Comparison between Janus-Base Nanotubes and Carbon Nanotubes: A Review on Synthesis, Physicochemical Properties, and Applications
Abstract
:1. Introduction
2. Structure and Morphology of Nanotubes
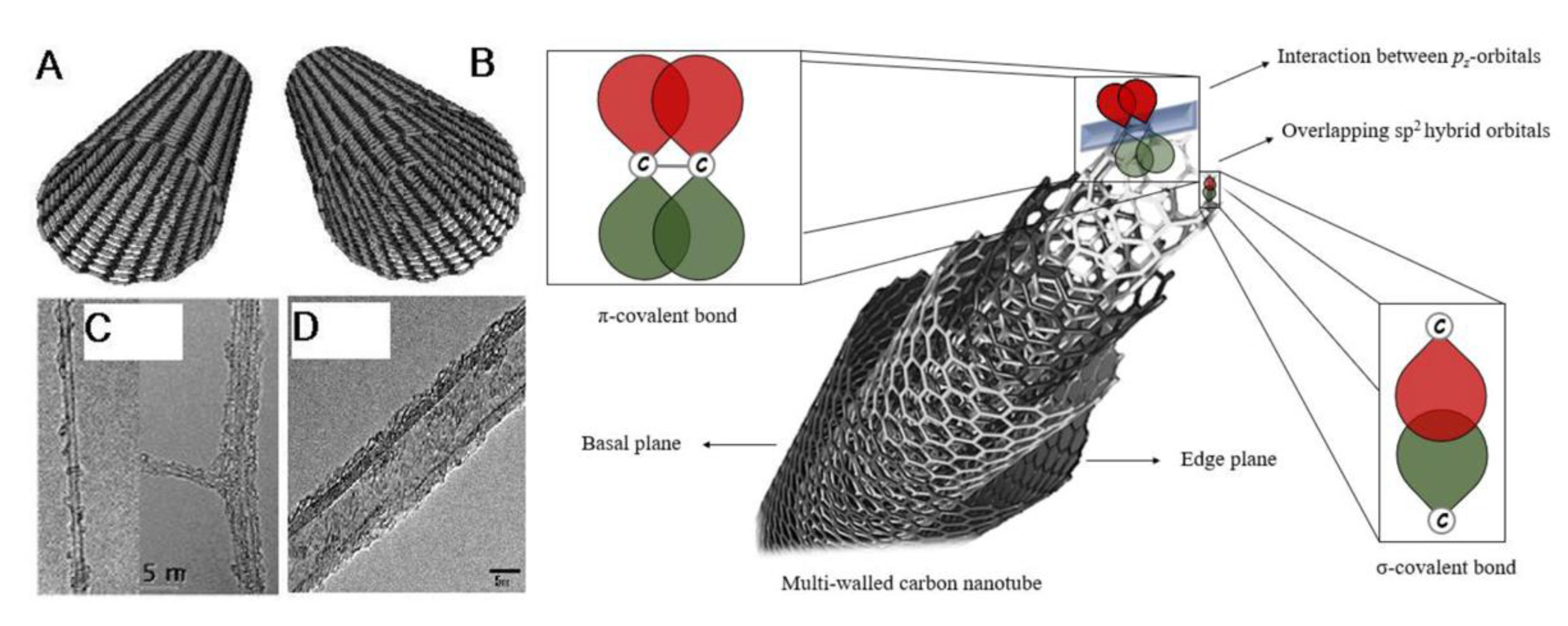
2.1. Structure and Morphology of Carbon Nanotubes
2.2. Structure and Morphology of Janus-Base Nanotubes
3. Synthesis of Nanotubes
3.1. Synthesis of Carbon Nanotubes
3.1.1. Chemical Vapor Deposition
3.1.2. Carbon Arc Discharge Method
3.1.3. Laser Ablation Technique
3.1.4. Functionalization of CNTs
3.2. Synthesis of Janus-Base Nanotubes
Functionalization of JBNt
4. Biomedical Applications
4.1. Drug Delivery
4.1.1. Drug Delivery via CNTs
4.1.2. Drug Delivery via JBNts
4.2. Electrical Conductivity
4.2.1. Electrical Conductivity of CNTs
4.2.2. Electrical Conductivity of JBNts
4.3. Scaffolding and Coating
4.3.1. Scaffolding and Coating with CNT
4.3.2. Scaffolding and Coating with JBNt
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Sands, I.; Lee, J.; Zhang, W.; Chen, Y. RNA Delivery via DNA-Inspired Janus Base Nanotubes for Extracellular Matrix Penetration. MRS Adv. 2020, 5, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Kitko, K.E.; Zhang, Q. Graphene-Based Nanomaterials: From Production to Integration With Modern Tools in Neuroscience. Front. Syst. Neurosci. 2019, 13, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.-Y.; Liu, T.-T.; Sang, Z.-T.; Lin, Z.-S.; Su, X.; Sun, X.-T.; Yang, H.-Z.; Wang, T.; Guo, S. Microfluidic Preparation of Janus Microparticles With Temperature and pH Triggered Degradation Properties. Front. Bioeng. Biotechnol. 2021, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Fenniri, H.; Mathivanan, P.; Vidale, K.L.; Sherman, D.M.; Hallenga, K.; Wood, K.V.; Stowell, J.G. Helical Rosette Nanotubes: Design, Self-Assembly, and Characterization. J. Am. Chem. Soc. 2001, 123, 3854–3855. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, W. Molecular Engineering of DNA-inspired Janus base nanomaterials. Juniper Online J. Mater. Sci. 2019, 5, 555670. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R. Physics of carbon nanotubes. Carbon 1995, 33, 883–891. Available online: https://www.sciencedirect.com/science/article/abs/pii/0008622395000178?via%3Dihub (accessed on 15 December 2021). [CrossRef]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Ebbesen, T.W. Nanometre-size tubes of carbon. Rep. Prog. Phys. 1997, 60, 1025. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Bai, X.; Wang, E.; Xie, S. Synthesis, structure, and properties of single-walled carbon nanotubes. Adv. Mater. 2009, 21, 4565–4583. [Google Scholar] [CrossRef]
- Fraczek-Szczypta, A.; Menaszek, E.; Syeda, T.B.; Misra, A.; Alavijeh, M.; Adu, J.; Blazewicz, S. Effect of MWCNT surface and chemical modification on in vitro cellular response. J. Nanoparticle Res. 2012, 14, 1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, T.M.B.F.; Morais, S. New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes. Appl. Sci. 2018, 8, 1925. [Google Scholar] [CrossRef] [Green Version]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1998. [Google Scholar]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, A.; Jain, A.; Gurnany, E.; Jain, R.; Modi, A.; Jain, A. Carbon nanotubes: A promising carrier for drug delivery and targeting. Nanoarchitecton. Smart Deliv. Drug Target. 2022. Available online: https://www.sciencedirect.com/science/article/pii/B9780323473477000173?via%3Dihub (accessed on 15 January 2022). [CrossRef]
- Song, S.; Chen, Y.; Yan, Z.; Fenniri, H.; Webster, T.J. Self-assembled rosette nanotubes for incorporating hydrophobic drugs in physiological environments. Int. J. Nanomed. 2011, 6, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Westhof, E.; Yusupov, M.; Yusupova, G. Recognition of Watson-Crick base pairs: Constraints and limits due to geometric selection and tautomerism. F1000Prime Rep. 2014, 6, 19. [Google Scholar] [CrossRef]
- Seeman, N.C.; Rosenberg, J.M.; Rich, A. Sequence-specific recognition of double helical nucleic acids by proteins. Proc. Natl. Acad. Sci. USA 1976, 73, 804–808. [Google Scholar] [CrossRef] [Green Version]
- Branda, N.; Kurz, G.; Lehn, J.-M. JANUS WEDGES: A new approach towards nucleobase-pair recognition. Chem. Commun. 1996, 2443–2444. Available online: https://pubs.rsc.org/en/content/articlelanding/1996/cc/cc9960002443/unauth (accessed on 15 January 2022). [CrossRef]
- Cheng, A.; Chen, W.W.; Fuhrmann, C.N.; Frankel, A.D. Recognition of Nucleic Acid Bases and Base-pairs by Hydrogen Bonding to Amino Acid Side-chains. J. Mol. Biol. 2003, 327, 781–796. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Yau, A.; Zhang, W.; Chen, Y. Fabrication of a Biomimetic Nano-Matrix with Janus Base Nanotubes and Fibronectin for Stem Cell Adhesion. J. Vis. Exp. 2020, 159, e61317. [Google Scholar] [CrossRef]
- Johnson, R.S.; Yamazaki, T.; Kovalenko, A.; Fenniri, H. Molecular Basis for Water-Promoted Supramolecular Chirality Inversion in Helical Rosette Nanotubes. J. Am. Chem. Soc. 2007, 129, 5735–5743. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral Drugs: An Overview. Int. J. Biomed. Sci. IJBS 2006, 2, 85–100. [Google Scholar]
- Hemraz, U.D.; El-Bakkari, M.; Yamazaki, T.; Cho, J.-Y.; Beingessner, R.L.; Fenniri, H. Chiromers: Conformation-driven mirror-image supramolecular chirality isomerism identified in a new class of helical rosette nanotubes. Nanoscale 2014, 6, 9421–9427. [Google Scholar] [CrossRef]
- José-Yacamán, M.; Miki-Yoshida, M.; Rendón, L.; Santiesteban, J.G. Catalytic growth of carbon microtubules with fullerene structure. Appl. Phys. Lett. 1993, 62, 657–659. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.H.; Kim, S.G.; Rinzler, A.G.; et al. Crystalline Ropes of Metallic Carbon Nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Hirlekar, R.S.; Yamagar, M.; Garse, H.; Vij, M.; Kadam, V.J.; Vidyapeeth, B. Carbon Nanotubes and Its Applications: A Review. Asian J. Pharm. Clin. Res. 2009, 2, 17–27. [Google Scholar]
- Rao, R.; Sharma, R.; Abild-Pedersen, F.; Nørskov, J.K.; Harutyunyan, A.R. Insights into carbon nanotube nucleation: Cap formation governed by catalyst interfacial step flow. Sci. Rep. 2014, 4, 6510. [Google Scholar] [CrossRef]
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; ul Haq Ali Shah, A.; Mian, S.A.; Ali, G. An Overview of the Recent Progress in the Synthesis and Applications of Carbon Nanotubes. C J. Carbon Res. 2019, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Vm, S.; Mohamed, A.R.; Abdullah, A.Z.; Chai, S.-P. Role of Reaction and Factors of Carbon Nanotubes Growth in Chemical Vapour Decomposition Process Using Methane—A Highlight. J. Nanomater. 2010, 2010, 395191. [Google Scholar] [CrossRef]
- Wang, X.-D.; Vinodgopal, K.; Dai, G.-P. Synthesis of Carbon Nanotubes by Catalytic Chemical Vapor Deposition. In Perspective of Carbon Nanotubes; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/67867 (accessed on 11 January 2022). [CrossRef] [Green Version]
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976, 32, 335–349. Available online: https://www.sciencedirect.com/science/article/pii/0022024876901159 (accessed on 13 January 2022). [CrossRef]
- Britannica, T. Editors of Encyclopaedia Nucleation. Encyclopedia Britannica. 11 February 2020. Available online: https://www.britannica.com/science/nucleation (accessed on 12 December 2021).
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1369800115301402?via%3Dihub (accessed on 13 December 2021). [CrossRef]
- Dahmen, K.-H. Chemical Vapor Deposition. In Encyclopedia of Physical Science and Technology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2004; Available online: https://www.sciencedirect.com/science/article/pii/B0122274105001022 (accessed on 21 December 2021).
- Xu, Z.; Yan, T.; Ding, F. Atomistic simulation of the growth of defect-free carbon nanotubes. Chem. Sci. 2015, 6, 4704–4711. Available online: https://pubs.rsc.org/en/Content/ArticleLanding/2015/SC/C5SC00938C (accessed on 5 January 2022). [CrossRef] [PubMed] [Green Version]
- Szabó, A.; Perri, C.; Csató, A.; Giordano, G.; Vuono, D.; Nagy, J.B. Synthesis Methods of Carbon Nanotubes and Related Materials. Materials 2010, 3, 3092–3140. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Franceschi, W.; Menezes, B.R.; Biagioni, A.F.; Coutinho, A.R.; Cividanes, L.S. Synthesis, Characterization, and Applications of Carbon Nanotubes. Carbon-Based Nanofill. Rubber Nanocompos. 2019, 1–45. Available online: https://www.sciencedirect.com/science/article/pii/B9780128132487000018 (accessed on 12 January 2022). [CrossRef]
- Das, R.; Shahnavaz, Z.; Ali, M.E.; Islam, M.M.; Abd Hamid, S.B. Can We Optimize Arc Discharge and Laser Ablation for Well-Controlled Carbon Nanotube Synthesis? Nanoscale Res. Lett. 2016, 11, 510. [Google Scholar] [CrossRef] [Green Version]
- Hokkanen, M.J.; Lautala, S.; Shao, D.; Turpeinen, T.; Koivistoinen, J.; Ahlskog, M. On-chip purification via liquid immersion of arc-discharge synthesized multiwalled carbon nanotubes. Appl. Phys. A 2016, 122, 634. [Google Scholar] [CrossRef] [Green Version]
- Batani, D.; Vinci, T.; Bleiner, D. Laser-ablation and induced nanoparticle synthesis. Laser Part. Beams 2014, 32, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Journet, C.; Maser, W.K.; Bernier, P.; Loiseau, A.; De La Chapelle, M.L.; Lefrant, S.; Deniard, P.; Lee, R.; Fischer, J.E. Large-scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 1997, 388, 756–758. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic growth of single-walled nanotubes by laser vaporization. Chem. Phys. Lett. 1995, 243, 49–54. Available online: https://www.sciencedirect.com/science/article/abs/pii/000926149500825O (accessed on 19 January 2022). [CrossRef]
- Arepalli, S. Laser ablation process for single-walled carbon nanotube production. J. Nanosci. Nanotechnol. 2004, 4, 317–325. [Google Scholar] [CrossRef]
- Scott, C.; Arepalli, S.; Nikolaev, P.; Smalley, R. Growth mechanisms for single-wall carbon nanotubes in a laser-ablation process. Appl. Phys. A 2001, 72, 573–580. [Google Scholar] [CrossRef]
- Song, Y.S.; Youn, J.R. Influence of dispersion states of carbon nanotubes on physical properties of epoxy nanocomposites. Carbon 2005, 43, 1378–1385. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0008622305000527 (accessed on 17 December 2021). [CrossRef]
- Ajayan, P.M.; Schadler, L.S.; Giannaris, C.; Rubio, A. Single-Walled Carbon Nanotube–Polymer Composites: Strength and Weakness. Adv. Mater. 2000, 12, 750–753. [Google Scholar] [CrossRef]
- Yau, A.; Lee, J.; Chen, Y. Nanomaterials for Protein Delivery in Anticancer Applications. Pharmaceutics 2021, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-Y.; Chang, D.W.; Kumar, N.A.; Baek, J.-B. Functionalization of Carbon Nanotubes, Carbon Nanotubes-Polymer Nanocomposites; Yellampalli, S., Ed.; IntechOpen: London, UK, 2011; Available online: https://www.intechopen.com/chapters/16991 (accessed on 15 December 2021). [CrossRef] [Green Version]
- Salah, L.S.; Ouslimani, N.; Bousba, D.; Huynen, I.; Danlée, Y.; Aksas, H. Carbon Nanotubes (CNTs) from Synthesis to Functionalized (CNTs) Using Conventional and New Chemical Approaches. J. Nanomater. 2021, 2021, 4972770. [Google Scholar] [CrossRef]
- Aslam, M.M.-A.; Kuo, H.-W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Nejabat, F.; Rayati, S. Surface modification of multi-walled carbon nanotubes to produce a new bimetallic Fe/Mn catalyst for the aerobic oxidation of hydrocarbons. J. Ind. Eng. Chem. 2019, 69, 324–330. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1226086X18308323?via%3Dihub (accessed on 20 December 2021). [CrossRef]
- Syrgiannis, Z.; Melchionna, M.; Prato, M. Covalent Carbon Nanotube Functionalization. In Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Kelly, K.; Chiang, I.; Mickelson, E.; Hauge, R.; Margrave, J.; Wang, X.; Scuseria, G.; Radloff, C.; Halas, N. Insight into the mechanism of sidewall functionalization of single-walled nanotubes: An STM study. Chem. Phys. Lett. 1999, 313, 445–450. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0009261499009732?via%3Dihub (accessed on 18 December 2021). [CrossRef]
- Chen, J.; Hamon, M.A.; Hu, H.; Chen, Y.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Solution Properties of Single-Walled Carbon Nanotubes. Science 1998, 282, 95–98. [Google Scholar] [CrossRef]
- Heister, E.; Neves, V.; Tîlmaciu, C.; Lipert, K.; Beltrán, V.S.; Coley, H.M.; Silva, S.R.P.; McFadden, J. Triple functionalisation of single-walled carbon nanotubes with doxorubicin, a monoclonal antibody, and a fluorescent marker for targeted cancer therapy. Carbon 2009, 47, 2152–2160. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0008622309001961?via%3Dihub (accessed on 13 January 2022). [CrossRef] [Green Version]
- Pérez, E.M.; Martín, N. Π–π Interactions in Carbon Nanostructures. Chem. Soc. Rev. 2015, 44, 6425–6433. Available online: https://pubs.rsc.org/en/content/articlelanding/2015/CS/C5CS00578G (accessed on 5 January 2022). [CrossRef] [PubMed]
- O’Connell, M.J.; Boul, P.; Ericson, L.M.; Huffman, C.; Wang, Y.; Haroz, E.; Kuper, C.; Tour, J.; Ausman, K.; Smalley, R.E. Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem. Phys. Lett. 2001, 342, 265–271. [Google Scholar] [CrossRef]
- Woods, L.M.; Badescu, S.C.; Reinecke, T.L. Adsorption of simple benzene derivatives on carbon nanotubes. Phys. Rev. B 2007, 75, 155415. Available online: https://journals.aps.org/prb/abstract/10.1103/PhysRevB.75.155415 (accessed on 20 December 2021). [CrossRef] [Green Version]
- Zheng, W.; Li, Q.; Su, L.; Yan, Y.; Zhang, J.; Mao, L. Direct Electrochemistry of Multi-Copper Oxidases at Carbon Nanotubes Noncovalently Functionalized with Cellulose Derivatives. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2006, 18, 587–594. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R.P. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campidelli, S.; Ballesteros, B.; Filoramo, A.; Díaz, D.D.; de la Torre, G.; Torres, T.; Rahman, G.M.A.; Ehli, C.; Kiessling, D.; Werner, F.; et al. Facile Decoration of Functionalized Single-Wall Carbon Nanotubes with Phthalocyanines via “Click Chemistry”. J. Am. Chem. Soc. 2008, 130, 11503–11509. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shum, K.T.; Burnett, J.C.; Rossi, J.J. Nanoparticle-Based Delivery of RNAi Therapeutics: Progress and Challenges. Pharmaceuticals 2013, 6, 85–107. [Google Scholar] [CrossRef] [Green Version]
- Jayasuriya, C.T.; Chen, Y.; Liu, W.; Chen, Q. The influence of tissue microenvironment on stem cell-based cartilage repair. Ann. N. Y. Acad. Sci. 2016, 1383, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Aboofazeli, R. Carbon nanotubes: A promising approach for drug delivery. Iran. J. Pharm. Res. IJPR 2010, 9, 1–3. [Google Scholar]
- Sun, M.; Lee, J.; Chen, Y.; Hoshino, K. Studies of nanoparticle delivery with in vitro bio-engineered microtissues. Bioact. Mater. 2020, 5, 924–937. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H. Advanced Biomedical Techniques for Gene Delivery. Recent Pat. Biomed. Eng. (Discontin.) 2012, 5, 23–28. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Y.; Yu, H.; Machan, J.T.; Alladin, A.; Ramirez, J.; Taliano, R.; Hart, J.; Chen, Q.; Terek, R.M. Anti-miRNA Oligonucleotide Therapy for Chondrosarcoma. Mol. Cancer Ther. 2019, 18, 2021–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martincic, M.; Tobias, G. Filled carbon nanotubes in biomedical imaging and drug delivery. Expert Opin. Drug Deliv. 2014, 12, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Azqhandi, M.H.A.; Farahani, B.V.; Dehghani, N. Encapsulation of methotrexate and cyclophosphamide in interpolymer complexes formed between poly acrylic acid and poly ethylene glycol on multi-walled carbon nanotubes as drug delivery systems. Mater. Sci. Eng. C 2017, 79, 841–847. [Google Scholar] [CrossRef]
- Masotti, A.; Miller, M.R.; Celluzzi, A.; Rose, L.; Micciulla, F.; Hadoke, P.W.; Bellucci, S.; Caporali, A. Regulation of angiogenesis through the efficient delivery of microRNAs into endothelial cells using polyamine-coated carbon nanotubes. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1511–1522. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Ren, J.; Qu, X. Carbon Nanomaterials and DNA: From Molecular Recognition to Applications. Accounts Chem. Res. 2016, 49, 461–470. [Google Scholar] [CrossRef]
- Hassan, H.A.; Smyth, L.; Rubio, N.; Ratnasothy, K.; Wang, T.-W.; Bansal, S.S.; Summers, H.D.; Diebold, S.S.; Lombardi, G.; Al-Jamal, K.T. Carbon nanotubes’ surface chemistry determines their potency as vaccine nanocarriers in vitro and in vivo. J. Control. Release 2016, 225, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Wieckowski, S.; Pastorin, G.; Benincasa, M.; Klumpp, C.; Briand, J.-P.; Gennaro, R.; Prato, M.; Bianco, A. argeted Delivery of Amphotericin B to Cells by Using Functionalized Carbon Nanotubes. Angew. Chem. Int. Ed. 2005, 44, 6358–6362. [Google Scholar] [CrossRef]
- Pastorin, G.; Wu, W.; Wieckowski, S.; Briand, J.-P.; Kostarelos, K.; Prato, M.; Bianco, A. Double functionalisation of carbon nanotubes for multimodal drug delivery. Chem. Commun. 2006, 11, 1182–1184. Available online: https://pubs.rsc.org/en/content/articlehtml/2006/cc/b516309a (accessed on 15 December 2021). [CrossRef]
- Al-Jamal, K.T.; Gherardini, L.; Bardi, G.; Nunes, A.; Guo, C.; Bussy, C.; Herrero, M.A.; Bianco, A.; Prato, M.; Kostarelos, K.; et al. Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc. Natl. Acad. Sci. USA 2011, 108, 10952–10957. Available online: https://www.pnas.org/content/108/27/10952 (accessed on 21 December 2021). [CrossRef] [Green Version]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef] [PubMed]
- Veetil, J.V.; Ye, K. ailored carbon nanotubes for tissue engineering applications. Biotechnol. Prog. 2009, 25, 709–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieland, D.; Vera-Hidalgo, M.; Seelajaroen, H.; Sariciftci, N.S.; Perez, E.M.; Whang, D.R. Mechanically Interlocked Carbon Nanotubes as a Stable Electrocatalytic Platform for Oxygen Reduction. ACS Appl. Mater. Interfaces 2020, 12, 32615–32621. [Google Scholar] [CrossRef] [PubMed]
- Guldi, D.; Martin, N. Carbon Nanotubes and Related Structues: Synthesis, Characterization, Functionalization, and Applications; Wiley-VCH: Weinheim, Germany, 2010; pp. 211–213. [Google Scholar]
- Luo, X.; Matranga, C.; Tan, S.; Alba, N.; Cui, X.T. Carbon nanotube nanoreservior for controlled release of anti-inflammatory dexamethasone. Biomaterials 2011, 32, 6316–6323. [Google Scholar] [CrossRef] [Green Version]
- Dehaghani, M.Z.; Yousefi, F.; Seidi, F.; Bagheri, B.; Mashhadzadeh, A.H.; Naderi, G.; Esmaeili, A.; Abida, O.; Habibzadeh, S.; Saeb, M.R.; et al. Encapsulation of an anticancer drug Isatin inside a host nano-vehicle SWCNT: A molecular dynamics simulation. Sci. Rep. 2021, 11, 18753. [Google Scholar] [CrossRef]
- Kaur, J.; Gill, G.S.; Jeet, K. Applications of carbon nanotubes in drug delivery: A comprehensive review. Charact. Biol. Nanomater. Drug Deliv. 2019, 113–135. Available online: https://www.sciencedirect.com/science/article/pii/B9780128140314000052 (accessed on 15 January 2022). [CrossRef]
- Sharifi, S.; Mirzadeh, H.; Imani, M.; Rong, Z.; Jamshidi, A.; Shokrgozar, M.; Atai, M.; Roohpour, N. Injectable in situ forming drug delivery system based on poly(ε-caprolactone fumarate) for tamoxifen citrate delivery: Gelation characteristics, in vitro drug release and anti-cancer evaluation. Acta Biomater. 2009, 5, 1966–1978. [Google Scholar] [CrossRef]
- Chen, Y.; Song, S.; Yan, Z.; Fenniri, H.; Webster, T.J. Self-assembled rosette nanotubes encapsulate and slowly release dexamethasone. Int. J. Nanomed. 2011, 6, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Rakotondradany, F.; Myles, A.J.; Fenniri, H.; Webster, T.J. Arginine-glycine-aspartic acid modified rosette nanotube-hydrogel composites for bone tissue engineering. Biomaterials 2009, 30, 1309–1320. [Google Scholar] [CrossRef]
- Chun, A.L.; Moralez, J.G.; Fenniri, H.; Webster, T.J. Helical rosette nanotubes: A more effective orthopedic implant material. Nanotechnology 2004, 15, S234. Available online: https://iopscience.iop.org/article/10.1088/0957-4484/15/4/022 (accessed on 12 January 2022). [CrossRef]
- Chen, Y. Self-Assembled Nanostructures for RNA Delivery Against Joint Inflammation. In Orthopaedic Proceedings; The British Editorial Society of Bone & Joint Surgery: London, UK, 15 January 2022; Available online: https://online.boneandjoint.org.uk (accessed on 21 December 2021).
- Zhou, L.; Rubin, L.E.; Liu, C.; Chen, Y. Short Interfering RNA (siRNA)-Based Therapeutics for Cartilage Diseases. Regen. Eng. Transl. Med. 2021, 7, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sands, I.; Zhang, W.; Zhou, L.; Chen, Y. DNA-inspired nanomaterials for enhanced endosomal escape. Proc. Natl. Acad. Sci. USA 2021, 7, 283–290. Available online: https://www.pnas.org/content/118/19/e2104511118 (accessed on 21 December 2021). [CrossRef] [PubMed]
- Yau, A.; Yu, H.; Chen, Y. mRNA Detection with Fluorescence-base Imaging Techniques for Arthritis Diagnosis. J. Rheumatol. Res. 2019, 1, 39–46. [Google Scholar] [PubMed]
- Malik, R.; Tomer, V.K.; Chaudhary, V. Chapter 16-Hybridized Graphene for Chemical Sensing. In Functionalized Graphene Nanocomposites and their Derivatives; Jawaid, M., Bouhfid, R., Kacem, Q.A.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 323–338. [Google Scholar]
- Bandaru, P.R. Electrical properties and applications of carbon nanotube structures. J. Nanosci. Nanotechnol. 2007, 7, 1239–1267. [Google Scholar] [CrossRef]
- Bareket-Keren, L.; Hanein, Y. Carbon nanotube-based multi electrode arrays for neuronal interfacing: Progress and prospects. Front. Neural Circuits 2013, 6, 122. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Ji, H.; Liu, J.; Tu, C.; Kappl, M.; Koynov, K.; Vogt, J.; Butt, J. Carbon Nanotube-Hydrogel Composites Facilitate Neuronal Differentiation While Maintaining Homeostasis of Network Activity. Adv. Mater. 2021, 33, 41. [Google Scholar] [CrossRef]
- Cho, Y.; Ben Borgens, R. Electrically controlled release of the nerve growth factor from a collagen–carbon nanotube composite for supporting neuronal growth. J. Mater. Chem. B 2013, 1, 4166–4170. [Google Scholar] [CrossRef]
- Zang, R.; Yang, S.-T. Multiwalled carbon nanotube-coated polyethylene terephthalate fibrous matrices for enhanced neuronal differentiation of mouse embryonic stem cells. J. Mater. Chem. B 2013, 1, 646–653. [Google Scholar] [CrossRef]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Wang, K.; Li, Y.; Yang, Y. Nanotopography promoted neuronal differentiation of human induced pluripotent stem cells. Colloids Surf. B Biointerfaces 2016, 148, 49–58. [Google Scholar] [CrossRef]
- Mooney, E.; Mackle, J.N.; Blond, D.J.; O’Cearbhaill, E.; Shaw, G.; Blau, W.J.; Barry, F.P.; Barron, V.; Murphy, J.M. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials 2012, 33, 6132–6139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, V.; Cellot, G.; Fabbro, A.; Bosi, S.; Mestroni, L.; Ballerini, L. Improving cardiac myocytes performance by carbon nanotubes platforms†. Front. Physiol. 2013, 4, 6132–6139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borzsonyi, G.; Beingessner, R.L.; Yamazaki, T.; Cho, J.-Y.; Myles, A.J.; Malac, M.; Egerton, R.; Kawasaki, M.; Ishizuka, K.; Kovalenko, A.; et al. Water-Soluble J-Type Rosette Nanotubes with Giant Molar Ellipticity. J. Am. Chem. Soc. 2010, 132, 15136–15139. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Chen, Y.; Zhang, Z.; Saunders, L.; Schipani, E.; Chen, Q.; Ma, P.X. Suppressing mesenchymal stem cell hypertrophy and endochondral ossification in 3D cartilage regeneration with nanofibrous poly(l-lactic acid) scaffold and matrilin-3. Acta Biomater. 2018, 76, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Gunputh, U.; Le, H. Composite coatings for implants and tissue engineering scaffolds. In Biomedical Composites; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 111–138. Available online: https://www.sciencedirect.com/science/article/pii/B9780081007525000068 (accessed on 10 January 2022).
- Corona-Gomez, J.; Chen, X.; Yang, Q. Effect of Nanoparticle Incorporation and Surface Coating on Mechanical Properties of Bone Scaffolds: A Brief Review. J. Funct. Biomater. 2016, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Bilgen, B.; Pareta, R.A.; Myles, A.J.; Fenniri, H.; Ciombor, D.M.; Aaron, R.K.; Webster, T.J. Self-assembled rosette nanotube/hydrogel composites for cartilage tissue engineering. Tissue Eng. Part C Methods 2010, 16, 1233–1243. [Google Scholar] [CrossRef]
- Maruyama, T. Carbon Nanotubes. Handbook of Carbon-Based Nanomaterials. 15 January 2022. Available online: https://www.sciencedirect.com/science/article/pii/B9780128219966000099 (accessed on 13 December 2021).
- Martin-Gallego, M.; Verdejo, R.; Khayet, M.; de Zarate, J.M.O.; Essalhi, M.; Lopez-Manchado, M.A. Thermal conductivity of carbon nanotubes and graphene in epoxy nanofluids and nanocomposites. Nanoscale Res. Lett 2011, 6, 610. [Google Scholar] [CrossRef] [Green Version]
- Gorain, B.; Choudhury, H.; Pandey, M.; Kesharwani, P.; Abeer, M.M.; Tekade, R.K.; Hussain, Z. Carbon nanotube scaffolds as emerging nanoplatform for myocardial tissue regeneration: A review of recent developments and therapeutic implications. Biomed. Pharmacother. 2022, 104, 496–508. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0753332218311314 (accessed on 12 January 2022). [CrossRef]
- Calabrese, L.; Khaskoussi, A.; Proverbio, E. Wettability and Anti-Corrosion Performances of Carbon Nanotube-Silane Composite Coatings. Fibers 2020, 8, 57. [Google Scholar] [CrossRef]
- Price, R.L.; Ellison, K.; Haberstroh, K.M.; Webster, T.J. Nanometer surface roughness increases select osteoblast adhesion on carbon nanofiber compacts. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 70, 129–138. [Google Scholar] [CrossRef]
- Imaninezhad, M.; Schobr, J.; Griggs, D.; Ruminski, P.; Kuljanishvili, I.; Zustiak, S.P. Cell Attachment and Spreading on Carbon Nanotubes Is Facilitated by Integrin Binding. Front. Bioeng. Biotechnol. 2018, 6, 129. [Google Scholar] [CrossRef] [Green Version]
- Yau, A.; Sands, I.; Chen, Y. Nano-Scale Surface Modifications to Advance Current Treatment Options for Cervical Degenerative Disc Disease (CDDD). J. Orthop. Res. Ther. 2019, 4, 1147. [Google Scholar] [PubMed]
- Zhang, L.; Chen, Y.; Rodriguez, J.; Fenniri, H.; Webster, T.J. Biomimetic helical rosette nanotubes and nanocrystalline hydroxyapatite coatings on titanium for improving orthopedic implants. Int. J. Nanomed. 2008, 3, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Cossman, J.; Jayasuriya, C.T.; Li, X.; Guan, Y.; Fonseca, V.; Yang, K.; Charbonneau, C.; Yu, H.; Kanbe, K.; et al. Deficient Mechanical Activation of Anabolic Transcripts and Post-Traumatic Cartilage Degeneration in Matrilin-1 Knockout Mice. PLoS ONE 2016, 11, e0156676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, W.; Lee, J.; Kuhn, L.; Chen, Y. Controlled Self-Assembly of DNA-Mimicking Nanotubes to Form a Layer-by-Layer Scaffold for Homeostatic Tissue Constructs. ACS Appl. Mater. Interfaces 2021, 13, 51321–51332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yau, A.; Yu, H.; Kuhn, L.; Guo, W.; Chen, Y. Self-assembled biomimetic Nano-Matrix for stem cell anchorage. J. Biomed. Mater. Res. Part A 2020, 108, 984–991. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griger, S.; Sands, I.; Chen, Y. Comparison between Janus-Base Nanotubes and Carbon Nanotubes: A Review on Synthesis, Physicochemical Properties, and Applications. Int. J. Mol. Sci. 2022, 23, 2640. https://doi.org/10.3390/ijms23052640
Griger S, Sands I, Chen Y. Comparison between Janus-Base Nanotubes and Carbon Nanotubes: A Review on Synthesis, Physicochemical Properties, and Applications. International Journal of Molecular Sciences. 2022; 23(5):2640. https://doi.org/10.3390/ijms23052640
Chicago/Turabian StyleGriger, Sydney, Ian Sands, and Yupeng Chen. 2022. "Comparison between Janus-Base Nanotubes and Carbon Nanotubes: A Review on Synthesis, Physicochemical Properties, and Applications" International Journal of Molecular Sciences 23, no. 5: 2640. https://doi.org/10.3390/ijms23052640
APA StyleGriger, S., Sands, I., & Chen, Y. (2022). Comparison between Janus-Base Nanotubes and Carbon Nanotubes: A Review on Synthesis, Physicochemical Properties, and Applications. International Journal of Molecular Sciences, 23(5), 2640. https://doi.org/10.3390/ijms23052640






