Balancing Damage via Non-Photochemical Quenching, Phenolic Compounds and Photorespiration in Ulva prolifera Induced by Low-Dose and Short-Term UV-B Radiation
Abstract
:1. Introduction
2. Results
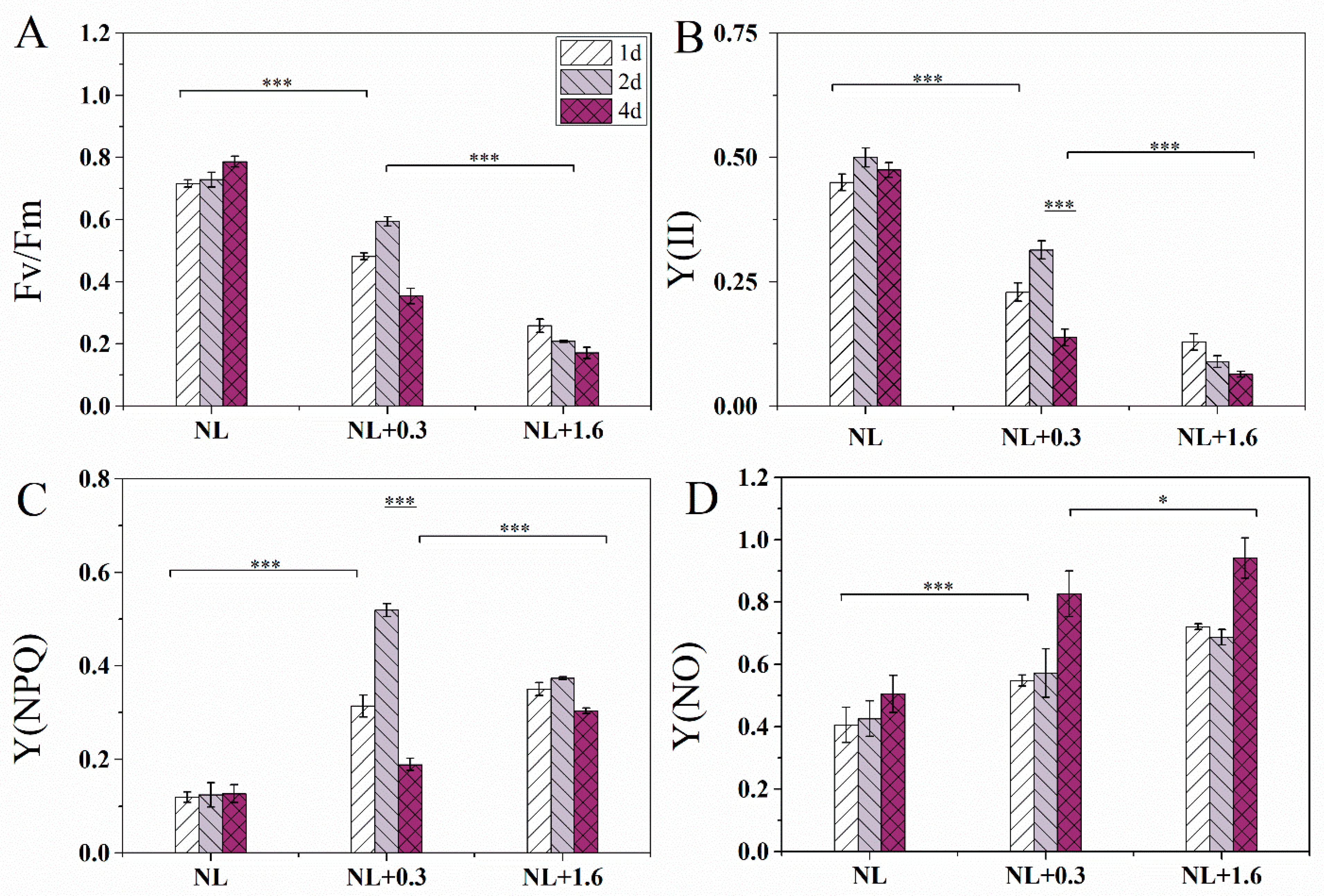
2.1. Changes of Fv/Fm, Y(II), Y(NPQ), and Y(NO) in U. prolifera under NL+0.3 and NL+1.6
2.2. Transmission Electron Microscopy Showed Changes in U. prolifera under NL+0.3 and NL+1.6
2.3. Changes of the Antioxidant System in U. prolifera under NL+0.3 and NL+1.6
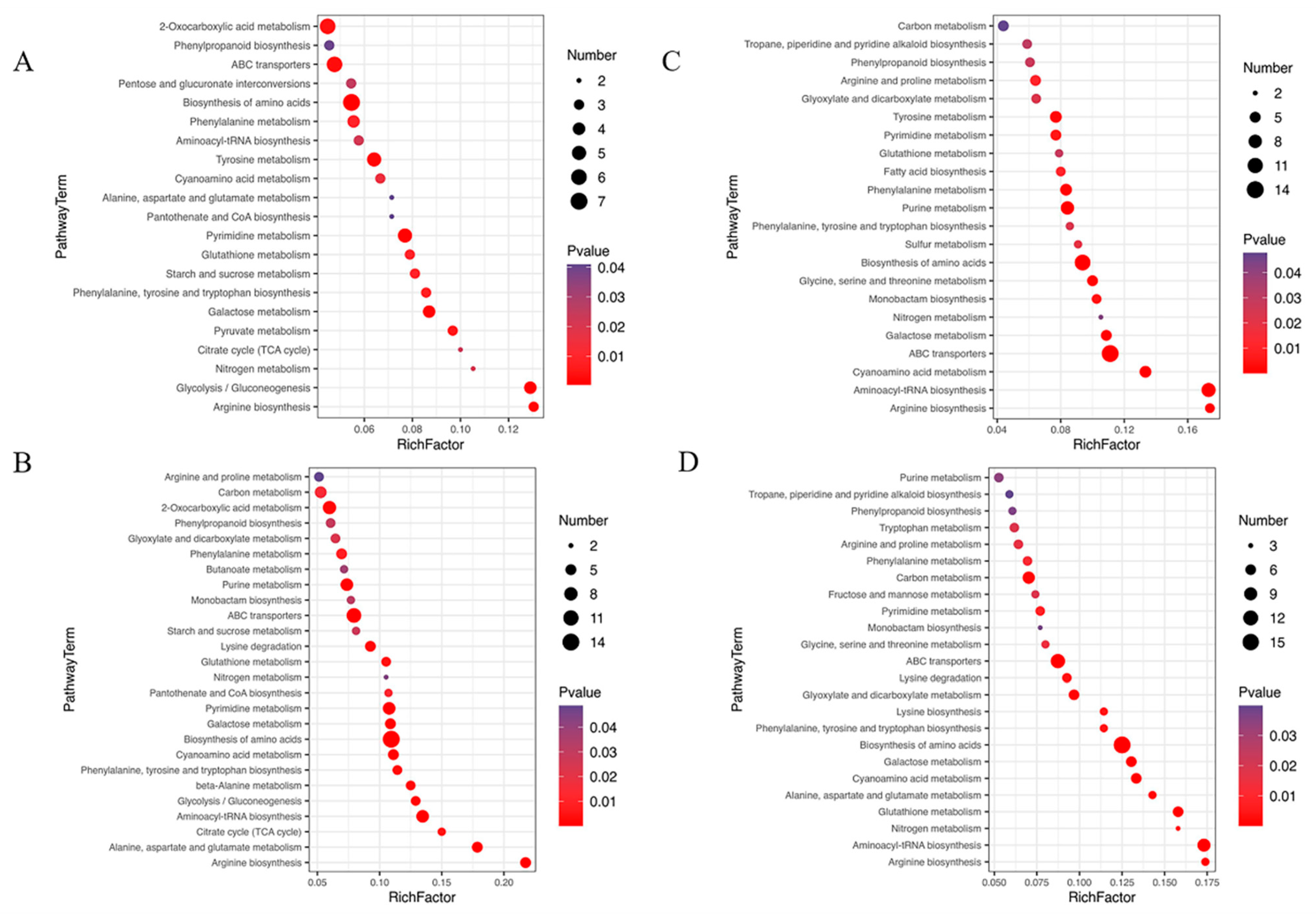
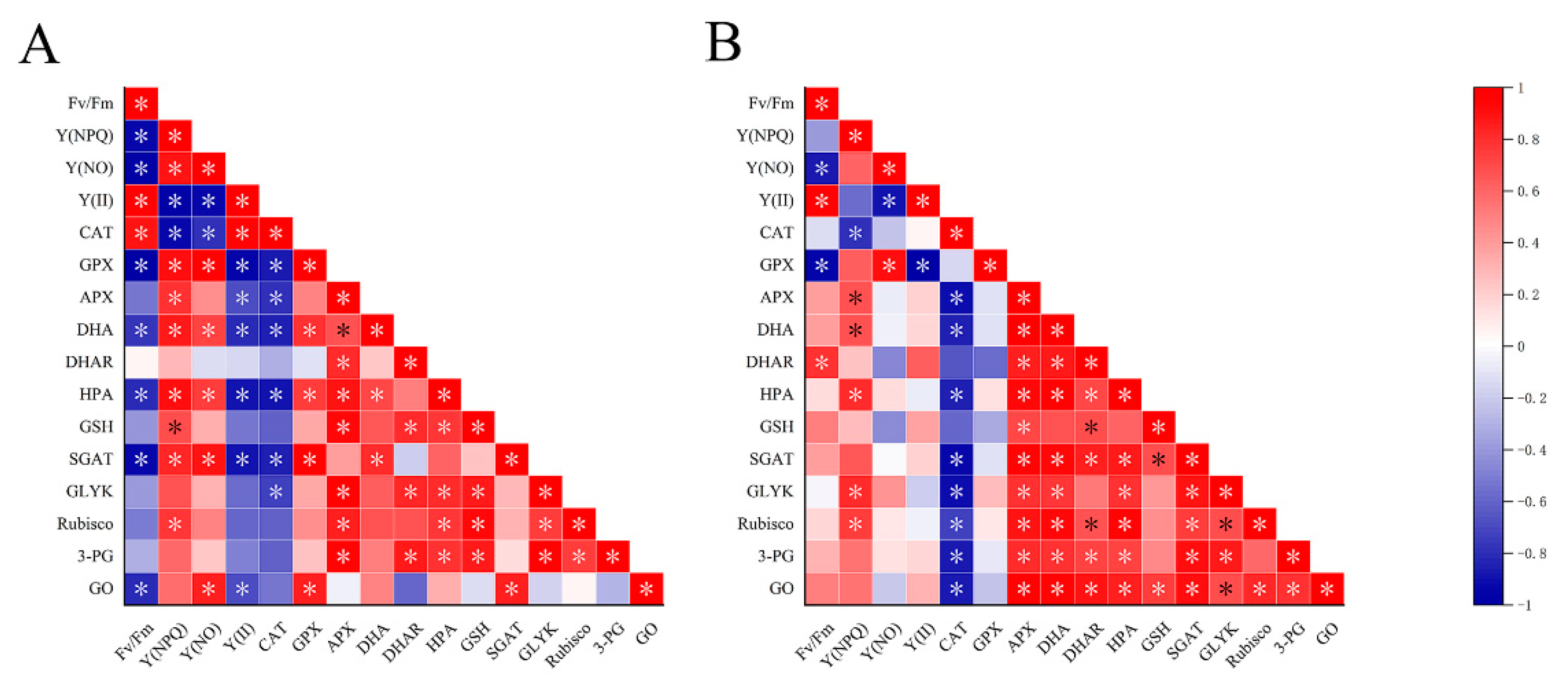
2.4. Metabonomics in U. prolifera under NL+0.3 and NL+1.6
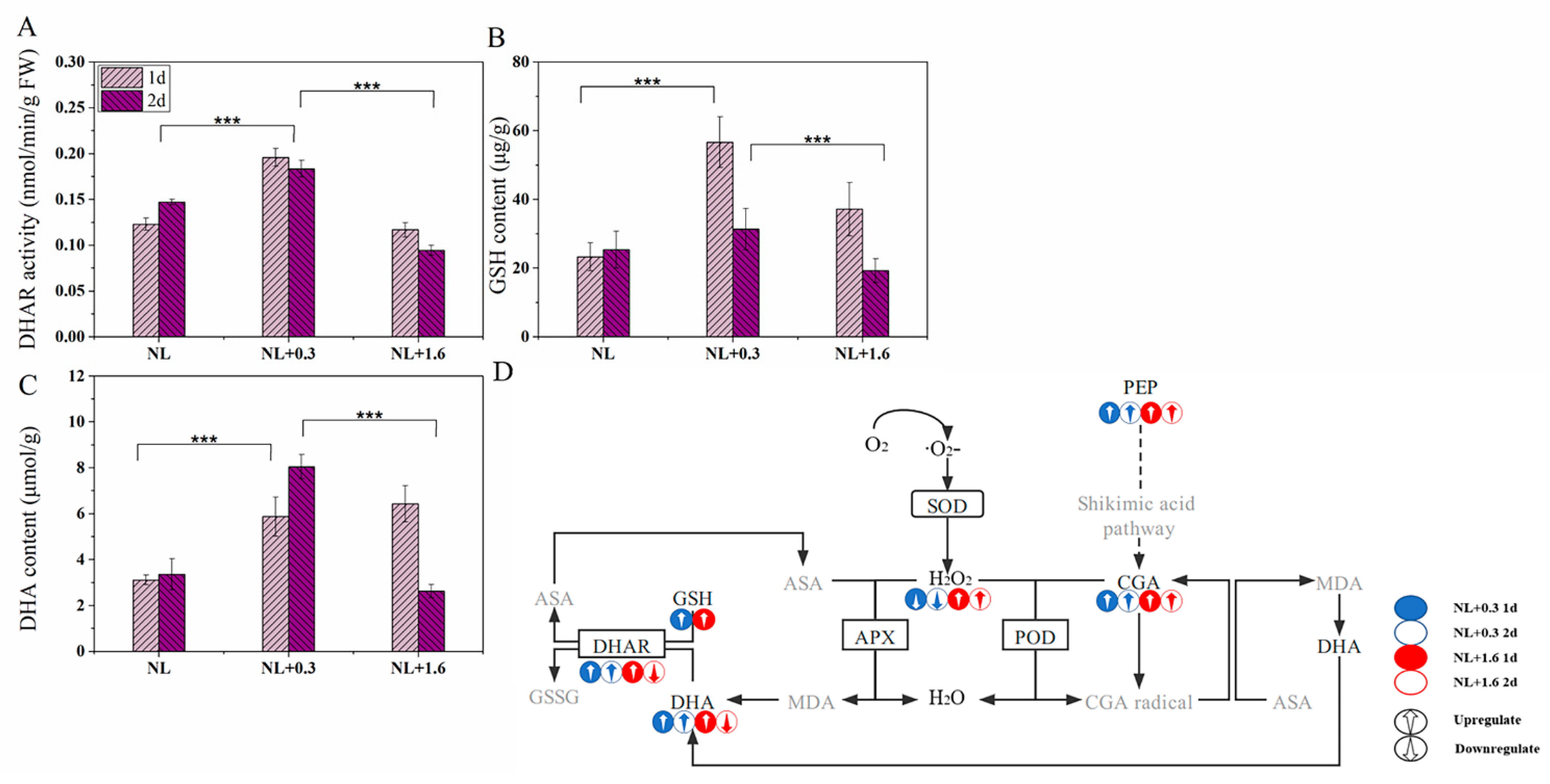
2.5. Changes of DHAR Activity, GSH and DHA Levels in U. prolifera under NL+0.3 and NL+1.6
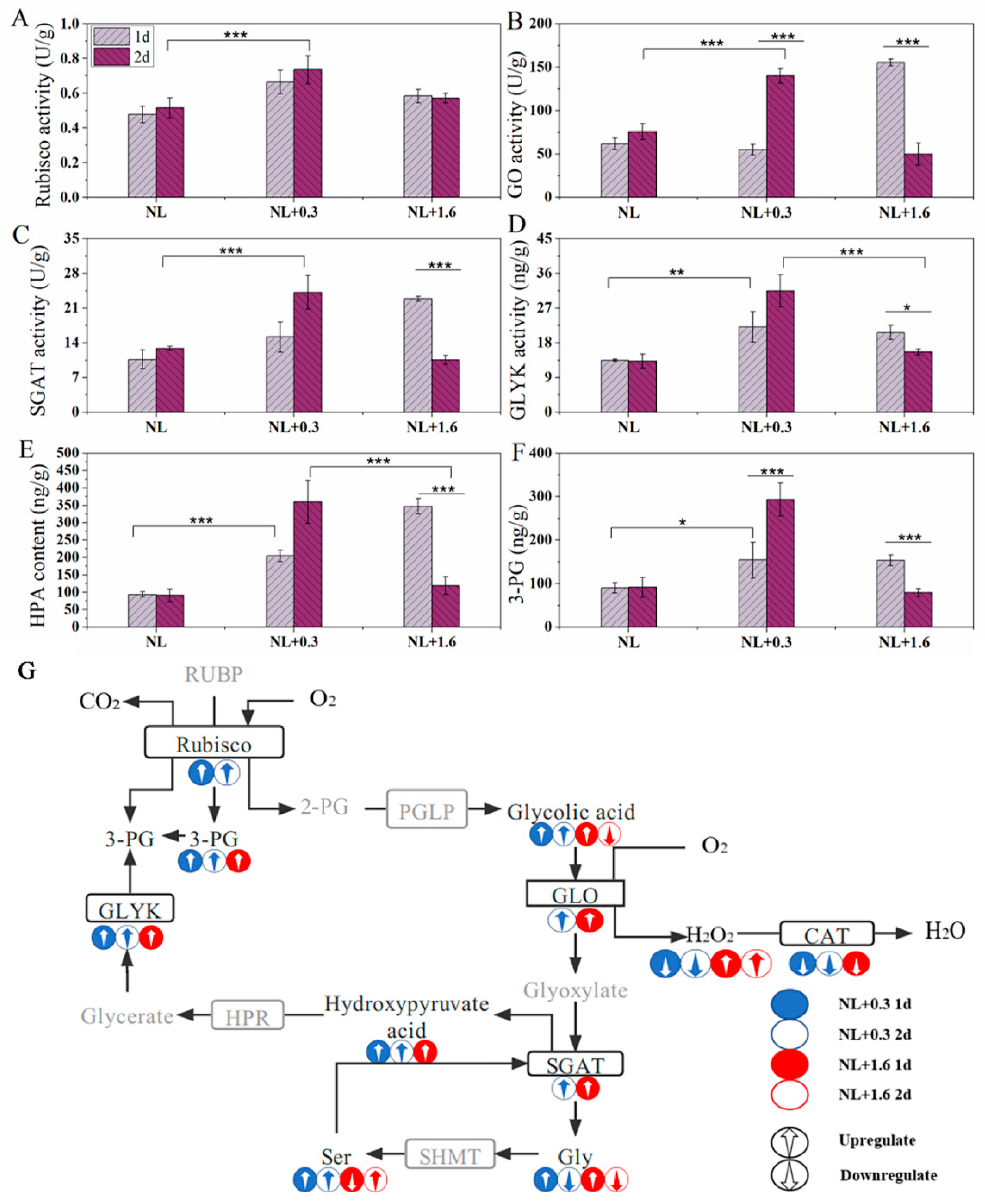
2.6. Changes of the Activities of Rubisco, GO, SGAT, and GLYK and the Contents of HPA and 3-PG in the Photorespiration in U. prolifera under NL+0.3 and NL+1.6
3. Discussion
3.1. Photosynthesis Was Influenced in U. prolifera under NL+0.3 and NL+1.6
3.2. Structural Alterations in U. prolifera under NL+0.3 and NL+1.6
3.3. Analysis of Antioxidant Enzyme Activity and Phenolic Compounds in U. prolifera under NL+0.3 and NL+1.6
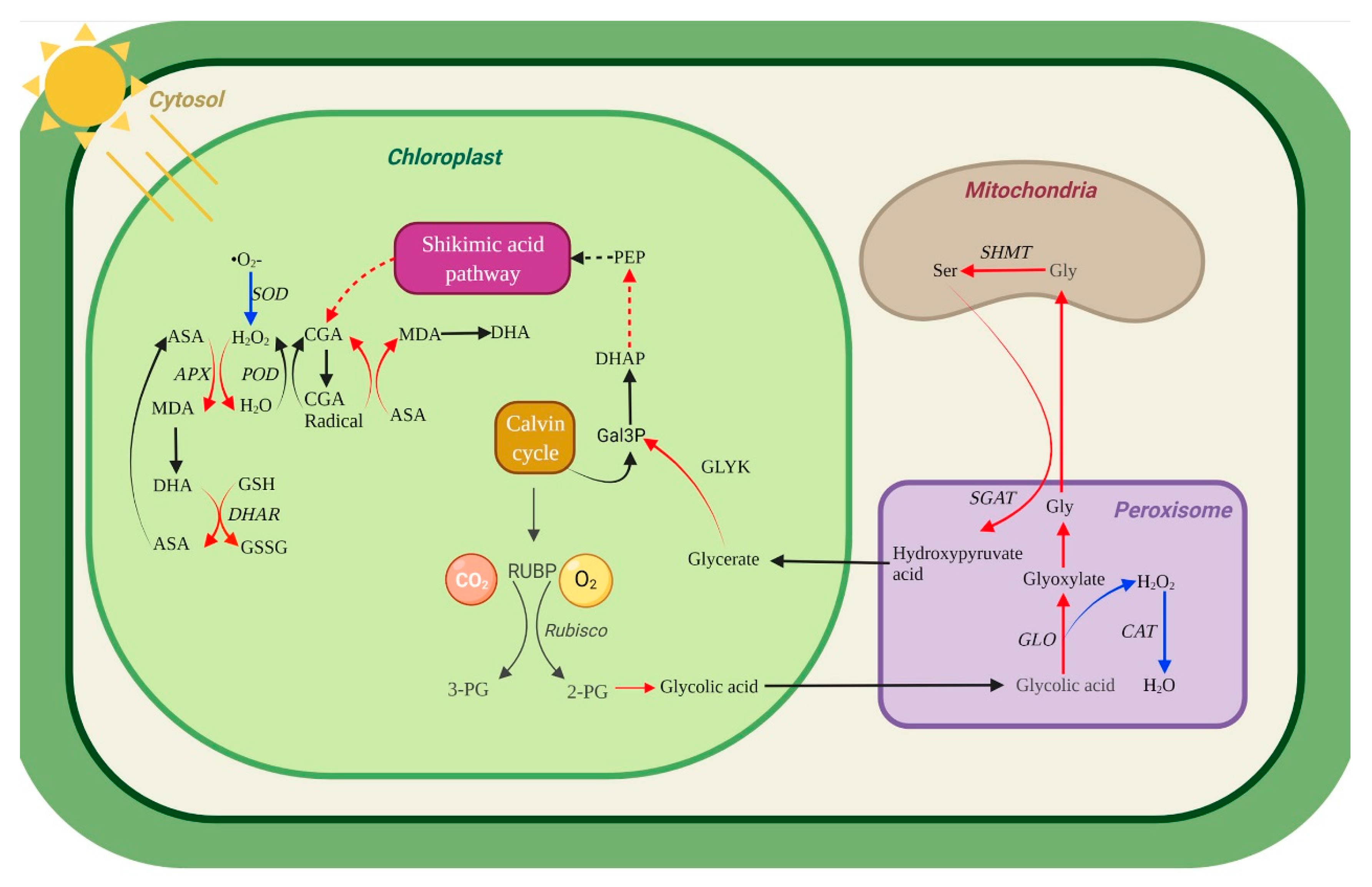
3.4. Effects of Photorespiratory Metabolism in U. prolifera under NL+0.3 and NL+1.6
3.5. Balancing Damage via NPQ, Phenolic Compounds and Photorespiration
4. Materials and Methods
4.1. Species and Treatments
4.2. Determination of Chlorophyll Fluorescence
4.3. Observations via Transmission Electron Microscopy
4.4. Measurement of H2O2 Content
4.5. Measurement of CAT, GPX, APX, DHAR, GSH and DHA
4.6. Metabolomics Profiling
4.7. Measurement of GO, SGAT, GLYK, HPA and 3-PG
4.8. Statistical Analysis
5. Conclusions
- (1)
- As one of the photoprotective mechanisms, efficient NPQ can ameliorate photosynthetic efficiency of U. prolifera.
- (2)
- Significant increases in the amounts of photoprotective structures such as electron-dense bodies were observed in the submicroscopic structure of the algae, observations that were supported by the significantly higher amounts of UV-absorbing phenolic compounds.
- (3)
- U. prolifera relies on phenolic compounds as substrates or directly as ROS scavengers, and they play roles in antioxidant together with enzymes in AsA-GSH cycle such as APX.
- (4)
- The enhanced photorespiration pathway can consume excess light energy. Moreover, photorespiration provides 3-PG for the Calvin cycle, thus maintaining high photosynthetic efficiency and ameliorating the ‘balancing damage’ of thalli.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schubert, H.; Forster, R.M. Sources of variability in the factors used for modelling primary productivity in eutrophic waters. Hydrobiologia 1997, 349, 75–85. [Google Scholar] [CrossRef]
- Hennige, S.J.; Coyne, K.J.; Macintyre, H.; Liefer, J.; Warner, M.E. The photobiology of Heterosigma akashiwo. Photoacclimation, diurnal periodicity, and its ability to rapidly exploit exposure to high light. J. Phycol. 2013, 49, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Blanding, C.R.; Simmons, S.J.; Casati, P.; Walbot, V.; Stapleton, A.E. Coordinated regulation of maize genes during increasing exposure to ultraviolet radiation: Identification of ultraviolet-responsive genes, functional processes and associated potential promoter motifs. Plant Biotechnol. J. 2007, 5, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.I.; Brown, B.A. UV-B perception and signal transduction. Light Plant Dev. 2007, 30, 155–182. [Google Scholar]
- Hanelt, D.; Roleda, M.Y. UVB radiation may ameliorate photoinhibition in specific shallow-water tropical marine macrophytes. Aquat. Bot. 2009, 91, 6–12. [Google Scholar] [CrossRef]
- Han, T.; Han, Y.; Kim, K.; Kim, J.; Shin, H.; Kain, J.M.; Callow, J.A.; Callow, M.E. Influences of light and UV-B on growth and sporulation of the green alga Ulva pertusa Kjellman. J. Exp. Mar. Biol. Ecol. 2003, 290, 115–131. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Wiencke, C.; Hanelt, D. Thallus morphology and optical characteristics affect growth and DNA damage by UV radiation in juvenile Arctic Laminaria sporophytes. Planta 2006, 223, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Franklin, L.A. Photoinhibition, UV-B and Algal Photosynthesis. In Photosynthesis in Algae; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Allorent, G.; Lefebvre-Legendre, L.; Chappuis, R.; Kuntz, M.; Truong, T.B.; Niyogi, K.K.; Ulm, R.; Goldschmidt-Clermont, M. UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2016, 113, 14864–14869. [Google Scholar] [CrossRef] [Green Version]
- Pescheck, F.; Lohbeck, K.T.; Roleda, M.Y.; Bilger, W. UVB-induced DNA and photosystem II damage in two intertidal green macroalgae: Distinct survival strategies in UV-screening and non-screening Chlorophyta. J. Photochem. Photobiol. B 2014, 132, 85–93. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yamamoto, H.; Allakhverdiev, S.I.; Inaba, M.; Yokota, A.; Murata, N. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 2001, 20, 5587–5594. [Google Scholar] [CrossRef]
- Pourcel, L.; Routaboul, J.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends. Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Osmond, C.B. What is photoinhibition? Some insights from comparisons of shade and sun plants. In Photoinhibition of Photo-Synthesis. From Molecular Mechanisms to the Field; BIOS Scientific Publishers: Oxford, UK, 1994; pp. 1–24. [Google Scholar]
- Cullen, J.J.; Neale, P.J.; Lesser, M.P. Biological weighting function for the inhibition of phytoplankton photosynthesis by ultraviolet radiation. Science 1992, 258, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Flores-Moya, A.; Hanelt, D.; Figueroa, F.; Altamirano, M.; Viñegla, B.; Salles, S. Involvement of solar UV-B radiation in recovery of inhibited photosynthesis in the brown alga Dictyota dichotoma (Hudson) Lamouroux. J. Photochem. Photobiol. B Biol. 1999, 49, 129–135. [Google Scholar] [CrossRef]
- Hanelt, D.; Hawes, I.; Rae, R. Reduction of UV-B radiation causes an enhancement of photoinhibition in high light stressed aquatic plants from New Zealand lakes. J. Photochem. Photobiol. B Biol. 2006, 84, 89–102. [Google Scholar] [CrossRef]
- Thomson, P.G.; Davidson, A.T.; Cadman, N. Temporal changes in effects of ambient UV radiation on natural communities of Antarctic marine protists. Aquat. Microb. Ecol. 2008, 52, 131–147. [Google Scholar] [CrossRef] [Green Version]
- Beggs, C.J.; Wellmann, E. Photocontrol of flavonoid biosynthesis. In Photomorphogenesis in Plants; Springer: Dordrecht, The Netherlands, 1994; pp. 733–751. [Google Scholar]
- Ballaré, C.L.; Barnes, P.W.; Kendrick, R.E. Photomorphogenic effects of UV-B radiation on hypocotyl elongation in wild type and stable-phytochrome-deficient mutant seedlings of cucumber. Physiol. Plant. 1991, 83, 652–658. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, Y.; Zhang, H.; Qu, T.; Jiang, Y.; Tang, X.; Wang, Y. Cooperation between photosynthetic and antioxidant systems: An important factor in the adaptation of Ulva prolifera to abiotic factors on the sea surface. Front. Plant Sci. 2019, 10, 648. [Google Scholar] [CrossRef]
- Hou, C.; Qu, T.; Zhao, X.; Xu, J.; Zhong, Y.; Guan, C.; Zhang, H.; Lin, Z.; Tang, X.; Wang, Y. Diel metabolism of Yellow Sea green tide algae alters bacterial community composition under in situ seawater acidification of coastal areas. Sci. Total Environ. 2022, 807, 150759. [Google Scholar] [CrossRef]
- Gao, S.; Gu, W.; Xiong, Q.; Ge, F.; Xie, X.; Li, J.; Chen, W.; Pan, G.; Wang, G. Desiccation enhances phosphorylation of PSII and affects the distribution of protein complexes in the thylakoid membrane. Physiol. Plant. 2015, 153, 492–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, M.A.; Gaba, V.; Greenberg, B.M. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends. Plant Sci. 1998, 3, 131–135. [Google Scholar] [CrossRef]
- Liepman, A.H.; Olsen, L.J. Peroxisomal alanine: Glyoxylate aminotransferase (AGT1) is a photorespiratory enzyme with multiple substrates in Arabidopsis thaliana. Plant J. 2001, 25, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Boldt, R.; Edner, C.; Kolukisaoglu, U.; Hagemann, M.; Weckwerth, W.; Wienkoop, S.; Morgenthal, K.; Bauwe, H. D-GLYCERATE 3-KINASE, the last unknown enzyme in the photorespiratory cycle in Arabidopsis, belongs to a novel kinase family. Plant Cell 2005, 17, 2413–2420. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zheng, W.; Qu, T.; Zhong, Y.; Xu, J.; Jiang, Y.; Zhang, H.; Tang, X.; Wang, Y. Dual roles of reactive oxygen species in intertidal macroalgae Ulva prolifera under ultraviolet-B radiation. Environ. Exp. Bot. 2021, 189, 104534. [Google Scholar] [CrossRef]
- Munekage, Y.; Hashimoto, M.; Endo, T.; Tasaka, M.; Shikanai, T. Cyclic electron flow around PSI is essential for ATP synthesis during photosynthesis. In Plant and Cell Physiology; Oxford University Press: Oxford, UK, 2003; Volume 44, p. S27. [Google Scholar]
- Munekage, Y.; Hashimoto, M.; Miyake, C.; Tomizawa, K.; Endo, T.; Tasaka, M.; Shikanai, T. Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 2004, 429, 579–582. [Google Scholar] [CrossRef]
- Li, X.; BjoÈrkman, O.; Shih, C.; Grossman, A.R.; Rosenquist, M.; Jansson, S.; Niyogi, K.K. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 2000, 403, 391–395. [Google Scholar] [CrossRef]
- Koziol, A.G.; Borza, T.; Ishida, K.; Keeling, P.; Lee, R.W.; Durnford, D.G. Tracing the evolution of the light-harvesting antennae in chlorophyll a/b-containing organisms. Plant Physiol. 2007, 143, 1802–1816. [Google Scholar] [CrossRef] [Green Version]
- Marija, S.; Dieter, H. Sensitivity of photosynthesis to UV radiation in several Cosmarium strains (Zygnematophyceae, Streptophyta) is related to their geographical distribution. Photoch. Photobiol. Sci. 2014, 13, 1066–1081. [Google Scholar] [CrossRef]
- Hernández-Pinzón, I.; Ross, J.H.; Barnes, K.A.; Damant, A.P.; Murphy, D.J. Composition and role of tapetal lipid bodies in the biogenesis of the pollen coat of Brassica napus. Planta 1999, 208, 588–598. [Google Scholar] [CrossRef]
- Recht, L.; Töpfer, N.; Batushansky, A.; Sikron, N.; Gibon, Y.; Fait, A.; Nikoloski, Z.; Boussiba, S.; Zarka, A. Metabolite profiling and integrative modeling reveal metabolic constraints for carbon partitioning under nitrogen starvation in the green algae Haematococcus pluvialis. J. Biol. Chem. 2014, 289, 30387–30403. [Google Scholar] [CrossRef] [Green Version]
- Küken, A.; Sommer, F.; Yaneva-Roder, L.; Mackinder, L.C.; Höhne, M.; Geimer, S.; Jonikas, M.C.; Schroda, M.; Stitt, M.; Nikoloski, Z. Effects of microcompartmentation on flux distribution and metabolic pools in Chlamydomonas reinhardtii chloroplasts. eLife 2018, 7, e37960. [Google Scholar] [CrossRef] [PubMed]
- Kuchitsu, K.; Tsuzuki, M.; Miyachi, S. Changes of starch localization within the chloroplast induced by changes in CO2 concentration during growth of Chlamydomonas reinhardtii: Independent regulation of pyrenoid starch and stroma starch. Plant Cell Physiol. 1988, 29, 1269–1278. [Google Scholar]
- Holzinger, A.; Roleda, M.Y.; Lütz, C. The vegetative arctic freshwater green alga Zygnema is insensitive to experimental UV exposure. Micron 2009, 40, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Pichrtová, M.; Remias, D.; Lewis, L.A.; Holzinger, A. Changes in phenolic compounds and cellular ultrastructure of Arctic and Antarctic strains of Zygnema (Zygnematophyceae, Streptophyta) after exposure to experimentally enhanced UV to PAR ratio. Microb. Ecol. 2013, 65, 68–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenwaelder, M.E. The occurrence and cellular significance of physodes in brown algae. Phycologia 2002, 41, 125–139. [Google Scholar] [CrossRef]
- Livingstone, D.R. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Asada, K. Production and action of active oxygen species in photosynthetic tissues. In Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants; CRC press: Boca Raton, FL, USA, 2019; pp. 77–104. [Google Scholar]
- Smith, M.D.; Licatalosi, D.D.; Thompson, J.E. Co-association of cytochrome f catabolites and plastid-lipid-associated protein with chloroplast lipid particles. Plant Physiol. 2000, 124, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Biswal, B. Carotenoid catabolism during leaf senescence and its control by light. J. Photochem. Photobiol. B Biol. 1995, 30, 3–13. [Google Scholar] [CrossRef]
- Takahama, U.; Oniki, T. Flavonoids and some other phenolics as substrates of peroxidase: Physiological significance of the redox reactions. J. Plant Res. 2000, 113, 301–309. [Google Scholar] [CrossRef]
- Warren, J.M.; Bassman, J.H.; Mattinson, D.S.; Fellman, J.K.; Edwards, G.E.; Robberecht, R. Alteration of foliar flavonoid chemistry induced by enhanced UV-B radiation in field-grown Pinus ponderosa, Quercus rubra and Pseudotsuga menziesii. J. Photochem. Photobiol. B Biol. 2002, 66, 125–133. [Google Scholar] [CrossRef]
- López, J.G. Flavonoids in Health and Disease. Curr. Med. Chem. 2019, 26, 6972–6975. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kawashima, M. Enhancement of the tolerance to oxidative stress in cucumber (Cucumis sativus L.) seedlings by UV-B irradiation: Possible involvement of phenolic compounds and antioxidative enzymes. J. Plant Res. 2000, 113, 311–317. [Google Scholar] [CrossRef]
- Noctor, G.; Arisi, A.M.; Jouanin, L.; Foyer, C.H. Photorespiratory glycine enhances glutathione accumulation in both the chloroplastic and cytosolic compartments. J. Exp. Bot. 1999, 50, 1157–1167. [Google Scholar] [CrossRef]
- Yang, L.; Han, H.; Liu, M.; Zuo, Z.; Zhou, K.; Lü, J.; Zhu, Y.; Bai, Y.; Wang, Y. Overexpression of the Arabidopsis photorespiratory pathway gene, serine: Glyoxylate aminotransferase (AtAGT1), leads to salt stress tolerance in transgenic duckweed (Lemna minor). Plant Cell Tissue Organ Cult. 2013, 113, 407–416. [Google Scholar] [CrossRef]
- Spencer, M.; von Caemmerer, S.; Hudson, G.S.; Andrews, T.J. Directed Mutation of the Rubisco Large Subunit of Tobacco Influences Photorespiration and Growth. Plant Physiol. 1999, 121, 579–588. [Google Scholar]
- Holzinger, A.; Albert, A.; Aigner, S.; Uhl, J.; Schmitt-Kopplin, P.; Trumhová, K.; Pichrtová, M. Arctic, Antarctic, and temperate green algae Zygnema spp. under UV-B stress: Vegetative cells perform better than pre-akinetes. Protoplasma 2018, 255, 1239–1252. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Tang, X.; Zhang, H.; Qu, T.; Wang, Y. Photosynthetic adaptation strategy of Ulva prolifera floating on the sea surface to environmental changes. Plant Physiol. Bioch. 2016, 107, 116–125. [Google Scholar] [CrossRef]
- Timm, S.; Hagemann, M. Photorespiration—how is it regulated and how does it regulate overall plant metabolism? J. Exp. Bot. 2020, 71, 3955–3965. [Google Scholar] [CrossRef]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bresson, J.; Vasseur, F.; Dauzat, M.; Koch, G.; Granier, C.; Vile, D. Quantifying spatial heterogeneity of chlorophyll fluorescence during plant growth and in response to water stress. Plant Methods 2015, 11, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genty, B.; Briantais, J.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta BBA-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, I.B.; Watkins, C.B.; Harman, J.E. Inhibition by calcium of senescence of detached cucumber cotyledons: Effect on ethylene and hydroperoxide production. Plant Physiol. 1983, 71, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cheng, Y.; Chen, T.; Zhang, Y.; Wang, X.; Zhao, A.; Jia, W.; Bo, Y.; Jin, C. GC/TOFMS analysis of endogenous metabolites in mouse fibroblast cells and its application in TiO2 nanoparticle-induced cytotoxicity study. Chromatographia 2012, 75, 1301–1310. [Google Scholar] [CrossRef]
- Li, C.; Ma, J.; Huang, D.; Ma, C.; Jin, J.; Yao, M.; Chen, L. Comprehensive dissection of metabolic changes in albino and green tea cultivars. J. Agric. Food Chem. 2018, 66, 2040–2048. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Xu, J.; Zhao, X.; Qu, T.; Guan, C.; Hou, C.; Tang, X.; Wang, Y. Balancing Damage via Non-Photochemical Quenching, Phenolic Compounds and Photorespiration in Ulva prolifera Induced by Low-Dose and Short-Term UV-B Radiation. Int. J. Mol. Sci. 2022, 23, 2693. https://doi.org/10.3390/ijms23052693
Zhong Y, Xu J, Zhao X, Qu T, Guan C, Hou C, Tang X, Wang Y. Balancing Damage via Non-Photochemical Quenching, Phenolic Compounds and Photorespiration in Ulva prolifera Induced by Low-Dose and Short-Term UV-B Radiation. International Journal of Molecular Sciences. 2022; 23(5):2693. https://doi.org/10.3390/ijms23052693
Chicago/Turabian StyleZhong, Yi, Jinhui Xu, Xinyu Zhao, Tongfei Qu, Chen Guan, Chengzong Hou, Xuexi Tang, and Ying Wang. 2022. "Balancing Damage via Non-Photochemical Quenching, Phenolic Compounds and Photorespiration in Ulva prolifera Induced by Low-Dose and Short-Term UV-B Radiation" International Journal of Molecular Sciences 23, no. 5: 2693. https://doi.org/10.3390/ijms23052693
APA StyleZhong, Y., Xu, J., Zhao, X., Qu, T., Guan, C., Hou, C., Tang, X., & Wang, Y. (2022). Balancing Damage via Non-Photochemical Quenching, Phenolic Compounds and Photorespiration in Ulva prolifera Induced by Low-Dose and Short-Term UV-B Radiation. International Journal of Molecular Sciences, 23(5), 2693. https://doi.org/10.3390/ijms23052693





