High Light Acclimation Mechanisms Deficient in a PsbS-Knockout Arabidopsis Mutant
Abstract
:1. Introduction
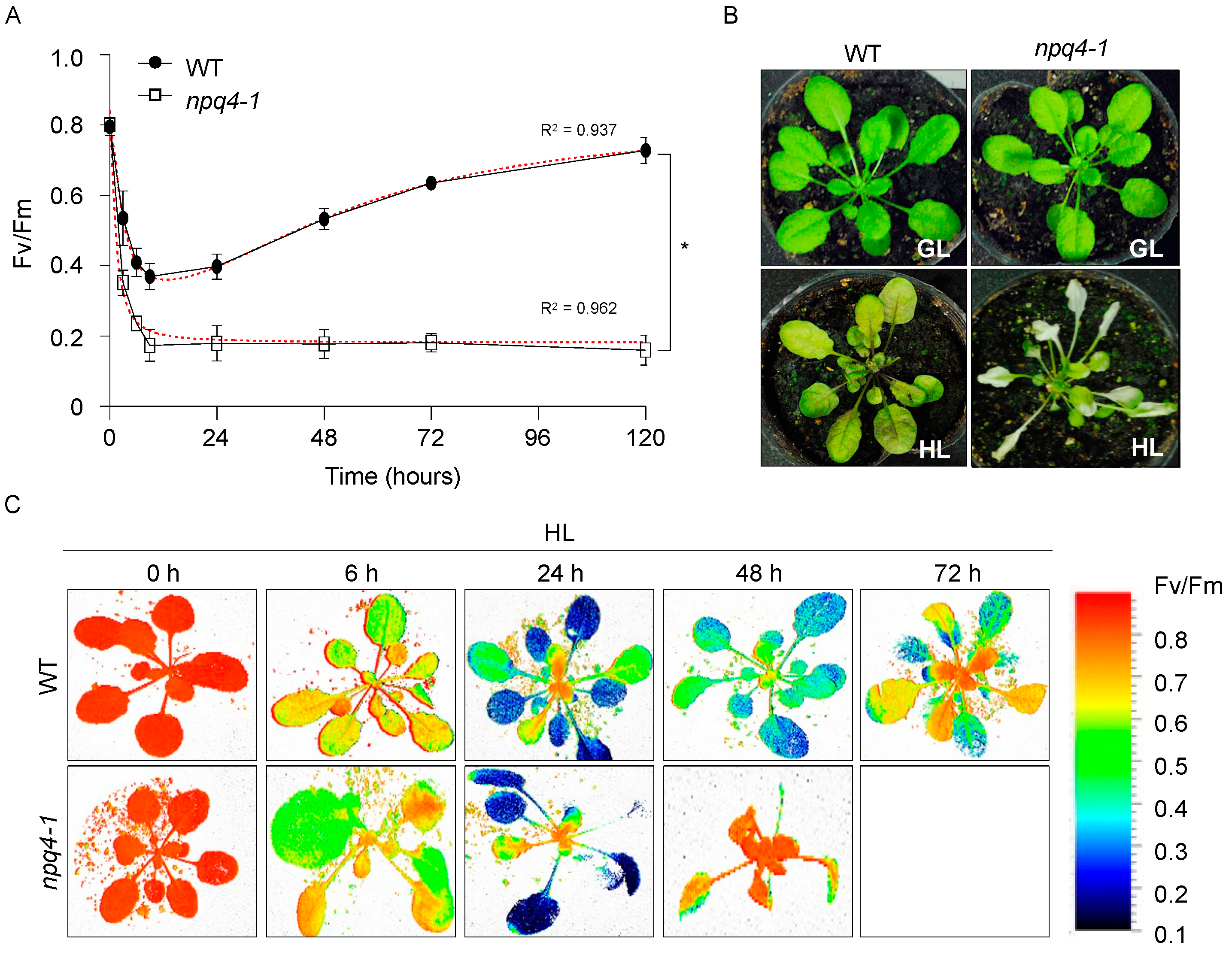
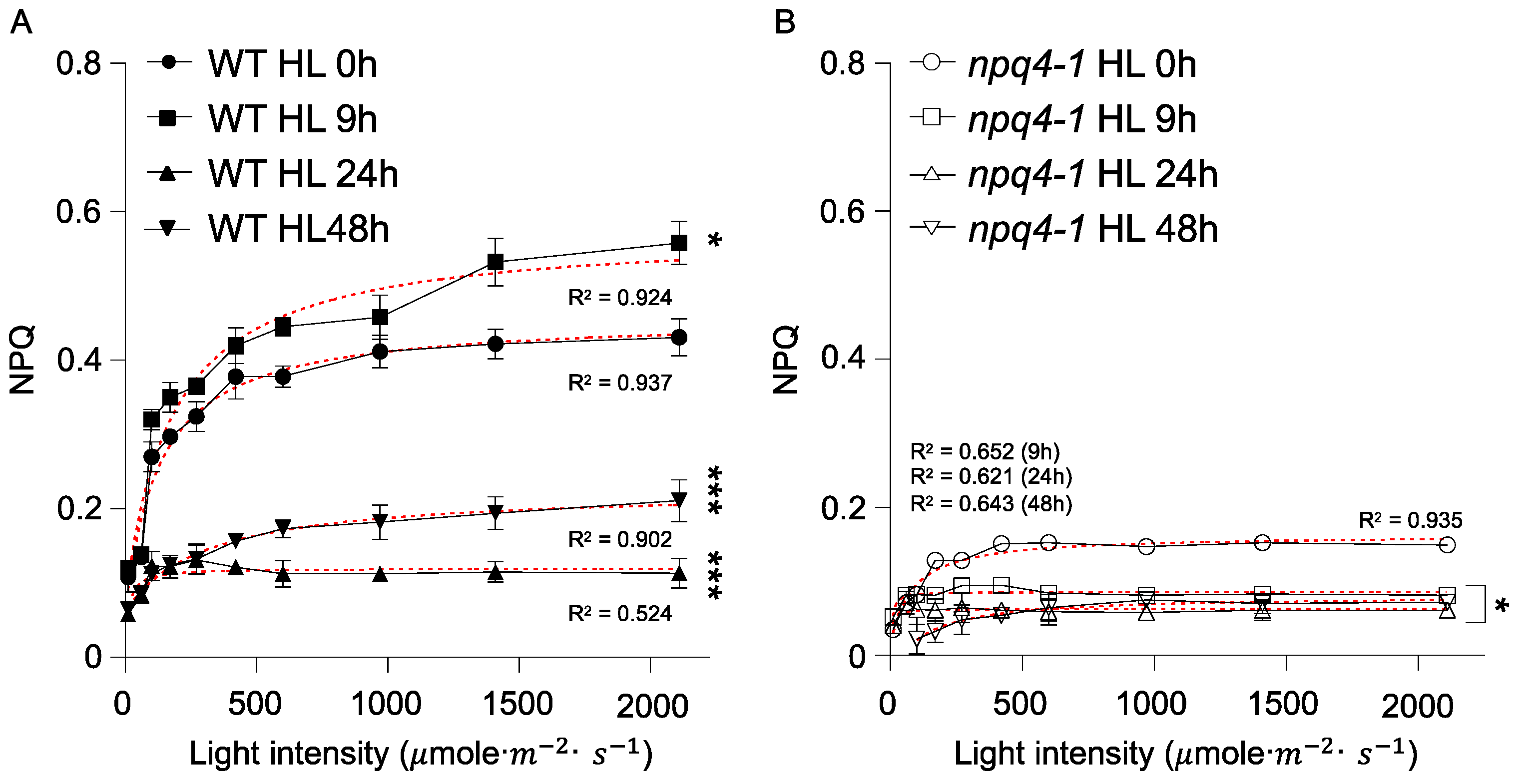
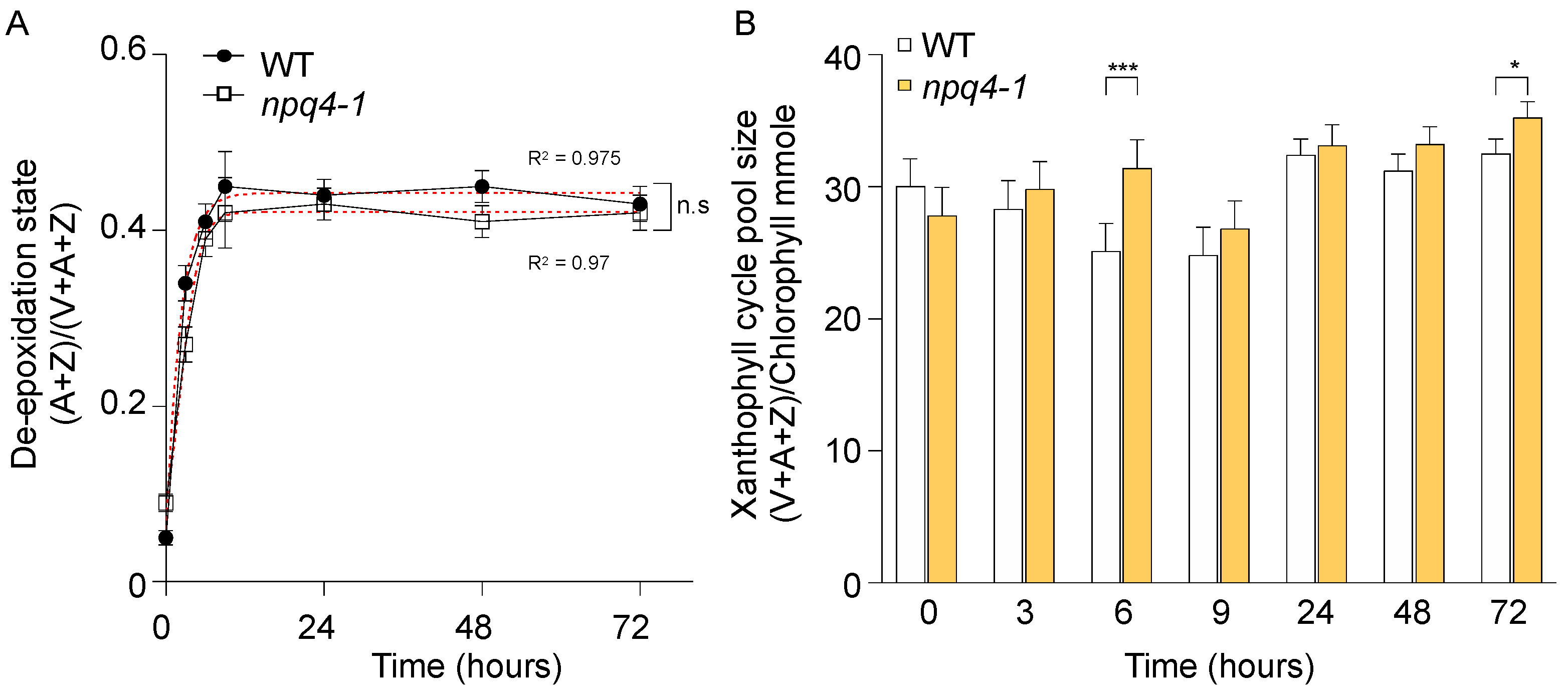
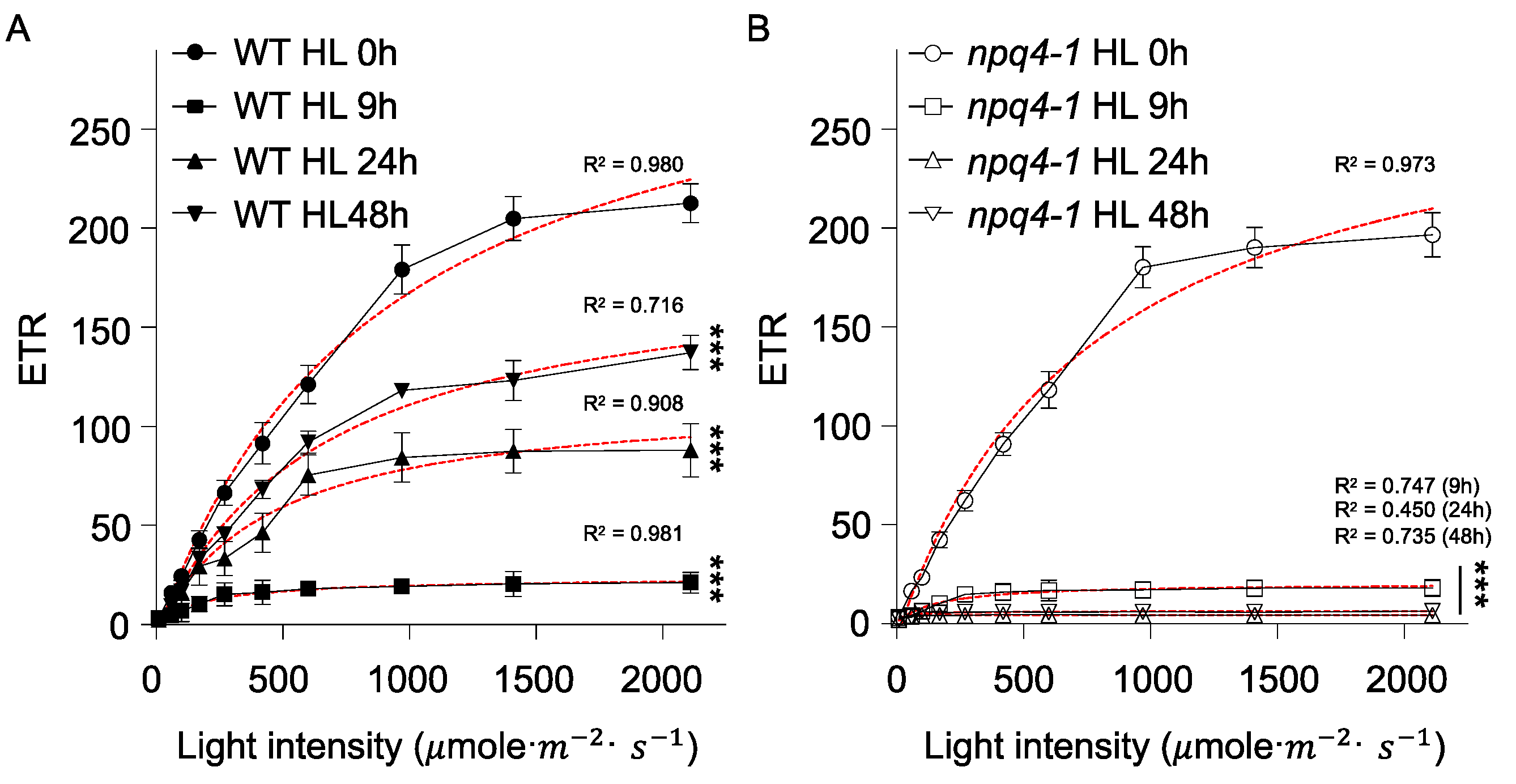
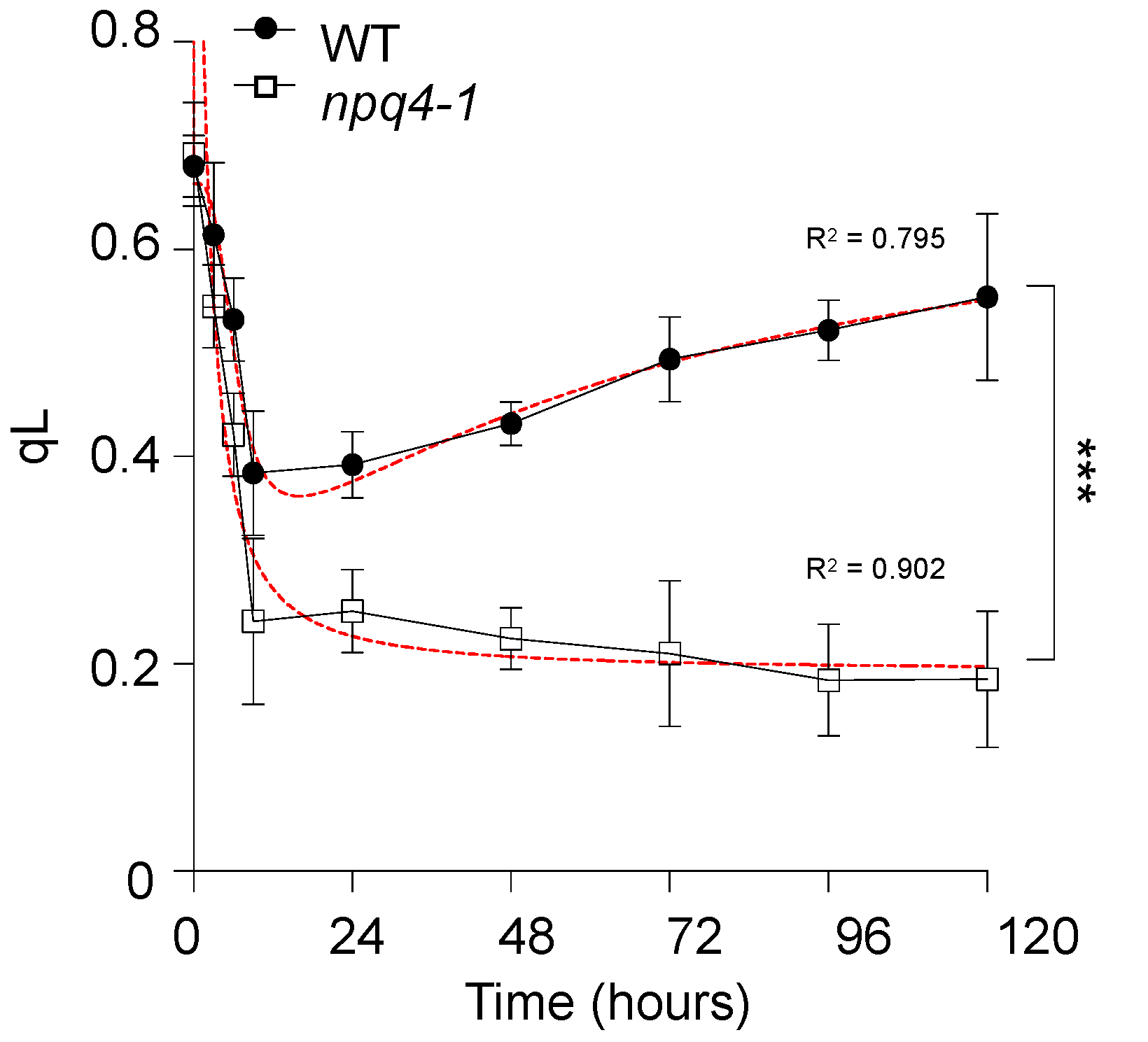
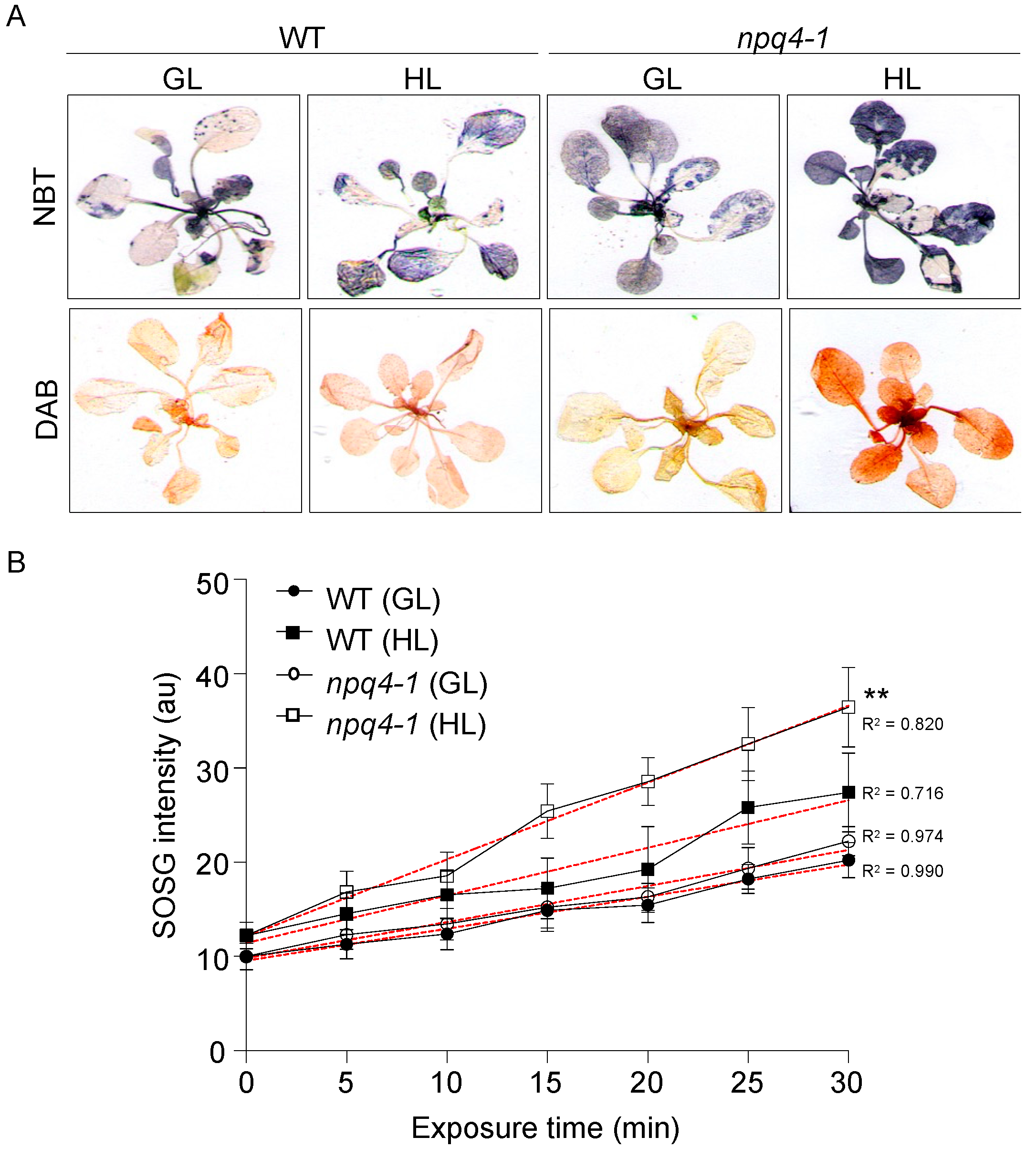
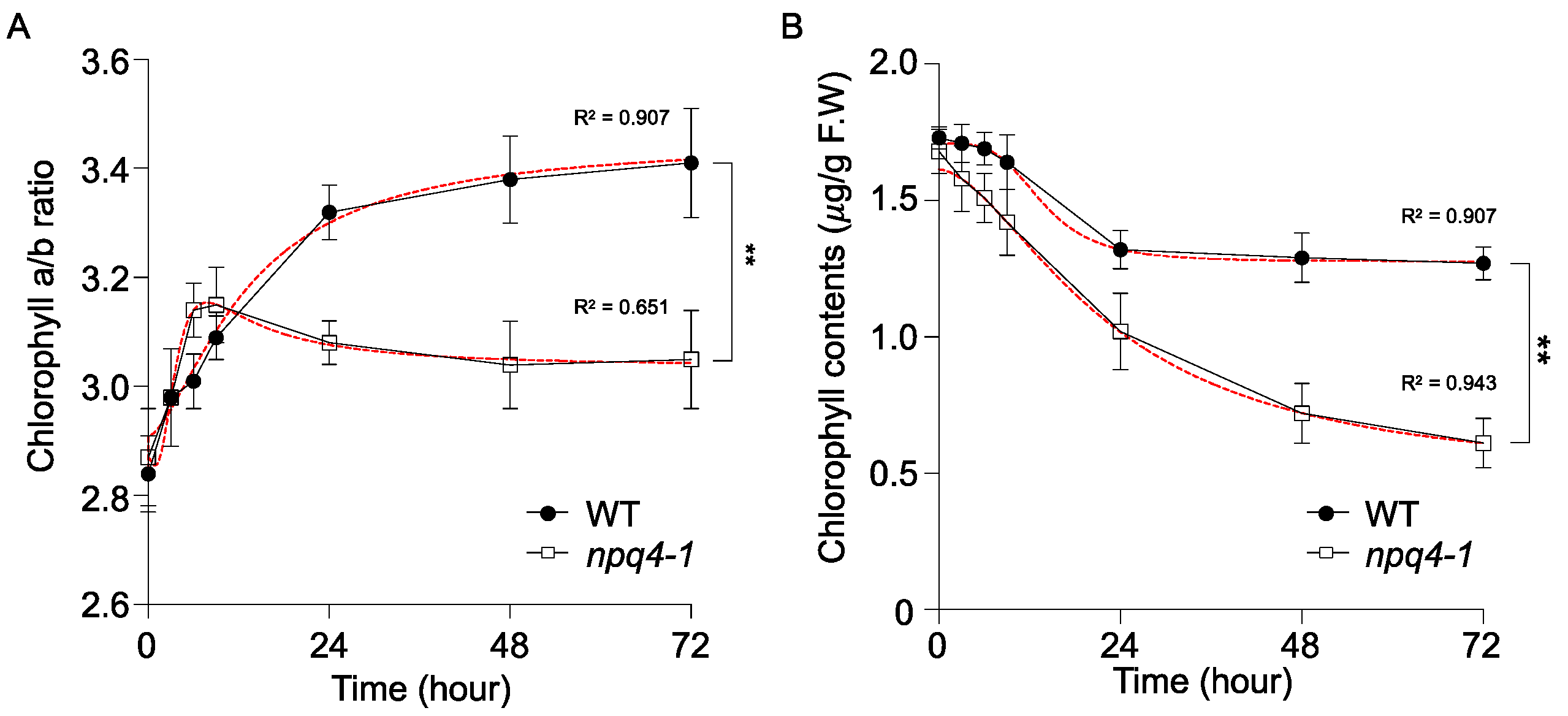
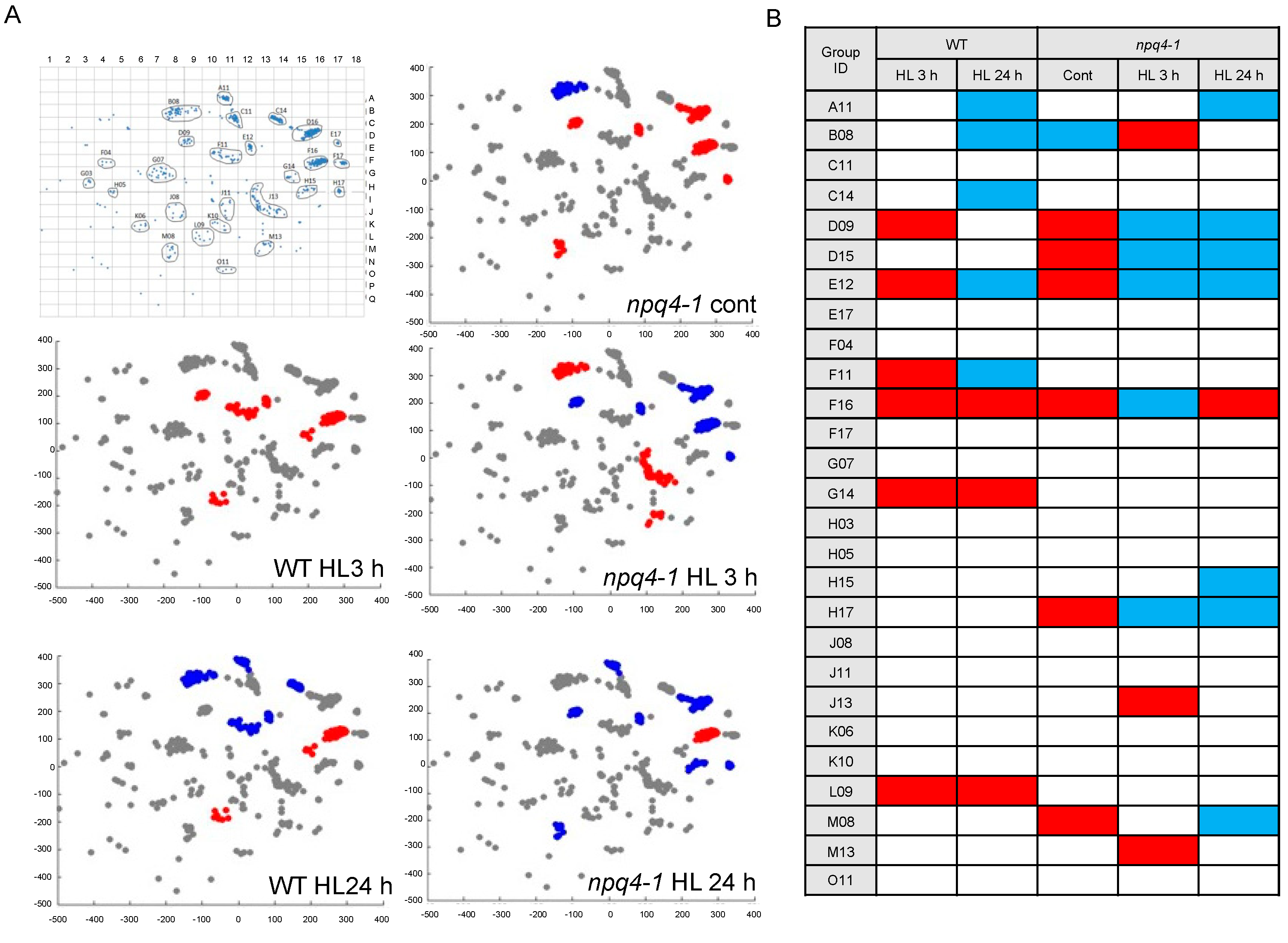
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth and HL Treatment Conditions
4.2. Chlorophyll Fluorescence
4.3. Thylakoid Membrane Isolation
4.4. Measurement of Photosynthetic Pigments by High Performance Liquid Chromatography
4.5. Histochemical Staining of Superoxide and Hydrogen Peroxide
4.6. Fluorometric Detection of Singlet Oxygen
4.7. RNA Isolation and Purification
4.8. Microarray Experiment, Image Acquisition, Data Acquisition and Normalization
4.9. GSEA Analysis Using Chloroplast-Specific Coexpression Gene Set Database
4.10. Gene Expression Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bassi, R.; Dall’Osto, L. Dissipation of Light Energy Absorbed in Excess: The Molecular Mechanisms. Annu. Rev. Plant Biol. 2021, 72, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Ksas, B.; Becuwe, N.; Chevalier, A.; Havaux, M. Plant tolerance to excess light energy and photooxidative damage relies on plastoquinone biosynthesis. Sci. Rep. 2015, 5, 10919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.P.; Phippard, A.; Pasari, J.; Niyogi, K.K. Structure-function analysis of photosystem II subunit S (PsbS) in vivo. Funct. Plant Biol. 2002, 29, 1131–1139. [Google Scholar] [CrossRef]
- Zulfugarov, I.S.; Ham, O.K.; Mishra, S.R.; Kim, J.Y.; Nath, K.; Koo, H.Y.; Kim, H.S.; Moon, Y.H.; An, G.; Lee, C.H. Dependence of reaction center-type energy-dependent quenching on photosystem II antenna size. Biochim. Biophys. Acta 2007, 1767, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Zulfugarov, I.S.; Tovuu, A.; Eu, Y.-J.; Dogsom, B.; Poudyal, R.S.; Nath, K.; Hall, M.; Banerjee, M.; Yoon, U.C.; Moon, Y.-H. Production of superoxide from Photosystem II in a rice (Oryza sativaL.) mutant lacking PsbS. BMC Plant Biol. 2014, 14, 242. [Google Scholar] [CrossRef] [Green Version]
- Zulfugarov, I.S.; Wu, G.; Tovuu, A.; Lee, C.H. Effect of oxygen on the non-photochemical quenching of vascular plants and potential oxygen deficiency in the stroma of PsbS-knock-out rice. Plant Sci. 2019, 286, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Bjorkman, O.; Shih, C.; Grossman, A.R.; Rosenquist, M.; Jansson, S.; Niyogi, K.K. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 2000, 403, 391–395. [Google Scholar] [CrossRef]
- Brooks, M.D.; Jansson, S.; Niyogi, K.K. PsbS-dependent non-photochemical quenching. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Springer: Berlin/Heidelberg, Germany, 2014; pp. 297–314. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Zhao, X.; Chory, J. The Arabidopsis Transcriptome Responds Specifically and Dynamically to High Light Stress. Cell Rep. 2019, 29, 4186–4199. [Google Scholar] [CrossRef] [Green Version]
- Rantala, M.; Tikkanen, M.; Aro, E.M. Proteomic characterization of hierarchical megacomplex formation in Arabidopsis thylakoid membrane. Plant J. 2017, 92, 951–962. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, E.M.; Tietz, S.; Froehlich, J.E. Photosystem I-LHCII megacomplexes respond to high light and aging in plants. Photosynth. Res. 2018, 136, 107–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikkanen, M.; Rantala, S.; Grieco, M.; Aro, E.-M. Comparative analysis of mutant plants impaired in the main regulatory mechanisms of photosynthetic light reactions-From biophysical measurements to molecular mechanisms. Plant Physiol. Biochem. 2017, 112, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, H. Structural changes of the thylakoid membrane network induced by high light stress in plant chloroplasts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulfugarov, I.S.; Tovuu, A.; Kim, J.-H.; Lee, C.-H. Detection of reactive oxygen species in higher plants. J. Plant Biol. 2011, 54, 351. [Google Scholar] [CrossRef]
- Pinnola, A.; Bassi, R. Molecular mechanisms involved in plant photoprotection. Biochem. Soc. Trans. 2018, 46, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Osmond, C. What is photoinhibition? Some insights from comparisons of shade and sun plants. Photoinhib. Photosynth. Mol. Mech. Field 1994, 1–24. [Google Scholar]
- Melis, A.; Neidhardt, J.; Benemann, J.R. Dunaliella salina (Chlorophyta) with small chlorophyll antenna sizes exhibit higher photosynthetic productivities and photon use efficiencies than normally pigmented cells. J. Appl. Phycol. 1998, 10, 515–525. [Google Scholar] [CrossRef]
- Baroli, I.; Melis, A. Photoinhibitory damage is modulated by the rate of photosynthesis and by the photosystem II light-harvesting chlorophyll antenna size. Planta 1998, 205, 288–296. [Google Scholar] [CrossRef]
- Roach, T.; Krieger-Liszkay, A. The role of the PsbS protein in the protection of photosystems I and II against high light in Arabidopsis thaliana. Biochim. Biophys. Acta 2012, 1817, 2158–2165. [Google Scholar] [CrossRef] [Green Version]
- Amesz, J. The function of plastoquinone in photosynthetic electron transport. Biochim. Biophys. Acta BBA Rev. Bioenerg. 1973, 301, 35–51. [Google Scholar] [CrossRef]
- Ksas, B.; Légeret, B.; Ferretti, U.; Chevalier, A.; Pospíšil, P.; Alric, J.; Havaux, M. The plastoquinone pool outside the thylakoid membrane serves in plant photoprotection as a reservoir of singlet oxygen scavengers. Plant Cell Environ. 2018, 41, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, Y.J.; Koo, M.Y.; Joo, H.J.; Ha-Lee, Y.M.; Lee, D.H. Comparative analysis of gene expression under cold acclimation, deacclimation and reacclimation in Arabidopsis. Physiol. Plant. 2014, 152, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K.; Grossman, A.R.; Björkman, O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 1998, 10, 1121–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulfugarov, I.S.; Tovuu, A.; Dogsom, B.; Lee, C.Y.; Lee, C.-H. PsbS-specific zeaxanthin-independent changes in fluorescence emission spectrum as a signature of energy-dependent non-photochemical quenching in higher plants. Photochem. Photobiol. Sci. 2010, 9, 697–703. [Google Scholar] [CrossRef]
- Miyake, C.; Amako, K.; Shiraishi, N.; Sugimoto, T. Acclimation of tobacco leaves to high light intensity drives the plastoquinone oxidation system--relationship among the fraction of open PSII centers, non-photochemical quenching of Chl fluorescence and the maximum quantum yield of PSII in the dark. Plant Cell Physiol. 2009, 50, 730–743. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide anion radical (O· 2), superoxide dismutases, and related matters. J. Biol. Chem. 1997, 272, 18515–18517. [Google Scholar] [CrossRef] [Green Version]
- Telfer, A.; Dhami, S.; Bishop, S.M.; Phillips, D.; Barber, J. beta-Carotene quenches singlet oxygen formed by isolated photosystem II reaction centers. Biochemistry 1994, 33, 14469–14474. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Cazzaniga, S.; Havaux, M.; Bassi, R. Enhanced photoprotection by protein-bound vs free xanthophyll pools: A comparative analysis of chlorophyll b and xanthophyll biosynthesis mutants. Mol. Plant 2010, 3, 576–593. [Google Scholar] [CrossRef]
- de Bianchi, S.; Betterle, N.; Kouril, R.; Cazzaniga, S.; Boekema, E.; Bassi, R.; Dall’Osto, L. Arabidopsis mutants deleted in the light-harvesting protein Lhcb4 have a disrupted photosystem II macrostructure and are defective in photoprotection. Plant Cell 2011, 23, 2659–2679. [Google Scholar] [CrossRef] [Green Version]
- Björkman, O. Comparative studies on photosynthesis in higher plants. In Photophysiology; Elsevier: Amsterdam, The Netherlands, 1973; pp. 1–63. [Google Scholar]
- Anderson, J.; Barrett, J. Light-harvesting pigment-protein complexes of algae. In Encyclopedia of Plant Physiology; Staehelin, L.A., Arntzen, C.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; Volume 19, pp. 269–285. [Google Scholar]
- Perrine, Z.; Negi, S.; Sayre, R.T. Optimization of photosynthetic light energy utilization by microalgae. Algal Res. 2012, 1, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Melis, A.; Harvey, G. Regulation of photosystem stoichiometry, chlorophyll a and chlorophyll b content and relation to chloroplast ultrastructure. Biochim. Biophys. Acta BBA Bioenerg. 1981, 637, 138–145. [Google Scholar] [CrossRef]
- Leong, T.Y.; Anderson, J.M. Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. I. Study on the distribution of chlorophyll-protein complexes. Photosynth. Res. 1984, 5, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, M.; Yang, D.H.; Andersson, B. Regulatory proteolysis of the major light-harvesting chlorophyll a/b protein of photosystem II by a light-induced membrane-associated enzymic system. Eur. J. Biochem. 1995, 231, 503–509. [Google Scholar] [CrossRef]
- Havaux, M.; Eymery, F.; Porfirova, S.; Rey, P.; Dormann, P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 2005, 17, 3451–3469. [Google Scholar] [CrossRef] [Green Version]
- Powles, S.B. Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 1984, 35, 15–44. [Google Scholar] [CrossRef]
- Osmond, B.; Förster, B. Photoinhibition: Then and now. In Photoprotection, Photoinhibition, Gene Regulation, and Environment; Springer: Berlin/Heidelberg, Germany, 2008; pp. 11–22. [Google Scholar]
- Chow, W.S.; Melis, A.; Anderson, J.M. Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc. Natl. Acad. Sci. USA 1990, 87, 7502–7506. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.; Walters, R.G.; Jansson, S.; Horton, P. Acclimation of Arabidopsis thaliana to the light environment: The existence of separate low light and high light responses. Planta 2001, 213, 794–801. [Google Scholar] [CrossRef]
- Kouřil, R.; Wientjes, E.; Bultema, J.B.; Croce, R.; Boekema, E.J. High-light vs. low-light: Effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim. Biophys. Acta BBA Bioenerg. 2013, 1827, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Wientjes, E.; van Amerongen, H.; Croce, R. LHCII is an antenna of both photosystems after long-term acclimation. Biochim. Biophys. Acta BBA Bioenerg. 2013, 1827, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.H.; Webster, J.; Adam, Z.; Lindahl, M.; Andersson, B. Induction of acclimative proteolysis of the light-harvesting chlorophyll a/b protein of photosystem II in response to elevated light intensities. Plant Physiol. 1998, 118, 827–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horie, Y.; Ito, H.; Kusaba, M.; Tanaka, R.; Tanaka, A. Participation of chlorophyll b reductase in the initial step of the degradation of light-harvesting chlorophyll a/b-protein complexes in Arabidopsis. J. Biol. Chem. 2009, 284, 17449–17456. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Szymanska, R. Singlet oxygen and non-photochemical quenching contribute to oxidation of the plastoquinone-pool under high light stress in Arabidopsis. Biochim. Biophys. Acta 2012, 1817, 705–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruk, J.; Trebst, A. Plastoquinol as a singlet oxygen scavenger in photosystem II. Biochim. Biophys. Acta 2008, 1777, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Gruszka, J.; Pawlak, A.; Kruk, J. Tocochromanols, plastoquinol, and other biological prenyllipids as singlet oxygen quenchers-determination of singlet oxygen quenching rate constants and oxidation products. Free Radical Bio. Med. 2008, 45, 920–928. [Google Scholar] [CrossRef]
- Szymańska, R.; Kruk, J. Plastoquinol is the main prenyllipid synthesized during acclimation to high light conditions in Arabidopsis and is converted to plastochromanol by tocopherol cyclase. Plant Cell Physiol. 2010, 51, 537–545. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Biosynthesis, accumulation and emission of carotenoids, alpha-tocopherol, plastoquinone, and isoprene in leaves under high photosynthetic irradiance. Photosynth. Res. 2007, 92, 163–179. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, S.-J.; Cho, S.H.; Chow, W.S.; Lee, C.-H. Photosystem I acceptor side limitation is a prerequisite for the reversible decrease in the maximum extent of P700 oxidation after short-term chilling in the light in four plant species with different chilling sensitivities. Physiol. Plantarum 2005, 123, 100–107. [Google Scholar] [CrossRef]
- Kramer, D.M.; Avenson, T.J.; Edwards, G.E. Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci. 2004, 9, 349–357. [Google Scholar] [CrossRef]
- Aro, E.M.; McCaffery, S.; Anderson, J.M. Photoinhibition and D1 Protein Degradation in Peas Acclimated to Different Growth Irradiances. Plant Physiol. 1993, 103, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta BBA Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Gilmore, A.M.; Yamamoto, H.Y. Resolution of lutein and zeaxanthin using a non-endcapped, lightly carbon-loaded C18 high-performance liquid chromatographic column. J. Chromatogr. 1991, 543, 137–145. [Google Scholar] [CrossRef]
- Fryer, M.J.; Oxborough, K.; Mullineaux, P.M.; Baker, N.R. Imaging of photo-oxidative stress responses in leaves. J. Exp. Bot. 2002, 53, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Kariola, T.; Brader, G.; Li, J.; Palva, E.T. Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell 2005, 17, 282–294. [Google Scholar] [CrossRef] [Green Version]
- Mahalingam, R.; Jambunathan, N.; Gunjan, S.K.; Faustin, E.; Weng, H.; Ayoubi, P. Analysis of oxidative signalling induced by ozone in Arabidopsis thaliana. Plant Cell Environ. 2006, 29, 1357–1371. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.N.; Le, T.T.L.; Hwang, J.-H.; Zulfugarov, I.S.; Kim, E.-H.; Kim, H.U.; Jeon, J.-S.; Lee, D.-H.; Lee, C.-H. High Light Acclimation Mechanisms Deficient in a PsbS-Knockout Arabidopsis Mutant. Int. J. Mol. Sci. 2022, 23, 2695. https://doi.org/10.3390/ijms23052695
Yang YN, Le TTL, Hwang J-H, Zulfugarov IS, Kim E-H, Kim HU, Jeon J-S, Lee D-H, Lee C-H. High Light Acclimation Mechanisms Deficient in a PsbS-Knockout Arabidopsis Mutant. International Journal of Molecular Sciences. 2022; 23(5):2695. https://doi.org/10.3390/ijms23052695
Chicago/Turabian StyleYang, Young Nam, Thi Thuy Linh Le, Ji-Hye Hwang, Ismayil S. Zulfugarov, Eun-Ha Kim, Hyun Uk Kim, Jong-Seong Jeon, Dong-Hee Lee, and Choon-Hwan Lee. 2022. "High Light Acclimation Mechanisms Deficient in a PsbS-Knockout Arabidopsis Mutant" International Journal of Molecular Sciences 23, no. 5: 2695. https://doi.org/10.3390/ijms23052695






