Kupffer Cells and Blood Monocytes Orchestrate the Clearance of Iron–Carbohydrate Nanoparticles from Serum
Abstract
1. Introduction
2. Results
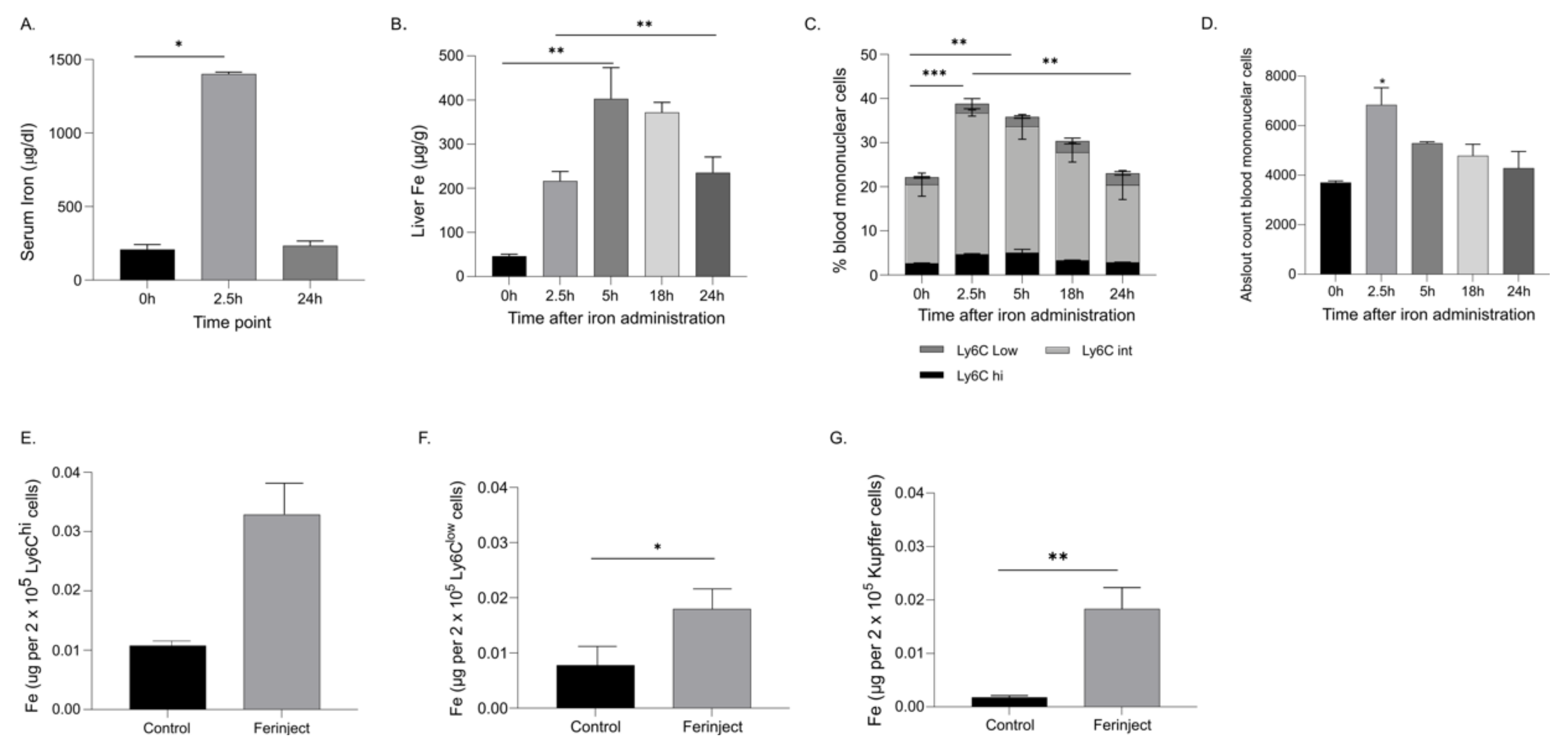
2.1. Clearance of Circulating Iron Carboxymaltose in the Serum Occured within 24 h
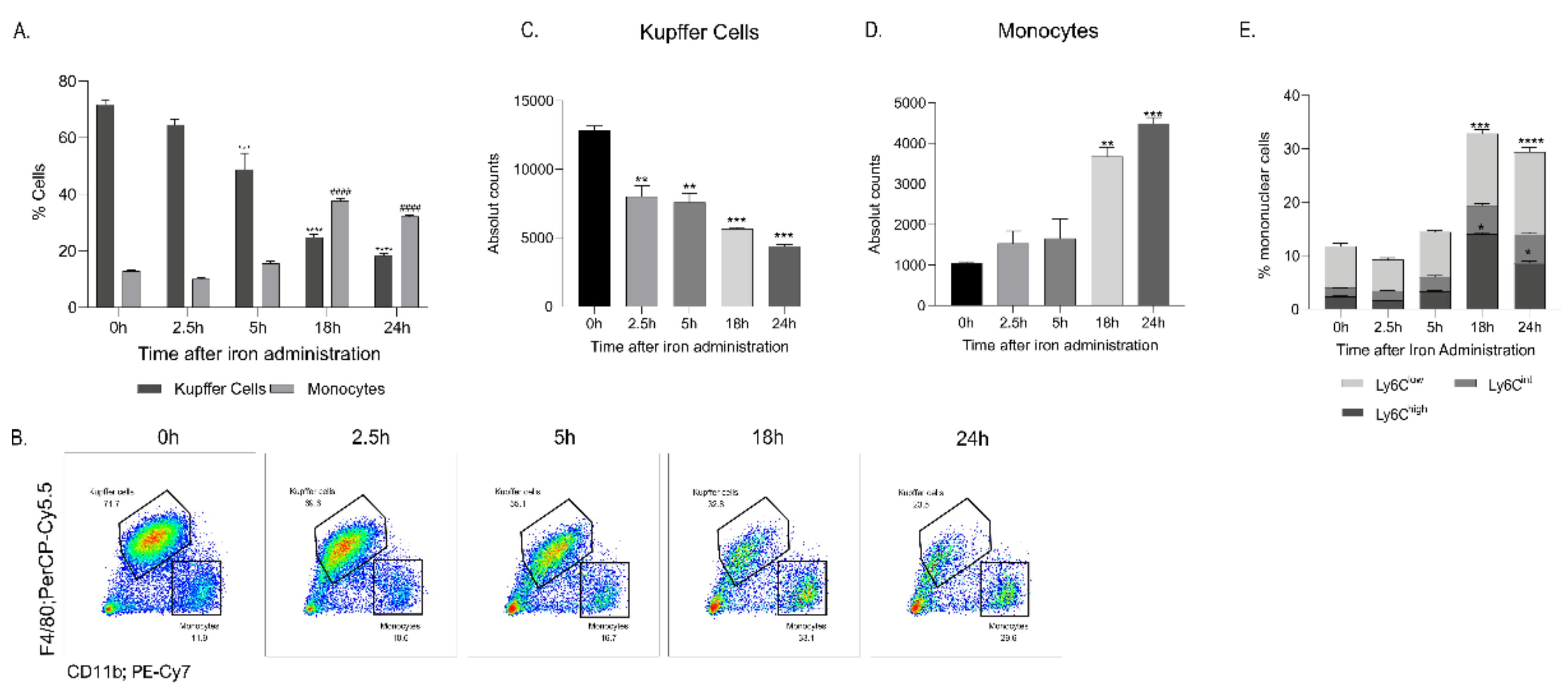
2.2. FCM Administration Increased Blood and Liver Monocytes and Simultaneously Decreased Liver Kupffer Cells
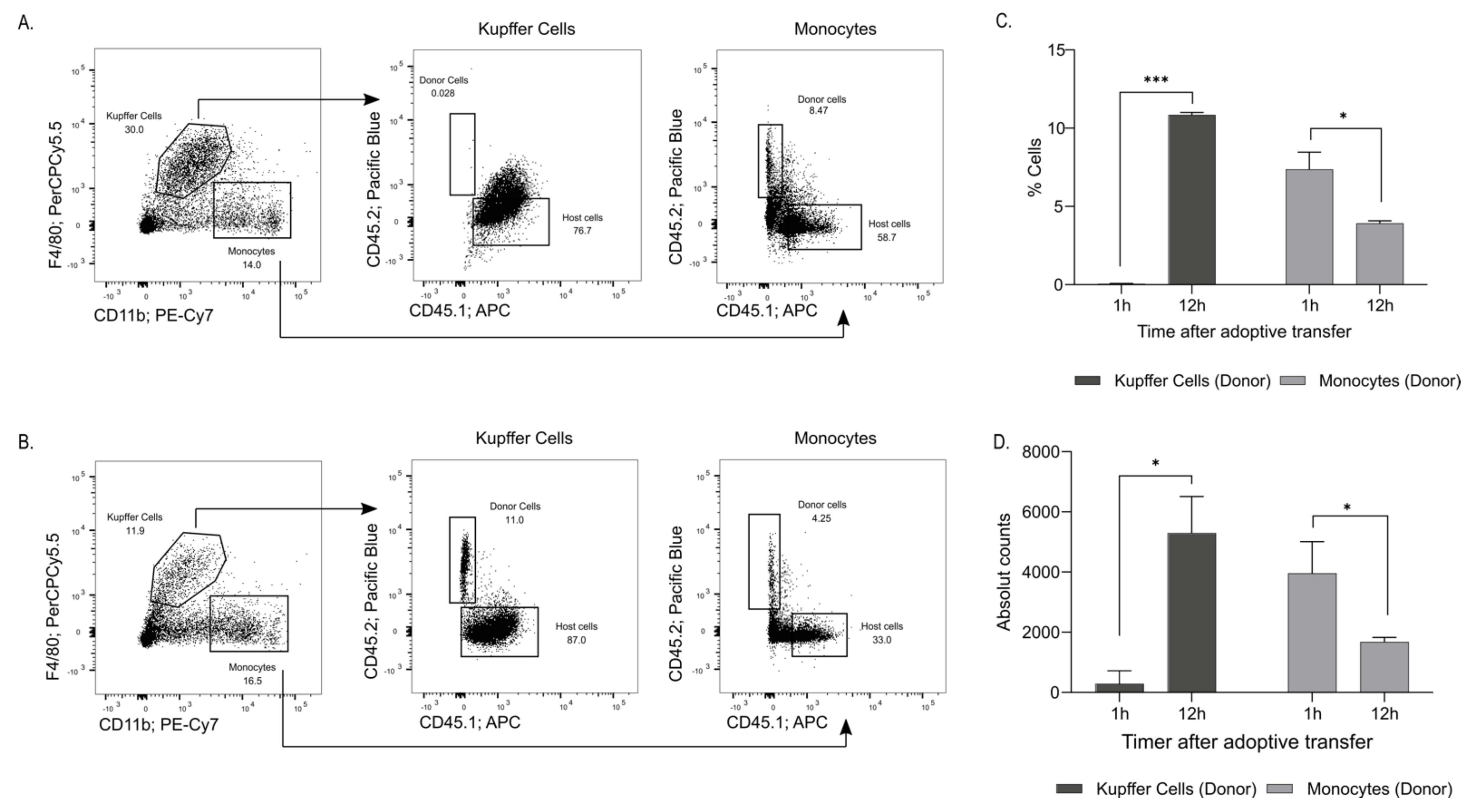
2.3. Adoptive Transfer Demonstrated Recruitment of Blood Monocytes to the Liver
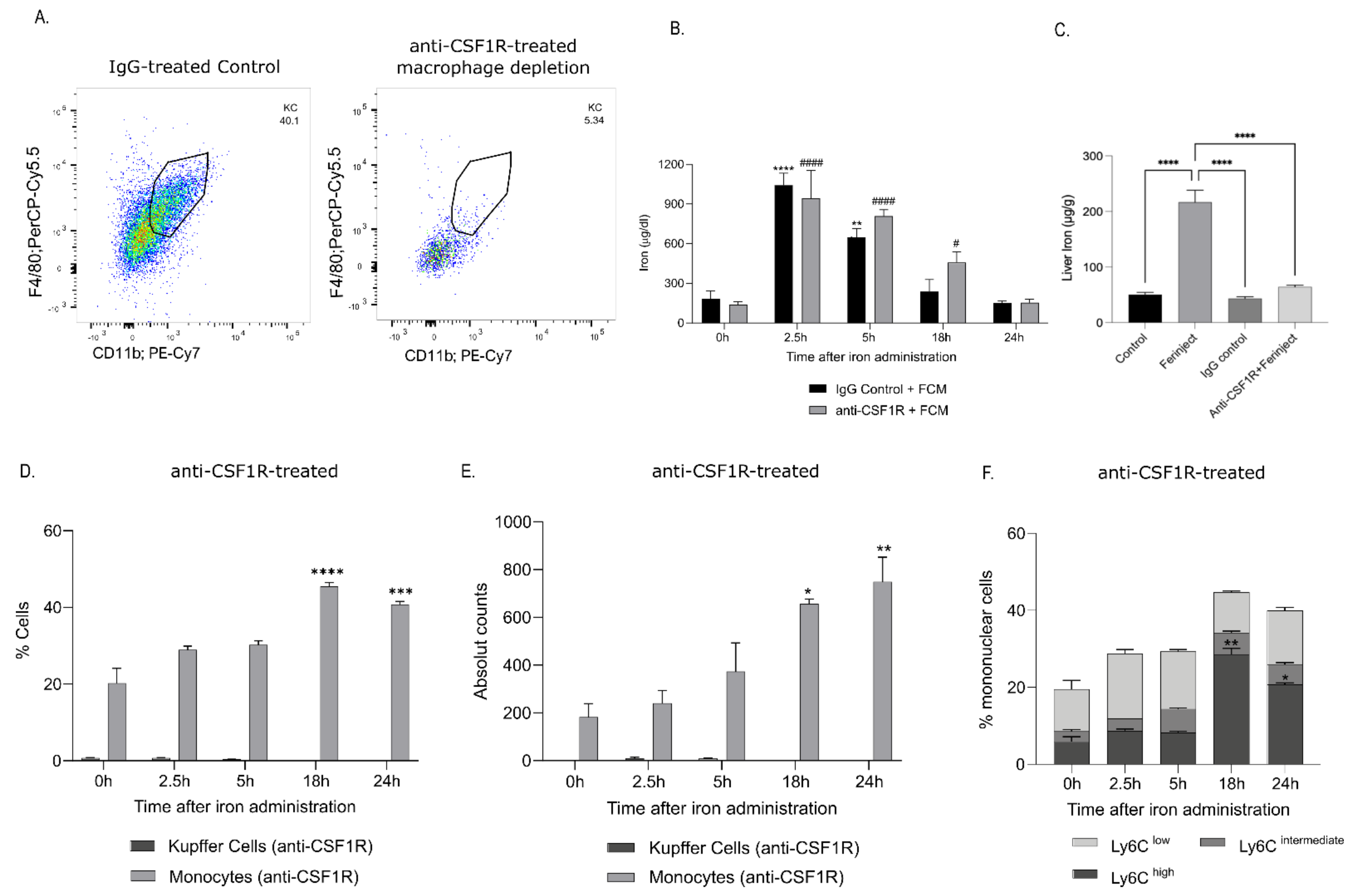
2.4. Anti-CSF1R Antibody Administration Depleted Tissue-Resident Macrophages Disrupting Iron Uptake and Metabolization
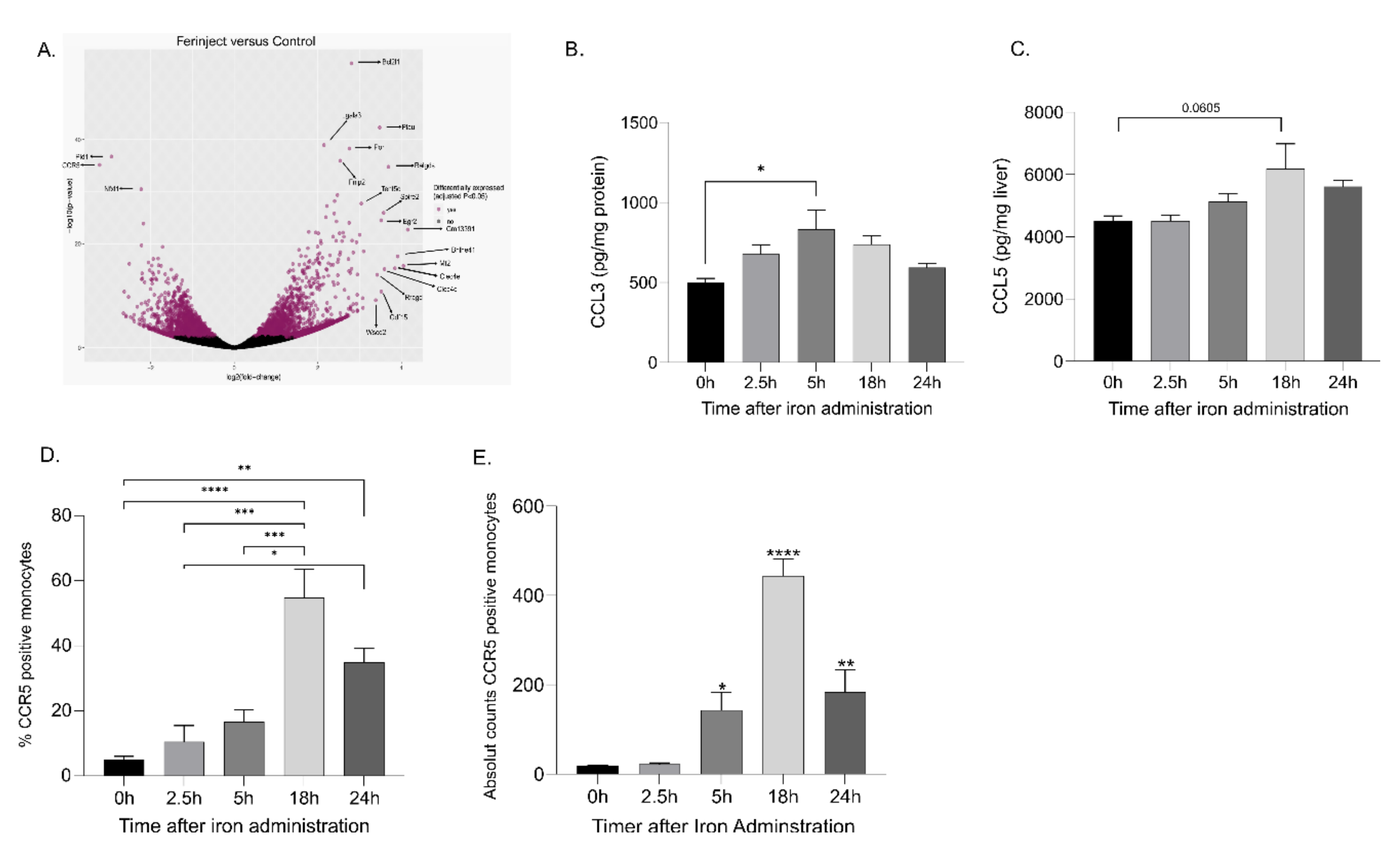
2.5. Recruitment of Blood Monocytes into the Liver Was CCR5-Mediated
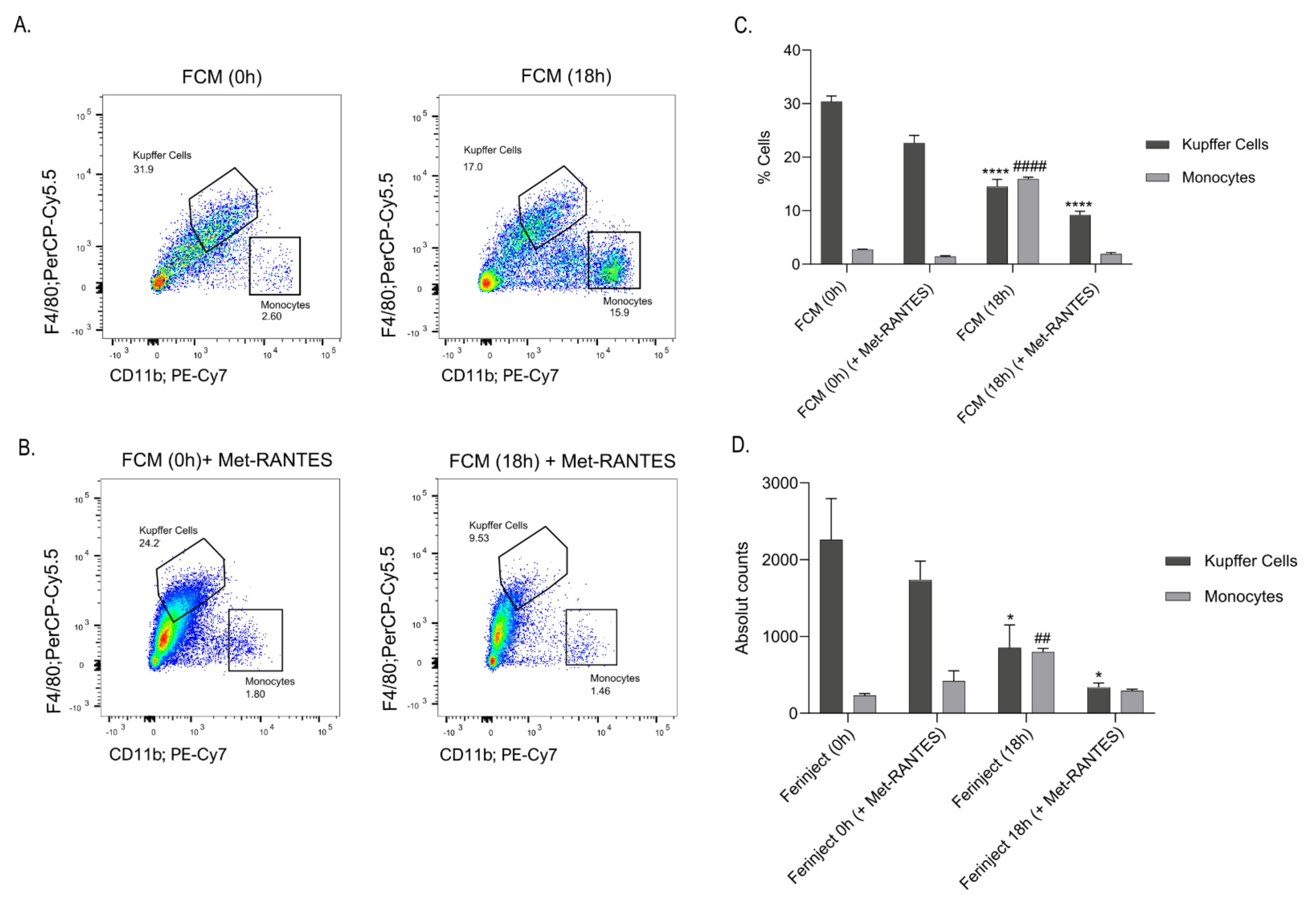
2.6. Blocking CCR5 Inhibited the Migration of Blood Monocytes to the Liver
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Iron Measurements
4.3. Collection of Liver Kupffer Cells
4.4. Collection of Blood and Blood Cells
4.5. RNA Extraction and Sequencing
4.6. ELISA
4.7. Inhibition of Monocyte Recruitment by Met-Rantes
4.8. Flow Cytometry and Cell Sorting
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gozzelino, R.; Arosio, P. Iron Homeostasis in Health and Disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron Deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Polin, V.; Coriat, R.; Perkins, G.; Dhooge, M.; Abitbol, V.; Leblanc, S.; Prat, F.; Chaussade, S. Iron Deficiency: From Diagnosis to Treatment. Dig. Liver Dis. 2013, 45, 803–809. [Google Scholar] [CrossRef]
- Olone, E.L.; Hodson, E.M.; Nistor, I.; Bolignano, D.; Webster, A.C.; Craig, J.C. Parenteral versus Oral Iron Therapy for Adults and Children with Chronic Kidney Disease. Cochrane Database Syst. Rev. 2019, 2019, 1–100. [Google Scholar] [CrossRef] [PubMed]
- Neiser, S.; Rentsch, D.; Dippon, U.; Kappler, A.; Weidler, P.G.; Göttlicher, J.; Steininger, R.; Wilhelm, M.; Braitsch, M.; Funk, F.; et al. Physico-Chemical Properties of the New Generation IV Iron Preparations Ferumoxytol, Iron Isomaltoside 1000 and Ferric Carboxymaltose. BioMetals 2015, 28, 615–635. [Google Scholar] [CrossRef]
- Funk, F.; Ryle, P.; Canclini, C.; Neiser, S. The new generation of intravenous iron: Chemistry, pharmacology, and toxicology of ferric carboxymaltose. Arzneimittelforschung 2010, 60, 353–545. [Google Scholar] [CrossRef]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
- Shepshelovich, D.; Rozen-Zvi, B.; Avni, T.; Gafter, U.; Gafter-Gvili, A. Intravenous Versus Oral Iron Supplementation for the Treatment of Anemia in CKD: An Updated Systematic Review and Meta-Analysis. Am. J. Kidney Dis. 2016, 68, 677–690. [Google Scholar] [CrossRef]
- Pietrangelo, A. Iron and the Liver. Liver Int. 2016, 36, 116–123. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Development, Surface Modification and Applications in Chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Alphandéry, E. Biodistribution and Targeting Properties of Iron Oxide Nanoparticles for Treatments of Cancer and Iron Anemia Disease. Nanotoxicology 2019, 13, 573–596. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron Deficiency Anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Hettinger, J.; Richards, D.M.; Hansson, J.; Barra, M.M.; Joschko, A.C.; Krijgsveld, J.; Feuerer, M. Origin of Monocytes and Macrophages in a Committed Progenitor. Nat. Immunol. 2013, 14, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Fell, L.H.; Seiler-Mußler, S.; Sellier, A.B.; Rotter, B.; Winter, P.; Sester, M.; Fliser, D.; Heine, G.H.; Zawada, A.M. Impact of Individual Intravenous Iron Preparations on the Differentiation of Monocytes towards Macrophages and Dendritic Cells. Nephrol. Dial. Transplant. 2016, 31, 1835–1845. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xue, W.; Liu, Y.; Zhang, N.; Yao, Y.; Ma, P.; Wen, H.; Huang, S.; Luo, Y.; Fan, H. Effects of Core Size and PEG Coating Layer of Iron Oxide Nanoparticles on the Distribution and Metabolism in Mice. Int. J. Nanomed. 2018, 13, 5719–5731. [Google Scholar] [CrossRef]
- Garbowski, M.W.; Bansal, S.; Porter, J.B.; Mori, C.; Burckhardt, S.; Hider, R.C. Intravenous Iron Preparations Transiently Generate Non-Transferrin-Bound Iron from Two Proposed Pathways. Haematologica 2021, 106, 2885–2896. [Google Scholar] [CrossRef]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and Tissue Specialization. Annu. Rev. Immunol. 2015, 33. [Google Scholar] [CrossRef]
- Graubardt, N.; Vugman, M.; Mouhadeb, O.; Caliari, G.; Pasmanik-Chor, M.; Reuveni, D.; Zigmond, E.; Brazowski, E.; David, E.; Chappell-Maor, L.; et al. Ly6Chi Monocytes and Their Macrophage Descendants Regulate Neutrophil Function and Clearance in Acetaminophen-Induced Liver Injury. Front. Immunol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Brempelis, K.J.; Crispe, I.N. Infiltrating Monocytes in Liver Injury and Repair. Clin. Transl. Immunol. 2016, 5, e113. [Google Scholar] [CrossRef]
- Tomczyk, M.; Kraszewska, I.; Dulak, J.; Jazwa-Kusior, A. Modulation of the Monocyte/Macrophage System in Heart Failure by Targeting Heme Oxygenase-Vascul. Pharmacol 2019, 112, 79–90. [Google Scholar] [CrossRef]
- Nairz, M.; Theurl, I.; Swirski, F.K.; Weiss, G. “Pumping Iron”—How Macrophages Handle Iron at the Systemic, Microenvironmental, and Cellular Levels. Pflugers Arch. Eur. J. Physiol. 2017, 469, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.L.; Guilliams, M. The Role of Kupffer Cells in Hepatic Iron and Lipid Metabolism. J. Hepatol. 2018, 69, 1197–1199. [Google Scholar] [CrossRef]
- Sukhbaatar, N.; Weichhart, T. Iron Regulation: Macrophages in Control. Pharmaceuticals 2018, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Danielson, B.G. Structure, Chemistry, and Pharmacokinetics of Intravenous Iron Agents. J. Am. Soc. Nephrol. 2004, 15, 93–98. [Google Scholar] [CrossRef]
- Guggenheim, E.J.; Rappoport, J.Z.; Lynch, I. Mechanisms for Cellular Uptake of Nanosized Clinical MRI Contrast Agents. Nanotoxicology 2020, 14, 504–532. [Google Scholar] [CrossRef]
- Geisser, P.; Burckhardt, S. The Pharmacokinetics and Pharmacodynamics of Iron Preparations. Pharmaceutics 2011, 3, 12–33. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, J.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Tang, D. Ferroptosis Is a Type of Autophagy-Dependent Cell Death. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Puengel, T.; Krenkel, O.; Kohlhepp, M.; Lefebvre, E.; Luedde, T.; Trautwein, C.; Tacke, F. Differential Impact of the Dual CCR2/CCR5 Inhibitor Cenicriviroc on Migration of Monocyte and Lymphocyte Subsets in Acute Liver Injury. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Blériot, C.; Ginhoux, F. Understanding the Heterogeneity of Resident Liver Macrophages. Front. Immunol. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Melino, M.; Gadd, V.L.; Alexander, K.A.; Beattie, L.; Lineburg, K.E.; Martinez, M.; Teal, B.; Le Texier, L.; Irvine, K.M.; Miller, G.C.; et al. Spatiotemporal Characterization of the Cellular and Molecular Contributors to Liver Fibrosis in a Murine Hepatotoxic-Injury Model. Am. J. Pathol. 2016, 186, 524–538. [Google Scholar] [CrossRef]
- Scott, C.L.; Zheng, F.; De Baetselier, P.; Martens, L.; Saeys, Y.; De Prijck, S.; Lippens, S.; Abels, C.; Schoonooghe, S.; Raes, G.; et al. Bone Marrow-Derived Monocytes Give Rise to Self-Renewing and Fully Differentiated Kupffer Cells. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Haschka, D.; Petzer, V.; Kocher, F.; Tschurtschenthaler, C.; Schaefer, B.; Seifert, M.; Sopper, S.; Sonnweber, T.; Feistritzer, C.; Arvedson, T.L.; et al. Classical and Intermediate Monocytes Scavenge Non-Transferrin-Bound Iron and Damaged Erythrocytes. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Oo, Y.H.; Shetty, S.; Adams, D.H. The Role of Chemokines in the Recruitment of Lymphocytes to the Liver. Dig. Dis. 2010, 28, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Theurl, I.; Hilgendorf, I.; Nairz, M.; Tymoszuk, P.; Haschka, D.; Asshoff, M.; He, S.; Gerhardt, L.M.S.; Holderried, T.A.W.; Seifert, M.; et al. On-Demand Erythrocyte Disposal and Iron Recycling Requires Transient Macrophages in the Liver. Nat. Med. 2016, 22, 945–951. [Google Scholar] [CrossRef]
- Li, S.; Huang, Y. Ferroptosis: An Iron-Dependent Cell Death Form Linking Metabolism, Diseases, Immune Cell and Targeted Therapy. Clin. Transl. Oncol. 2021, 24, 1–12. [Google Scholar] [CrossRef]
- Cheng, X.; Tian, X.; Wu, A.; Li, J.; Tian, J.; Chong, Y.; Chai, Z.; Zhao, Y.; Chen, C.; Ge, C. Protein Corona Influences Cellular Uptake of Gold Nanoparticles by Phagocytic and Nonphagocytic Cells in a Size-Dependent Manner. ACS Appl. Mater. Interfaces 2015, 7, 20568–20575. [Google Scholar] [CrossRef]
- Branstetter, D.; Bonham, L.; Palmer, J.S.; MacDonald, K.P.A.; Smith, J.; Hill, G.R.; Clouston, A.; Pettit, A.R.; Hume, D.A.; Cronau, S.; et al. An Antibody against the Colony-Stimulating Factor 1 Receptor Depletes the Resident Subset of Monocytes and Tissue- and Tumor-Associated Macrophages but Does Not Inhibit Inflammation. Blood 2010, 116, 3955–3963. [Google Scholar] [CrossRef]
- Wan, X.; McCarthy, D.P.; Unanue, E.R.; Vomund, A.N.; Carrero, J.A.; Hu, H.; Zinselmeyer, B.H.; Ferris, S.T. Resident Macrophages of Pancreatic Islets Have a Seminal Role in the Initiation of Autoimmune Diabetes of NOD Mice. Proc. Natl. Acad. Sci. USA 2017, 114, E10418–E10427. [Google Scholar] [CrossRef]
- Xu, F.; Zhen, P.; Zheng, Y.; Lijuan, F.; Aiting, Y.; Min, C.; Hong, Y.; Jidong, J. Preparation of Kupffer Cell Enriched Non-Parenchymal Liver Cells with High Yield and Reduced Damage of Surface Markers by a Modified Method for Flow Cytometry. Cell Biol. Int. 2013, 37, 284–291. [Google Scholar] [CrossRef]
- Anders, H.-J.; Frink, M.; Linde, Y.; Banas, B.; Wörnle, M.; Cohen, C.D.; Vielhauer, V.; Nelson, P.J.; Gröne, H.-J.; Schlöndorff, D. CC Chemokine Ligand 5/RANTES Chemokine Antagonists Aggravate Glomerulonephritis Despite Reduction of Glomerular Leukocyte Infiltration. J. Immunol. 2003, 170, 5658–5666. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arsiwala, T.; Vogt, A.-C.S.; Barton, A.E.; Manolova, V.; Funk, F.; Flühmann, B.; Bachmann, M.F. Kupffer Cells and Blood Monocytes Orchestrate the Clearance of Iron–Carbohydrate Nanoparticles from Serum. Int. J. Mol. Sci. 2022, 23, 2666. https://doi.org/10.3390/ijms23052666
Arsiwala T, Vogt A-CS, Barton AE, Manolova V, Funk F, Flühmann B, Bachmann MF. Kupffer Cells and Blood Monocytes Orchestrate the Clearance of Iron–Carbohydrate Nanoparticles from Serum. International Journal of Molecular Sciences. 2022; 23(5):2666. https://doi.org/10.3390/ijms23052666
Chicago/Turabian StyleArsiwala, Tasneem, Anne-Cathrine S. Vogt, Amy E. Barton, Vania Manolova, Felix Funk, Beat Flühmann, and Martin F. Bachmann. 2022. "Kupffer Cells and Blood Monocytes Orchestrate the Clearance of Iron–Carbohydrate Nanoparticles from Serum" International Journal of Molecular Sciences 23, no. 5: 2666. https://doi.org/10.3390/ijms23052666
APA StyleArsiwala, T., Vogt, A.-C. S., Barton, A. E., Manolova, V., Funk, F., Flühmann, B., & Bachmann, M. F. (2022). Kupffer Cells and Blood Monocytes Orchestrate the Clearance of Iron–Carbohydrate Nanoparticles from Serum. International Journal of Molecular Sciences, 23(5), 2666. https://doi.org/10.3390/ijms23052666






