Nitrite Concentration in the Striated Muscles Is Reversely Related to Myoglobin and Mitochondrial Proteins Content in Rats
Abstract
1. Introduction
2. Results
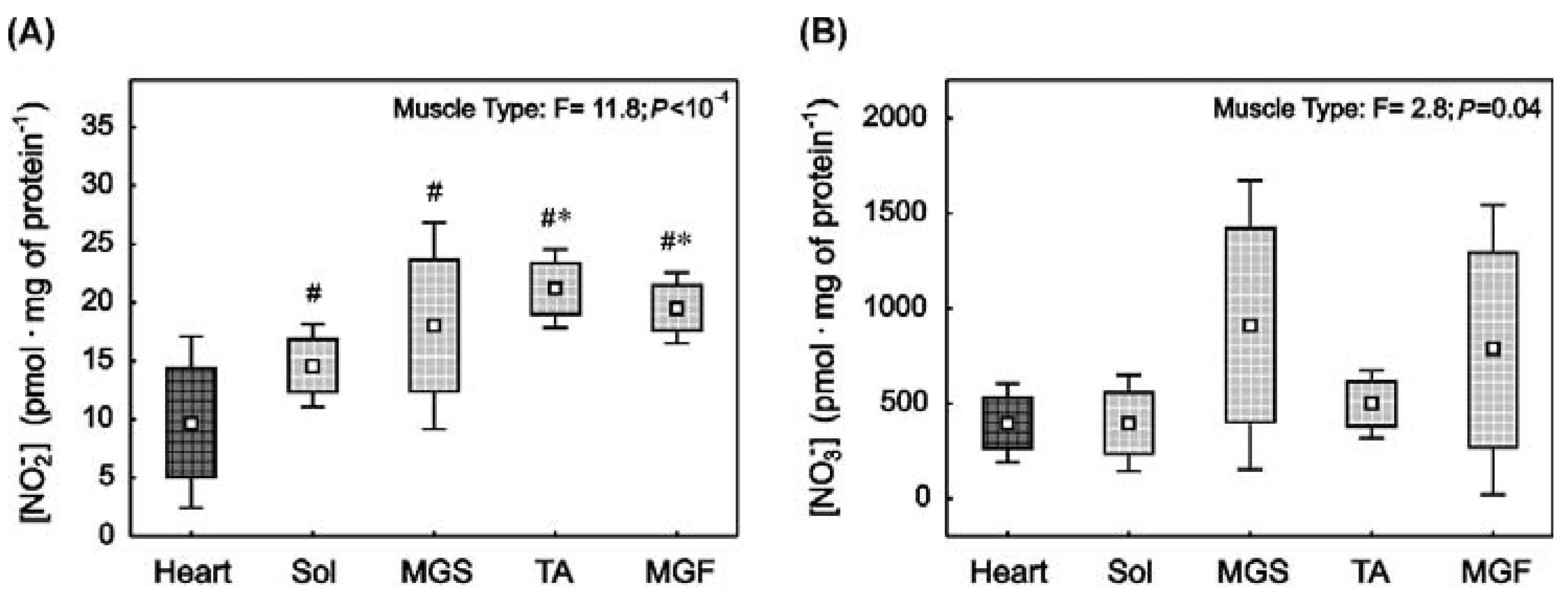
2.1. Nitrite and Nitrate Concentrations in the Heart and in the Skeletal Muscles with Varied Muscle Fibre Type Composition
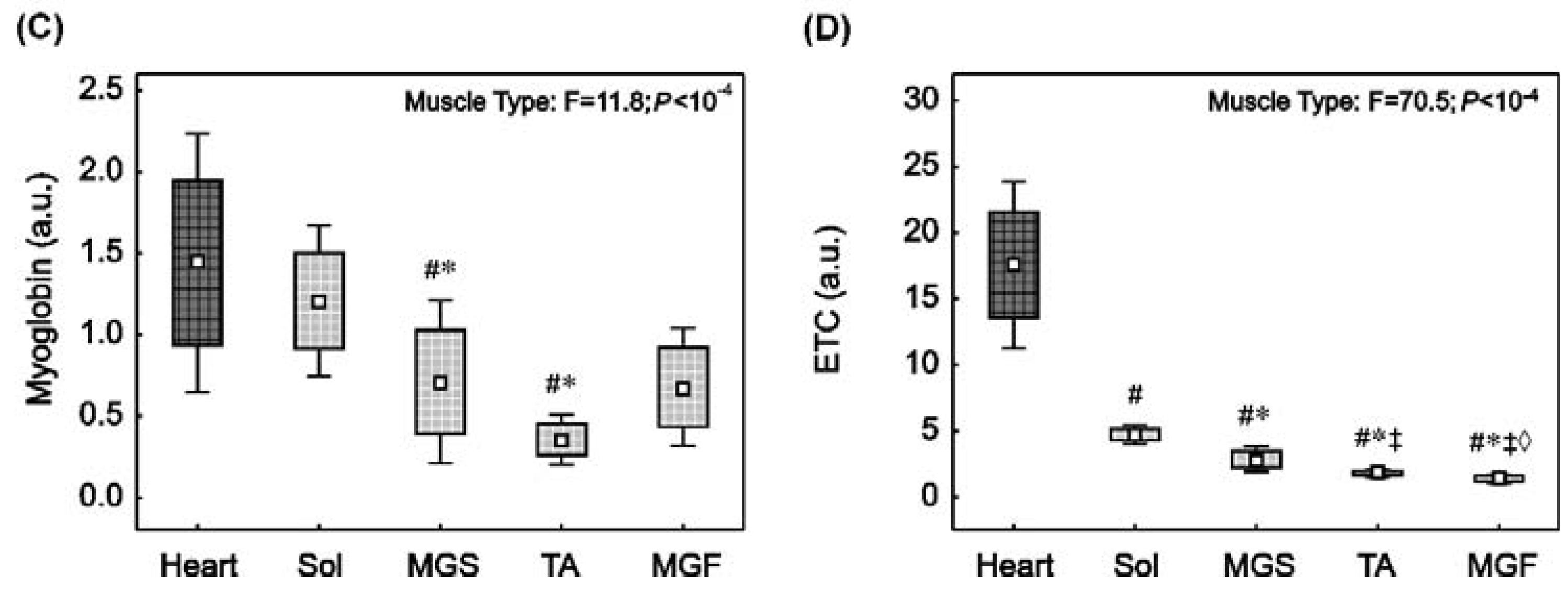
2.2. Myoglobin and ETC Proteins Content in the Heart and in the Skeletal Muscles with Varied Muscle Fibre Type Composition
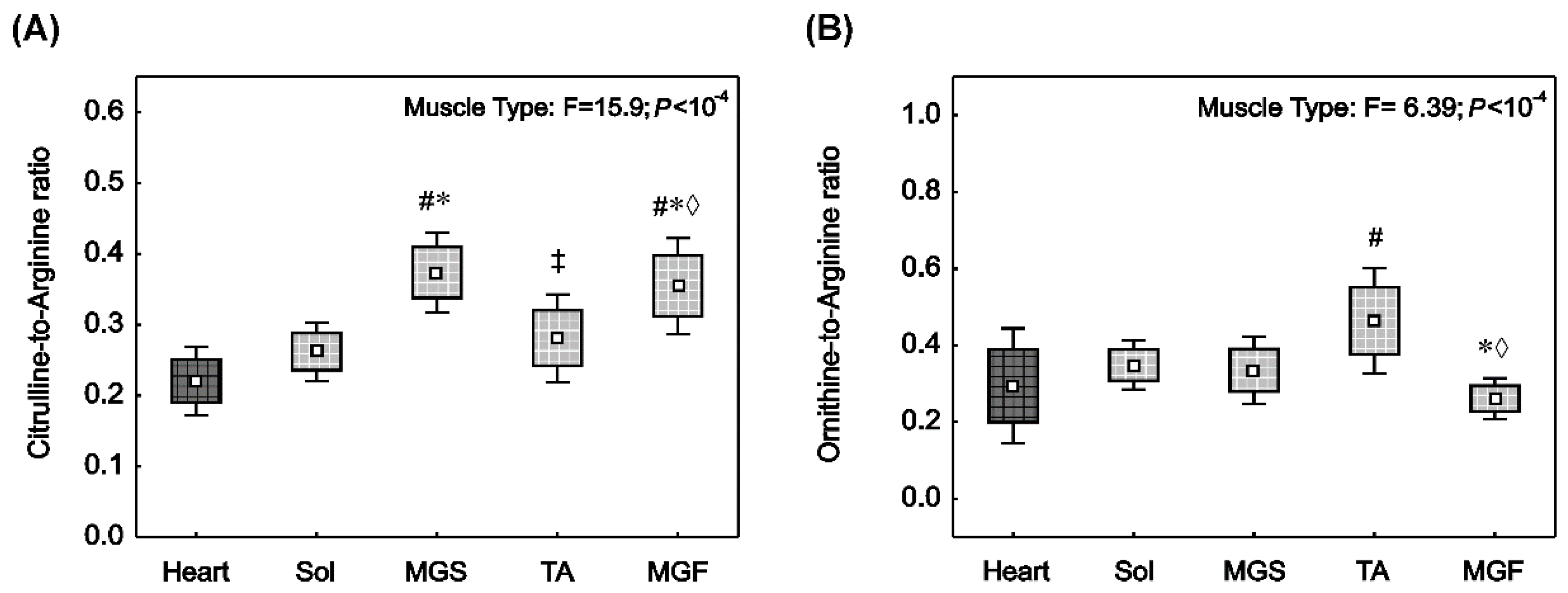
2.3. NO• Synthase and Arginase Activities in the Heart and in the Skeletal Muscles with Varied Muscle Fibre Type Composition
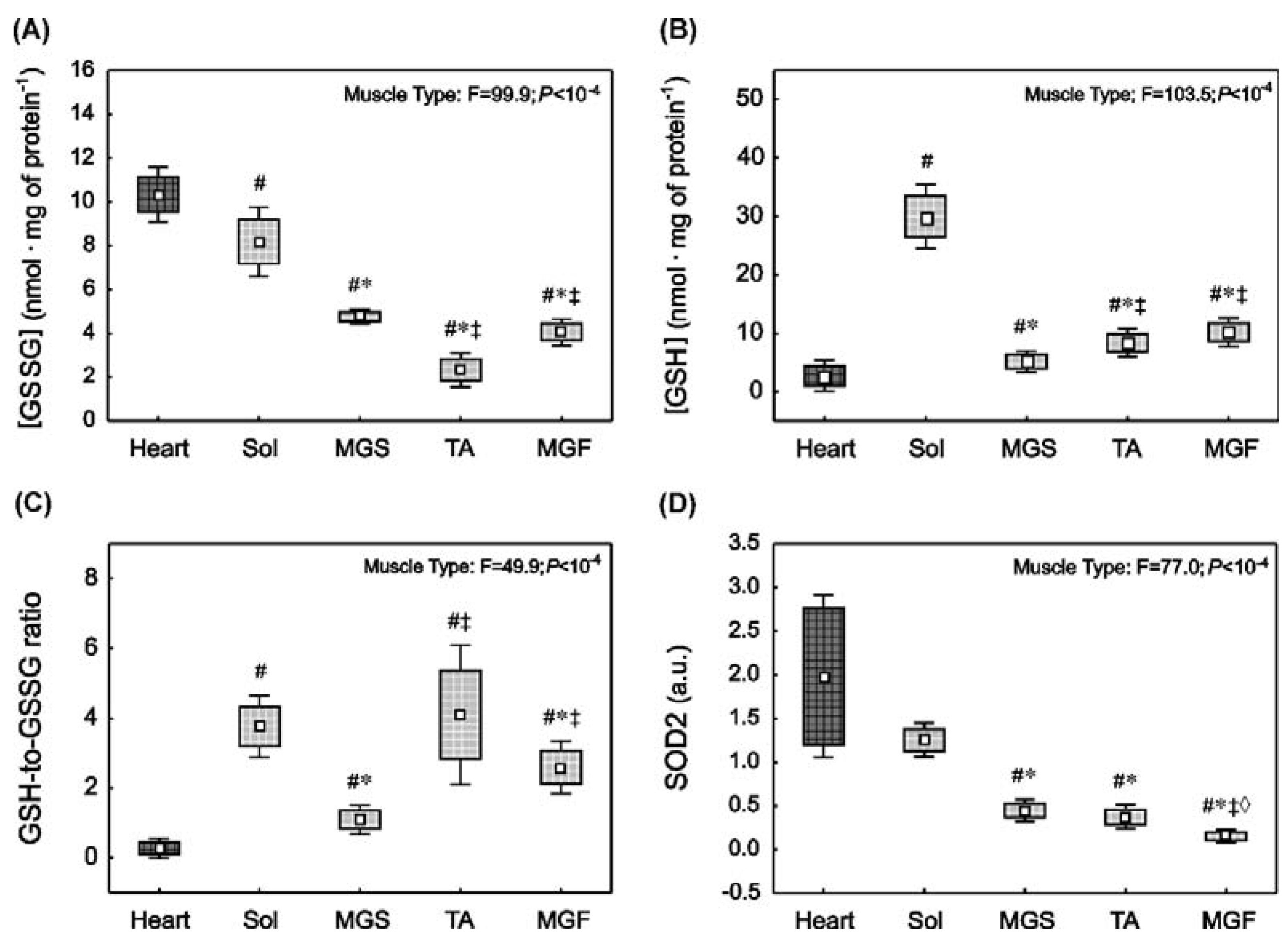
2.4. Oxidative Stress and Antioxidant Capacity Markers in the Heart and in the Skeletal Muscles with Varied Muscle Fibre Type Composition
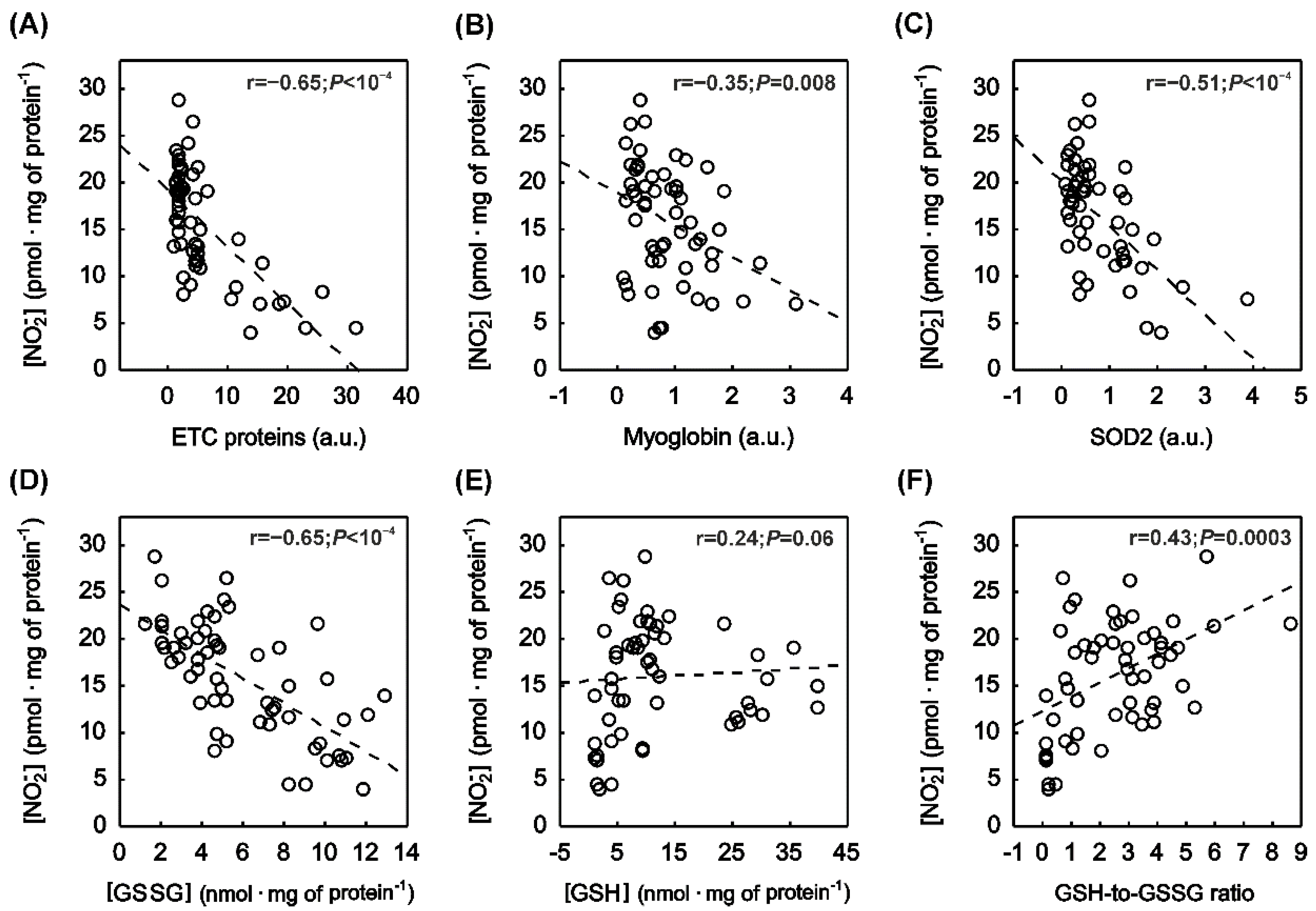
2.5. Correlation between Muscle NOx Concentration and Muscle Protein Content
2.6. Correlation between Muscle NOx Concentration and Muscle Oxidative Stress as Well as Antioxidant Capacity
3. Discussion
3.1. Nitrite and Nitrate Concentration in the Striated Muscles of Varied Muscle Fibre Type Composition
3.2. Relationship between the Myoglobin Content and the Nitrite Concentration in Striated Muscles
3.3. Nitrite Content in Relation to Redox Environment in the Striated Muscles of Varied Muscle Fibre Type Composition
4. Materials and Methods
4.1. Animals
4.2. Muscle Tissue Extraction
4.3. Muscle Sample Preparation for NO• Metabolite and Targeted Metabolomic Analysis
4.3.1. NO• Metabolite Quantification
4.3.2. Targeted Metabolomic Analysis
4.4. Protein Extraction and Western Immunoblotting Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moncada, S.; Higgs, E.A. Endogenous nitric oxide: Physiology, pathology and clinical relevance. Eur. J. Clin. Investig. 1991, 21, 361–374. [Google Scholar] [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Majerczak, J.; Grandys, M.; Frołow, M.; Szkutnik, Z.; Zakrzewska, A.; Niżankowski, R.; Duda, K.; Chlopicki, S.; Zoladz, J.A. Age-dependent impairment in endothelial function and arterial stiffness in former high class male athletes is no different to that in men with no history of physical training. J. Am. Heart Assoc. 2019, 8, e012670. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Kaplon, R.E.; Gioscia-Ryan, R.A.; LaRocca, T.J. You’re only as old as your arteries: Translational strategies for preserving vascular endothelial function with aging. Physiology 2014, 29, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Clementi, E.; Brown, G.C.; Feelisch, M.; Moncada, S. Persistent inhibition of cell respiration by nitric oxide: Crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. USA 1998, 95, 7631–7636. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Nitric oxide and mitochondrial respiration. Biochim. Biophys. Acta 1999, 1411, 351–369. [Google Scholar] [CrossRef]
- Stamler, J.S.; Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Schechter, A.N.; Kim-Shapiro, D.B.; Patel, R.P.; Hogg, N.; Shiva, S.; Cannon, R.O., 3rd; Kelm, M.; Wink, D.A.; Espey, M.G.; et al. The emerging biology of the nitrite anion. Nat. Chem. Biol. 2005, 1, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Rassaf, T.; Maloney, R.E.; Rodriguez, C.M.; Saijo, F.; Rodriguez, J.R.; Feelisch, M. Cellular targets and mechanisms of nitros(yl)ation: An insight into their nature and kinetics in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 4308–4313. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Park, J.W.; Swanson, K.M.; Dey, S.; Noguchi, C.T.; Schechter, A.N. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide 2015, 47, 10–16. [Google Scholar] [CrossRef]
- Majerczak, J.; Guzik, M.; Zakrzewska, A.; Kij, A.; Chlopicki, S.; Zoladz, J.A. Eight weeks of voluntary wheel running enhances nitrite concentration in fast skeletal muscles of mice. In Proceedings of the 25th Kraków Conference on Endothelium, Krakow, Poland, 20–21 October 2017; p. 74. [Google Scholar]
- Modin, A.; Björne, H.; Herulf, M.; Alving, K.; Weitzberg, E.; Lundberg, J.O. Nitrite-derived nitric oxide: A possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol. Scand. 2001, 171, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S. Nitrite: A physiological store of nitric oxide and modulator of mitochondrial function. Redox Biol. 2013, 1, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Fernandez, B.O.; Bauer, S.M.; Garcia-Saura, M.F.; Milsom, A.B.; Rassaf, T.; Maloney, R.E.; Bharti, A.; Rodriguez, J.; Feelisch, M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat. Chem. Biol. 2005, 1, 290–297. [Google Scholar] [CrossRef]
- Stamm, P.; Oelze, M.; Steven, S.; Kröller-Schön, S.; Kvandova, M.; Kalinovic, S.; Jasztal, A.; Kij, A.; Kuntic, M.; Bayo Jimenez, M.T.; et al. Direct comparison of inorganic nitrite and nitrate on vascular dysfunction and oxidative damage in experimental arterial hypertension. Nitric Oxide 2021, 113–114, 57–69. [Google Scholar] [CrossRef]
- Jansson, E.A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlén, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Staniek, K.; Nohl, H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999, 454, 127–130. [Google Scholar] [CrossRef]
- Hernández, A.; Schiffer, T.A.; Ivarsson, N.; Cheng, A.J.; Bruton, J.D.; Lundberg, J.O.; Weitzberg, E.; Westerblad, H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J. Physiol. 2012, 590, 3575–3583. [Google Scholar] [CrossRef]
- Angelone, T.; Gattuso, A.; Imbrogno, S.; Mazza, R.; Tota, B. Nitrite is a positive modulator of the Frank-Starling response in the vertebrate heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1271–R1281. [Google Scholar] [CrossRef][Green Version]
- Borlaug, B.A.; Koepp, K.E.; Melenovsky, V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2015, 66, 1672–1682. [Google Scholar] [CrossRef]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Duda, K.; Majerczak, J.; Nieckarz, Z.; Heymsfield, S.B.; Zoladz, J.A. Human body composition and muscle mass. In Muscle and Exercise Physiology; Zoladz, J.A., Ed.; Elsevier: London, UK, 2019; pp. 3–26. [Google Scholar]
- Jones, A.M.; Ferguson, S.K.; Bailey, S.J.; Vanhatalo, A.; Poole, D.C. Fiber type-specific effects of dietary nitrate. Exerc. Sport Sci. Rev. 2016, 44, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013, 591, 547–557. [Google Scholar] [CrossRef]
- Sargeant, A.J.; de Haan, A. Human muscle fatigue: The significance of muscle fibre type variability studied using a micro-dissection approach. J. Physiol. Pharmacol. 2006, 57 (Suppl. 10), 5–16. [Google Scholar]
- Zoladz, J.A.; Gladden, L.B.; Hogan, M.C.; Nieckarz, Z.; Grassi, B. Progressive recruitment of muscle fibers is not necessary for the slow component of VO2 kinetics. J. Appl. Physiol. 2008, 105, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Majerczak, J.; Szkutnik, Z.; Duda, K.; Komorowska, M.; Kolodziejski, L.; Karasinski, J.; Zoladz, J.A. Effect of pedaling rates and myosin heavy chain composition in the vastus lateralis muscle on the power generating capability during incremental cycling in humans. Physiol. Res. 2008, 57, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Grassi, B.; Rossiter, H.B.; Zoladz, J.A. Skeletal muscle fatigue and decreased efficiency: Two sides of the same coin? Exerc. Sport Sci. Rev. 2015, 43, 75–83. [Google Scholar] [CrossRef]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef]
- Webb, A.; Bond, R.; McLean, P.; Uppal, R.; Benjamin, N.; Ahluwalia, A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. USA 2004, 101, 13683–13688. [Google Scholar] [CrossRef]
- Rassaf, T.; Flögel, U.; Drexhage, C.; Hendgen-Cotta, U.; Kelm, M.; Schrader, J. Nitrite reductase function of deoxymyoglobin: Oxygen sensor and regulator of cardiac energetics and function. Circ. Res. 2007, 100, 1749–1754. [Google Scholar] [CrossRef]
- Zweier, J.L.; Wang, P.; Samouilov, A.; Kuppusamy, P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1995, 1, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Hendgen-Cotta, U.B.; Luedike, P.; Berenbrink, M.; Klare, J.P.; Steinhoff, H.J.; Semmler, D.; Shiva, S.; Williams, D.; Kipar, A.; et al. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation 2012, 126, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, K.; Palmskog, G.; Hultman, E. Adenine nucleotide and IMP contents of the quadriceps muscle in man after exercise. Pflugers Arch. 1978, 374, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.B.; Pereira, V.; Mira, L.; Moura, J.J. Nitrite reductase activity of rat and human xanthine oxidase, xanthine dehydrogenase, and aldehyde oxidase: Evaluation of their contribution to NO formation in vivo. Biochemistry 2015, 54, 685–710. [Google Scholar] [CrossRef] [PubMed]
- McDonough, P.; Behnke, B.J.; Padilla, D.J.; Musch, T.I.; Poole, D.C. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J. Physiol. 2005, 563, 903–913. [Google Scholar] [CrossRef]
- Tanaka, Y.; Poole, D.C.; Kano, Y. pH homeostasis in contracting and recovering skeletal muscle: Integrated function of the microcirculation with the interstitium and intramyocyte milieu. Curr. Top. Med. Chem. 2016, 16, 2656–2663. [Google Scholar] [CrossRef]
- Punkt, K.; Fritzsche, M.; Stockmar, C.; Hepp, P.; Josten, C.; Wellner, M.; Schering, S.; Buchwalow, I.B. Nitric oxide synthase in human skeletal muscles related to defined fibre types. Histochem. Cell Biol. 2006, 125, 567–573. [Google Scholar] [CrossRef]
- Staron, R.S.; Kraemer, W.J.; Hikida, R.S.; Fry, A.C.; Murray, J.D.; Campos, G.E. Fiber type composition of four hindlimb muscles of adult Fisher 344 rats. Histochem. Cell Biol. 1999, 111, 117–123. [Google Scholar] [CrossRef]
- Gruner, J.A.; Altman, J.; Spivack, N. Effects of arrested cerebellar development on locomotion in the rat. Cinematographic and electromyographic analysis. Exp. Brain Res. 1980, 40, 361–373. [Google Scholar] [CrossRef]
- Park, J.W.; Thomas, S.M.; Schechter, A.N.; Piknova, B. Control of rat muscle nitrate levels after perturbation of steady state dietary nitrate intake. Nitric Oxide 2021, 109–110, 42–49. [Google Scholar] [CrossRef]
- Bellavia, L.; Kim-Shapiro, D.B.; King, S.B. Detecting and monitoring NO, SNO and nitrite in vivo. Future Sci. OA 2015, 1, FSO36. [Google Scholar] [CrossRef] [PubMed]
- Copp, S.W.; Holdsworth, C.T.; Ferguson, S.K.; Hirai, D.M.; Poole, D.C.; Musch, T.I. Muscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill running. J. Physiol. 2013, 591, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S.; Huang, Z.; Grubina, R.; Sun, J.; Ringwood, L.A.; MacArthur, P.H.; Xu, X.; Murphy, E.; Darley-Usmar, V.M.; Gladwin, M.T. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007, 100, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Brunori, M. Nitric oxide, cytochrome-c oxidase and myoglobin. Trends. Biochem. Sci. 2001, 26, 21–23. [Google Scholar] [CrossRef]
- Flögel, U.; Merx, M.W.; Godecke, A.; Decking, U.K.; Schrader, J. Myoglobin: A scavenger of bioactive NO. Proc. Natl. Acad. Sci. USA 2001, 98, 735–740. [Google Scholar] [CrossRef]
- Gödecke, A.; Molojavyi, A.; Heger, J.; Flögel, U.; Ding, Z.; Jacoby, C.; Schrader, J. Myoglobin protects the heart from inducible nitric-oxide synthase (iNOS)-mediated nitrosative stress. J. Biol. Chem. 2003, 278, 21761–21766. [Google Scholar] [CrossRef]
- Hendgen-Cotta, U.B.; Merx, M.W.; Shiva, S.; Schmitz, J.; Becher, S.; Klare, J.P.; Steinhoff, H.J.; Goedecke, A.; Schrader, J.; Gladwin, M.T.; et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2008, 105, 10256–10261. [Google Scholar] [CrossRef]
- Hendgen-Cotta, U.B.; Kelm, M.; Rassaf, T. Myoglobin functions in the heart. Free Radic. Biol. Med. 2014, 73, 252–259. [Google Scholar] [CrossRef]
- Ordway, G.A.; Garry, D.J. Myoglobin: An essential hemoprotein in striated muscle. J. Exp. Biol. 2004, 207, 3441–3446. [Google Scholar] [CrossRef]
- Richardson, R.S.; Noyszewski, E.A.; Kendrick, K.F.; Leigh, J.S.; Wagner, P.D. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J. Clin. Investig. 1995, 96, 1916–1926. [Google Scholar] [CrossRef]
- Chung, Y.; Molé, P.A.; Sailasuta, N.; Tran, T.K.; Hurd, R.; Jue, T. Control of respiration and bioenergetics during muscle contraction. Am. J. Physiol. Cell Physiol. 2005, 288, C730–C738. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Furuichi, Y.; Takakura, H.; Hashimoto, T.; Hanai, Y.; Jue, T.; Masuda, K. Interaction between myoglobin and mitochondria in rat skeletal muscle. J. Appl. Physiol. 2013, 114, 490–497. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quesnelle, K.; Guimaraes, D.A.; Rao, K.; Singh, A.B.; Wang, Y.; Hogg, N.; Shiva, S. Myoglobin promotes nitrite-dependent mitochondrial S-nitrosation to mediate cytoprotection after hypoxia/reoxygenation. Nitric Oxide 2020, 104–105, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Jansson, E.; Sylvén, C. Myoglobin concentration in single type I and type II muscle fibres in man. Histochemistry 1983, 78, 121–124. [Google Scholar] [CrossRef]
- Schaper, J.; Meiser, E.; Stämmler, G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ. Res. 1985, 56, 377–391. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Duncker, D.J.; Bache, R.J.; Merkus, D.; Laughlin, M.H. Exercise and the Coronary Circulation. In Muscle and Exercise Physiology; Zoladz, J.A., Ed.; Elsevier: London, UK, 2019; pp. 467–503. [Google Scholar]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Dominiak, K.; Galganski, L.; Budzinska, A.; Woyda-PLoSzczyca, A.; Zoladz, J.A.; Jarmuszkiewicz, W. Effects of Endurance Training on the Coenzyme Q Redox State in Rat Heart, Liver, and Brain at the Tissue and Mitochondrial Levels: Implications for Reactive Oxygen Species Formation and Respiratory Chain Remodeling. Int. J. Mol. Sci. 2022, 23, 896. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C.; Kostrominova, T.Y.; Mann, M.; Murgia, M. Mitochondrial specialization revealed by single muscle fiber proteomics: Focus on the Krebs cycle. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. 4), 41–48. [Google Scholar] [CrossRef]
- Liu, J.; Yeo, H.C.; Overvik-Douki, E.; Hagen, T.; Doniger, S.J.; Chyu, D.W.; Brooks, G.A.; Ames, B.N. Chronically and acutely exercised rats: Biomarkers of oxidative stress and endogenous antioxidants. J. Appl. Physiol. 2000, 89, 21–28. [Google Scholar] [CrossRef]
- Gouspillou, G.; Sgarioto, N.; Norris, B.; Barbat-Artigas, S.; Aubertin-Leheudre, M.; Morais, J.A.; Burelle, Y.; Taivassalo, T.; Hepple, R.T. The relationship between muscle fiber type-specific PGC-1α content and mitochondrial content varies between rodent models and humans. PLoS ONE 2014, 9, e103044. [Google Scholar] [CrossRef] [PubMed]
- Jarmuszkiewicz, W.; Dominiak, K.; Galganski, L.; Galganska, H.; Kicinska, A.; Majerczak, J.; Zoladz, J.A. Lung mitochondria adaptation to endurance training in rats. Free Radic. Biol. Med. 2020, 161, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Kus, K.; Kij, A.; Zakrzewska, A.; Jasztal, A.; Stojak, M.; Walczak, M.; Chlopicki, S. Alterations in arginine and energy metabolism, structural and signalling lipids in metastatic breast cancer in mice detected in plasma by targeted metabolomics and lipidomics. Breast Cancer Res. 2018, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Majerczak, J.; Filipowska, J.; Tylko, G.; Guzik, M.; Karasinski, J.; Piechowicz, E.; Pyza, E.; Chlopicki, S.; Zoladz, J.A. Impact of long-lasting spontaneous physical activity on bone morphogenetic protein 4 in the heart and tibia in murine model of heart failure. Physiol. Rep. 2020, 8, e14412. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majerczak, J.; Kij, A.; Drzymala-Celichowska, H.; Kus, K.; Karasinski, J.; Nieckarz, Z.; Grandys, M.; Celichowski, J.; Szkutnik, Z.; Hendgen-Cotta, U.B.; et al. Nitrite Concentration in the Striated Muscles Is Reversely Related to Myoglobin and Mitochondrial Proteins Content in Rats. Int. J. Mol. Sci. 2022, 23, 2686. https://doi.org/10.3390/ijms23052686
Majerczak J, Kij A, Drzymala-Celichowska H, Kus K, Karasinski J, Nieckarz Z, Grandys M, Celichowski J, Szkutnik Z, Hendgen-Cotta UB, et al. Nitrite Concentration in the Striated Muscles Is Reversely Related to Myoglobin and Mitochondrial Proteins Content in Rats. International Journal of Molecular Sciences. 2022; 23(5):2686. https://doi.org/10.3390/ijms23052686
Chicago/Turabian StyleMajerczak, Joanna, Agnieszka Kij, Hanna Drzymala-Celichowska, Kamil Kus, Janusz Karasinski, Zenon Nieckarz, Marcin Grandys, Jan Celichowski, Zbigniew Szkutnik, Ulrike B. Hendgen-Cotta, and et al. 2022. "Nitrite Concentration in the Striated Muscles Is Reversely Related to Myoglobin and Mitochondrial Proteins Content in Rats" International Journal of Molecular Sciences 23, no. 5: 2686. https://doi.org/10.3390/ijms23052686
APA StyleMajerczak, J., Kij, A., Drzymala-Celichowska, H., Kus, K., Karasinski, J., Nieckarz, Z., Grandys, M., Celichowski, J., Szkutnik, Z., Hendgen-Cotta, U. B., & Zoladz, J. A. (2022). Nitrite Concentration in the Striated Muscles Is Reversely Related to Myoglobin and Mitochondrial Proteins Content in Rats. International Journal of Molecular Sciences, 23(5), 2686. https://doi.org/10.3390/ijms23052686





