens, S.; Mathur, A.S.; Tuli, D.K.; Barrow, C.J. Characterization of a new zeaxanthin producing strain of Chlorella saccharophila isolated from New Zealand marine waters. Bioresour. Technol. 2013, 143, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 125398. [Google Scholar] [CrossRef]
- Cui, D.; Hu, C.; Zou, Z.; Sun, X.; Shi, J.; Xu, N. Comparative transcriptome analysis unveils mechanisms underlying the promoting effect of potassium iodide on astaxanthin accumulation in Haematococcus pluvialis under high light stress. Aquaculture 2020, 525, 735279. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Krishnamoorthy, R.; Tao, Y.; Chu, D.-T.; Show, P.L. Liquid biphasic flotation for the purification of C-phycocyanin from Spirulina platensis microalga. Bioresour. Technol. 2019, 288, 121519. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.R.; Chew, K.W.; Show, P.L.; Xia, A.; Ho, S.-H.; Lim, J.W. Spirulina platensis based biorefinery for the production of value-added products for food and pharmaceutical applications. Bioresour. Technol. 2019, 289, 121727. [Google Scholar] [CrossRef] [PubMed]
- Porav, A.S.; Bocăneală, M.; Fălămaş, A.; Bogdan, D.F.; Barbu-Tudoran, L.; Hegeduş, A.; Dragoş, N. Sequential aqueous two-phase system for simultaneous purification of cyanobacterial phycobiliproteins. Bioresour. Technol. 2020, 315, 123794. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, X.; Si, T.; Zhou, F.; Zhou, J.; Cen, K. Pore fractal structures and combustion dynamics of cokes derived from the pyrolysis of typical Chinese power coals. Fuel Process. Technol. 2016, 149, 49–54. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Vijayan, D.; Praveenkumar, R.; Han, J.-I.; Lee, K.; Park, J.-Y.; Chang, W.-S.; Lee, J.-S.; Oh, Y.-K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Ljubic, A.; Jacobsen, C.; Holdt, S.L.; Jakobsen, J. Microalgae Nannochloropsis oceanica as a future new natural source of vitamin D3. Food Chem. 2020, 320, 126627. [Google Scholar] [CrossRef] [PubMed]
- Tarento, T.D.C.; McClure, D.D.; Vasiljevski, E.; Schindeler, A.; Dehghani, F.; Kavanagh, J.M. Microalgae as a source of vitamin K1. Algal Res. 2018, 36, 77–87. [Google Scholar] [CrossRef]
- Edelmann, M.; Aalto, S.; Chamlagain, B.; Kariluoto, S.; Piironen, V. Riboflavin, niacin, folate and vitamin B12 in commercial microalgae powders. J. Food Compos. Anal. 2019, 82, 103226. [Google Scholar] [CrossRef]
- Papachristou, I.; Akaberi, S.; Silve, A.; Navarro-López, E.; Wüstner, R.; Leber, K.; Nazarova, N.; Müller, G.; Frey, W. Analysis of the lipid extraction performance in a cascade process for Scenedesmus almeriensis biorefinery. Biotechnol. Biofuels 2021, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- González-Balderas, R.M.; Velásquez-Orta, S.B.; Valdez-Vazquez, I.; Orta Ledesma, M.T. Intensified recovery of lipids, proteins, and carbohydrates from wastewater-grown microalgae Desmodesmus sp. by using ultrasound or ozone. Ultrason. Sonochem. 2020, 62, 104852. [Google Scholar] [CrossRef]
- Mirsiaghi, M.; Reardon, K.F. Conversion of lipid-extracted Nannochloropsis salina biomass into fermentable sugars. Algal Res. 2015, 8, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Bai, M.-D.; Cheng, C.-H.; Wan, H.-M.; Lin, Y.-H. Microalgal pigments potential as byproducts in lipid production. J. Taiwan Inst. Chem. Eng. 2011, 42, 783–786. [Google Scholar] [CrossRef]
- Cheng, J.; Li, K.; Yang, Z.; Zhou, J.; Cen, K. Enhancing the growth rate and astaxanthin yield of Haematococcus pluvialis by nuclear irradiation and high concentration of carbon dioxide stress. Bioresour. Technol. 2016, 204, 49–54. [Google Scholar] [CrossRef]
- Hemlata, G.P.; Bano, F.; Fatma, T. Studies on Anabaena sp. nccu-9 with special reference to phycocyanin. J Algal Biomass Util. 2011, 2, 30–51. [Google Scholar]
- Lin, W.-R.; Tan, S.-I.; Hsiang, C.-C.; Sung, P.-K.; Ng, I.S. Challenges and opportunity of recent genome editing and multi-omics in cyanobacteria and microalgae for biorefinery. Bioresour. Technol. 2019, 291, 121932. [Google Scholar] [CrossRef] [PubMed]
- Mobin, S.; Alam, F. Some promising microalgal species for commercial applications: A review. Energy Procedia 2017, 110, 510–517. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Kao, P.-C.; Tsai, C.-J.; Lee, D.-J.; Chang, J.-S. Engineering strategies for simultaneous enhancement of C-phycocyanin production and CO2 fixation with Spirulina platensis. Bioresour. Technol. 2013, 145, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Coelho, A.; Hemaiswarya, S.; Kumar, P.; Carvalho, I.S.; Alagarsamy, A. Applications of microalgal paste and powder as food and feed: An update using text mining tool. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 740–747. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- El-fayoumy, E.A.; Shanab, S.M.; Hassan, O.M.A.; Shalaby, E.A. Enhancement of active ingredients and biological activities of Nostoc linckia biomass cultivated under modified BG-110 medium composition. Biomass Convers. Biorefin. 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Moore, R.E.; Patterson, G.M.L.; Xu, C.; Clardy, J. Scytophycins, cytotoxic and antimycotic agents from the cyanophyte Scytonema pseudohofmanni. J. Org. Chem. 1986, 51, 5300–5306. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Hirsch, C.F.; Sesin, D.F.; Flor, J.E.; Chartrain, M.; Fromtling, R.E.; Harris, G.H.; Salvatore, M.J.; Liesch, J.M.; Yudin, K. Pharmaceuticals from cultured algae. J. Ind. Microbiol. Biotechnol. 1990, 5, 113–123. [Google Scholar] [CrossRef]
- Kobayashi, J.; Ishibashi, M.; Nakamura, H.; Ohizumi, Y.; Yamasu, T.; Sasaki, T.; Hirata, Y. Amphidinolide-A, a novel antineoplastic macrolide from the marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 1986, 27, 5755–5758. [Google Scholar] [CrossRef]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.; Ianora, A.; Russo, G.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Hildebrand, M.; Manandhar-Shrestha, K.; Abbriano, R. Effects of chrysolaminarin synthase knockdown in the diatom Thalassiosira pseudonana: Implications of reduced carbohydrate storage relative to green algae. Algal Res. 2017, 23, 66–77. [Google Scholar] [CrossRef]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.-B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappo, M.; Berkov, S.; Massucco, C.; Di Maria, V.; Bastida, J.; Codina, C.; Avila, C.; Messina, P.; Zupo, V.; Zupo, S. Apoptotic activity of the marine diatom Cocconeis scutellum and eicosapentaenoic acid in BT20 cells. Pharm. Biol. 2012, 50, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Li, X.-F.; Kang, K.-H.; Ryu, B.; Kim, S.K. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 2014, 47, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, B.; Li, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling: Implication for chronic articular disease. Chem. Biol. Interact. 2009, 179, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.W.; Ko, J.-Y.; Lee, J.-H.; Kwon, O.-N.; Kim, S.-W.; Jeon, Y.-J. Apoptotic anticancer activity of a novel fatty alcohol ester isolated from cultured marine diatom, Phaeodactylum tricornutum. J. Funct. Foods 2014, 6, 231–240. [Google Scholar] [CrossRef]
- Andrianasolo, E.H.; Haramaty, L.; Vardi, A.; White, E.; Lutz, R.; Falkowski, P. Apoptosis-inducing galactolipids from a cultured marine diatom, Phaeodactylum tricornutum. J. Nat. Prod. 2008, 71, 1197–1201. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, M.; Wanezaki, S.; Miyauchi, K.; Kurihara, H.; Kohno, H.; Kawabata, J.; Odashima, S.; Takahashi, K. Apoptosis-inducing effect of fucoxanthin on human leukemia cell line HL-60. Food Sci. Technol. Res. 1999, 5, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int. J. Biol. Macromol. 1995, 17, 293–297. [Google Scholar] [CrossRef]
- Sun, Z.; Mohamed, M.A.A.; Park, S.Y.; Yi, T.H. Fucosterol protects cobalt chloride induced inflammation by the inhibition of hypoxia-inducible factor through PI3K/Akt pathway. Int. Immunopharmacol. 2015, 29, 642–647. [Google Scholar] [CrossRef]
- Bauer, S.; Jin, W.; Zhang, F.; Linhardt, R.J. The application of seaweed polysaccharides and their derived products with potential for the treatment of Alzheimer’s disease. Mar. Drugs 2021, 19, 89. [Google Scholar] [CrossRef]
- Márquez-Escobar, V.A.; Bañuelos-Hernández, B.; Rosales-Mendoza, S. Expression of a Zika virus antigen in microalgae: Towards mucosal vaccine development. J. Biotechnol. 2018, 282, 86–91. [Google Scholar] [CrossRef]
- Hans, N.; Malik, A.; Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour. Technol. Rep. 2020, 13, 100623. [Google Scholar] [CrossRef] [PubMed]
- Sami, N.; Ahmad, R.; Fatma, T. Exploring algae and cyanobacteria as a promising natural source of antiviral drug against SARS-CoV-2. Biomed. J. 2021, 44, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. The anti-inflammatory effect of algae-derived lipid extracts on lipopolysaccharide (LPS)-stimulated human THP-1 macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef] [Green Version]
- Jyonouchi, H.; Zhang, L.; Tomita, Y. Studies of immunomodulating actions of carotenoids. II. astaxanthin enhances in vitro antibody production to T-dependent antigens without facilitating polyclonal B-cell activation. Nutr. Cancer 1993, 19, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.S.; Capper, E.A.; Roshak, A.K.; Marquez, B.; Eichman, C.; Jackson, J.R.; Mattern, M.; Gerwick, W.H.; Jacobs, R.S.; Marshall, L.A. The identification and characterization of the marine natural product scytonemin as a novel antiproliferative pharmacophore. J. Pharmacol. Exp. Ther. 2002, 303, 858–866. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abdeen, A. Protective effects of rosuvastatin and vitamin E against fipronil-mediated oxidative damage and apoptosis in rat liver and kidney. Food Chem. Toxicol. 2018, 114, 69–77. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.-J. Hypolipidemic, Antioxidant, and Antiinflammatory Activities of Microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef] [Green Version]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2020, 283, 124657. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, S. Pesticides: Problems and Remedial Measures. In Evaluation of Environmental Contaminants and Natural Products: A Human Health Perspective; Sharma, A., Kumar, M., Kaur, S., Nagpal, A., Eds.; Bentham Science Publishers: Singapore, 2019; pp. 94–115. [Google Scholar]
- Butu, M.; Stef, R.; Grozea, I.; Corneanu, M.; Butnariu, M. Biopesticides: Clean and Viable Technology for Healthy Environment. In Bioremediation and Biotechnology; Hakeem, K.R., Bhat, R.A., Quadri, H., Eds.; Springer: Cham, Switzerland, 2020; pp. 107–151. [Google Scholar]
- Righini, H.; Roberti, R. Algae and Cyanobacteria as Biocontrol Agents of Fungal Plant Pathogens. In Plant Microbe Interface; Varma, A., Tripathi, S., Prasa, R., Eds.; Springer: Cham, Switzerland, 2019; pp. 219–238. [Google Scholar]
- Gupta, V.; Ratha, S.K.; Sood, A.; Chaudhary, V.; Prasanna, R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)—Prospects and challenges. Algal Res. 2013, 2, 79–97. [Google Scholar] [CrossRef]
- Fidor, A.; Konkel, R.; Mazur-Marzec, H. Bioactive peptides produced by cyanobacteria of the genus Nostoc: A review. Mar. Drugs 2019, 17, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latif, S.; Chiapusio, G.; Weston, L. Allelopathy and the role of allelochemicals in plant defence. Adv. Bot. Res. 2017, 82, 19–54. [Google Scholar]
- Shanmugam, S.; Mathimani, T.; Anto, S.; Sudhakar, M.P.; Kumar, S.S.; Pugazhendhi, A. Cell density, Lipidomic profile, and fatty acid characterization as selection criteria in bioprospecting of microalgae and cyanobacterium for biodiesel production. Bioresour. Technol. 2020, 304, 123061. [Google Scholar] [CrossRef] [PubMed]
- Khaldi, H.; Maatoug, M.; Dube, C.S.; Ncube, M.; Tandlich, R.; Heilmeier, H.; Laubscher, R.K.; Dellal, A. Efficiency of wastewater treatment by a mixture of sludge and microalgae. J. Fundam. Appl. Sci. 2017, 9, 1454–1472. [Google Scholar] [CrossRef]
- Chandra, R.; Pradhan, S.; Patel, A.; Ghosh, U.K. An approach for dairy wastewater remediation using mixture of microalgae and biodiesel production for sustainable transportation. J. Environ. Manag. 2021, 297, 113210. [Google Scholar] [CrossRef] [PubMed]
- Craggs, R.J.; Lundquist, T.J.; Benemann, J.R. Wastewater Treatment and Algal Biofuel Production. In Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 153–163. [Google Scholar]
- Quinn, J.C.; Davis, R. The potentials and challenges of algae based biofuels: A review of the techno-economic, life cycle, and resource assessment modeling. Bioresour. Technol. 2015, 184, 444–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naira, V.R.; Das, D.; Maiti, S.K. Real time light intensity based carbon dioxide feeding for high cell-density microalgae cultivation and biodiesel production in a bubble column photobioreactor under outdoor natural sunlight. Bioresour. Technol. 2019, 284, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Aden, A.; Pienkos, P.T. Techno-economic analysis of autotrophic microalgae for fuel production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar] [CrossRef]
- Hoffman, J.; Pate, R.C.; Drennen, T.; Quinn, J.C. Techno-economic assessment of open microalgae production systems. Algal Res. 2017, 23, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.; Olivieri, G.; De Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.; Kleinegris, D.M.; Wijffels, R.H.; Barbosa, M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef] [Green Version]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the production of bulk chemicals and biofuels. Biofuels Bioprod. Biorefin. 2010, 4, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Gifuni, I.; Pollio, A.; Safi, C.; Marzocchella, A.; Olivieri, G. Current bottlenecks and challenges of the microalgal biorefinery. Trends Biotechnol. 2019, 37, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Pott, R.W.M.; Howe, C.J.; Dennis, J.S. The purification of crude glycerol derived from biodiesel manufacture and its use as a substrate by Rhodopseudomonas palustris to produce hydrogen. Bioresour. Technol. 2014, 152, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Belay, A.; Ota, Y.; Miyakawa, K.; Shimamatsu, H. Current knowledge on potential health benefits of Spirulina. J. Appl. Phycol. 1993, 5, 235–241. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Liu, X.; Chen, F.; Chen, Z. Current microalgal health food R & D activities in China. In Asian Pacific Phycology in the 21st Century: Prospects and Challenges; Ang, P.O., Jr., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 45–48. [Google Scholar]
- Finney, K.; Pomeranz, Y.; Bruinsma, B. Use of algae Dunaliella as a protein supplement in bread. Cereal Chem. 1984, 61, 402–406. [Google Scholar]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Res. 2014, 3, 61–65. [Google Scholar] [CrossRef] [Green Version]

| Microalgae Species | Carbohydrate (%) | Lipid (%) | Protein (%) | References |
|---|---|---|---|---|
| Anabaena cylindrica | 25–30 | 4–7 | 43–56 | [23] |
| Arthrospira platensis | 15–25 | 4–7 | 55–70 | [24] |
| Chaetoceros calcitrans | 10 | 39 | 58 | [25] |
| Chaetoceros muellerii | 11–19 | 33 | 44–65 | [25] |
| Chaetoceros muelleri | 12–19 | 22–33 | 46–64 | [26] |
| Chlamydomonas rheinhardii | 17 | 21 | 48 | [27] |
| Chlorogloeopsis fritschii | 7 | 50 | 44 | [28] |
| Chlorella protothecoides | 10–15 | 55 | 10–52 | [25] |
| Chlorella vulgaris | 9–17 | 14–25 | 51–58 | [27] |
| Chlorellapyrenoidosa | 26 | 2 | 57 | [27] |
| Dunaliella salina | 32 | 6 | 57 | [27] |
| Dunaliella bioculata | 4 | 8 | 49 | [27] |
| Euglena gracilis | 14–20 | 14–18 | 39–61 | [29] |
| Euglena gracilis | 14–18 | 4–20 | 39–61 | [25] |
| Isochrysis galbana Parke | 7–25 | 21–38 | 30–45 | [25] |
| Nannochloropsis gaditana | 9.31 | 23.3 | 48.3 | [30] |
| Porphyridium cruentum | 40–57 | 9–14 | 28–39 | [27] |
| Porphyridium cruentum | 40–57 | 9–14 | 28–39 | [23] |
| Prymnesium parvum | 25–33 | 22–38 | 28–45 | [27] |
| Scenedesmus dimorphus | 18–52 | 16–43 | 8–18 | [28] |
| Scenedesmus quadricauda | – | 1.9 | 47 | [27] |
| Scenedesmus obliquus | 10–17 | 35–55 | 50–56 | [23] |
| Spirogyrasp. | 33–64 | 11–21 | 6–20 | [27] |
| Synechoccus sp. | 15 | 11 | 63 | [27] |
| Spirulina maxima | 13–16 | 6–7 | 60–71 | [23] |
| Spirulina platensis | 8–20 | 4–9 | 46–65 | [25] |
| Tetraselmis maculata | 15 | 3 | 52 | [27] |
| Microalgal Strains | Pretreatment | Fermentative Microorganism | Fermentation Condition | Ethanol Production | References |
|---|---|---|---|---|---|
| Chlamydomonas reinhardtii | Enzymatic | Saccharomyces cerevisiae S288C | SSF, Temp: 30 °C, Time: 40 h, 160 rpm | 0.235 (g/g algae) | [50] |
| Chlorella | Chemical (HCI and MgCI2) | Saccharomyces cerevisiae Y01 | Temp: 30 °C, Time: 48 h, 200 rpm | 22.60 (g/dm3) | [51] |
| Chlorellavariabilis | Enzymatic | Escherichiacoli KO11 | Temp: 35 °C, Time: 72 h, pH: 6.5, 150 rpm | 0.326 (g/g carbohydrate consumed) | [52] |
| Chlorellavulgaris | Chemical (H2SO4) | Escherichiacoli SJL2526 | SHF, Temp: 37 °C, pH: 7, 170 rpm | 0.4 (g/g algae) | [53] |
| Chlorellavulgaris FSP-E | Chemical (H2SO4) | Zymomonas mobilis ATCC 29191 | SHF, Temp: 30 °C, Time: 12 h, pH: 5–6 | 11.66 (g/dm3) | [54] |
| Chlorococcum infusionum | Chemical (NaOH) | Saccharomyces cerevisiae | Time: 12 h, 150 rpm | 0.26 (g/g algae) | [55] |
| Dunaliella tertiolecta | Chemical HCl/H2SO4 | S. cerevisiae | SHF, Temp: 30°C, Time: 12 h, 200 rpm | 0.14 g/g algae | [56] |
| Porphyridium cruemtum | Enzymatic | Saccharomyces cerevisiae KCTC 7906 | SSF, Temp: 37 °C Time: 9 h, pH: 4.8 | 2.77 (g/dm3) (seawater) 2.98 (g/dm3) (freshwater) | [57] |
| Scenedesmus obliquus CNW-N | Chemical (H2SO4) | Zymomonas mobilis ATCC29191 | SHF, Temp: 30 °C Time: 4 h, pH: 6 | 8.55 (g/dm3) | Ho et al., 2013 |
| Microalgal Species | Products | Extraction and Purification Methods | Yield or Extraction Efficiency | Remarks | References |
|---|---|---|---|---|---|
| Scenedesmus almeriensis | Lipid | Solvent extraction [Soxhlet method: methanol–chloroform 2:1 (v/v)] | 8.0 DW% | Need of organic solvent | [84] |
| Scenedesmus almeriensis | Lipid | ethanol:hexane (1:0.41 vol/vol) | 19 DW% | Need of organic solvent | [118] |
| Desmodesmus sp. | Lipid | Chloroform:methanol (2:1 v/v) | 5.6 g/L | Need of organic solvents | [119] |
| Chlorella sp. | Lipid | Isotonic extraction | 19 wt.% | Energy intensive High capital cost | [17] |
| Desmodesmus sp. | Carbohydrates | Ultrsound + H2SO4 (10%) | 5.2 g/L | Energy intensive and low extraction yield | [119] |
| Chlorococcum infusionum | Carbohydrates | Chemical hydrolysis (chemical pretreatment) | 89.6% (sugar) | Relatively inexpensive | [93] |
| Nannochloropsis salina | Carbohydrates | H2SO4 (10%) | 11.9 g/L | Cost effective | [120] |
| Isochrysis aff. galbana | Chlorophyll | Solvent extraction | 5.6% | Organic solvent needed | [121] |
| Haematococcus pluviali | Astaxanthin | Solvent extraction | 46 mg/L | Highest yield obtained with 6% CO2 | [122] |
| Chlorella saccharophila | β-Carotene | Ultrasonication and cell disruption | 37.3% (5.1 mg/g) | Improved extraction method | [107] |
| Anabaena sp. NCCU-9 | Phycocyanin | Repeated freezing and thawing | 128 mg/g | Optimization of culture conditions | [123] |
| Chlorella saccharophila | Zeaxanthin | Ultrasonication and cell disruption | 72.2% (11.3 mg/g) | Improved extraction method | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivaramakrishnan, R.; Suresh, S.; Kanwal, S.; Ramadoss, G.; Ramprakash, B.; Incharoensakdi, A. Microalgal Biorefinery Concepts’ Developments for Biofuel and Bioproducts: Current Perspective and Bottlenecks. Int. J. Mol. Sci. 2022, 23, 2623. https://doi.org/10.3390/ijms23052623
Sivaramakrishnan R, Suresh S, Kanwal S, Ramadoss G, Ramprakash B, Incharoensakdi A. Microalgal Biorefinery Concepts’ Developments for Biofuel and Bioproducts: Current Perspective and Bottlenecks. International Journal of Molecular Sciences. 2022; 23(5):2623. https://doi.org/10.3390/ijms23052623
Chicago/Turabian StyleSivaramakrishnan, Ramachandran, Subramaniyam Suresh, Simab Kanwal, Govindarajan Ramadoss, Balasubramani Ramprakash, and Aran Incharoensakdi. 2022. "Microalgal Biorefinery Concepts’ Developments for Biofuel and Bioproducts: Current Perspective and Bottlenecks" International Journal of Molecular Sciences 23, no. 5: 2623. https://doi.org/10.3390/ijms23052623






