1-Isobutanoil-2-isopropylisothiourea Phosphate, T1082: A Safe and Effective Prevention of Radiotherapy Complications in Oncology
Abstract
:1. Introduction
2. Results
2.1. Changing the Salt-Forming Acid from HBr to H3PO4 Does Not Change the Toxic Characteristics of the Active Substance
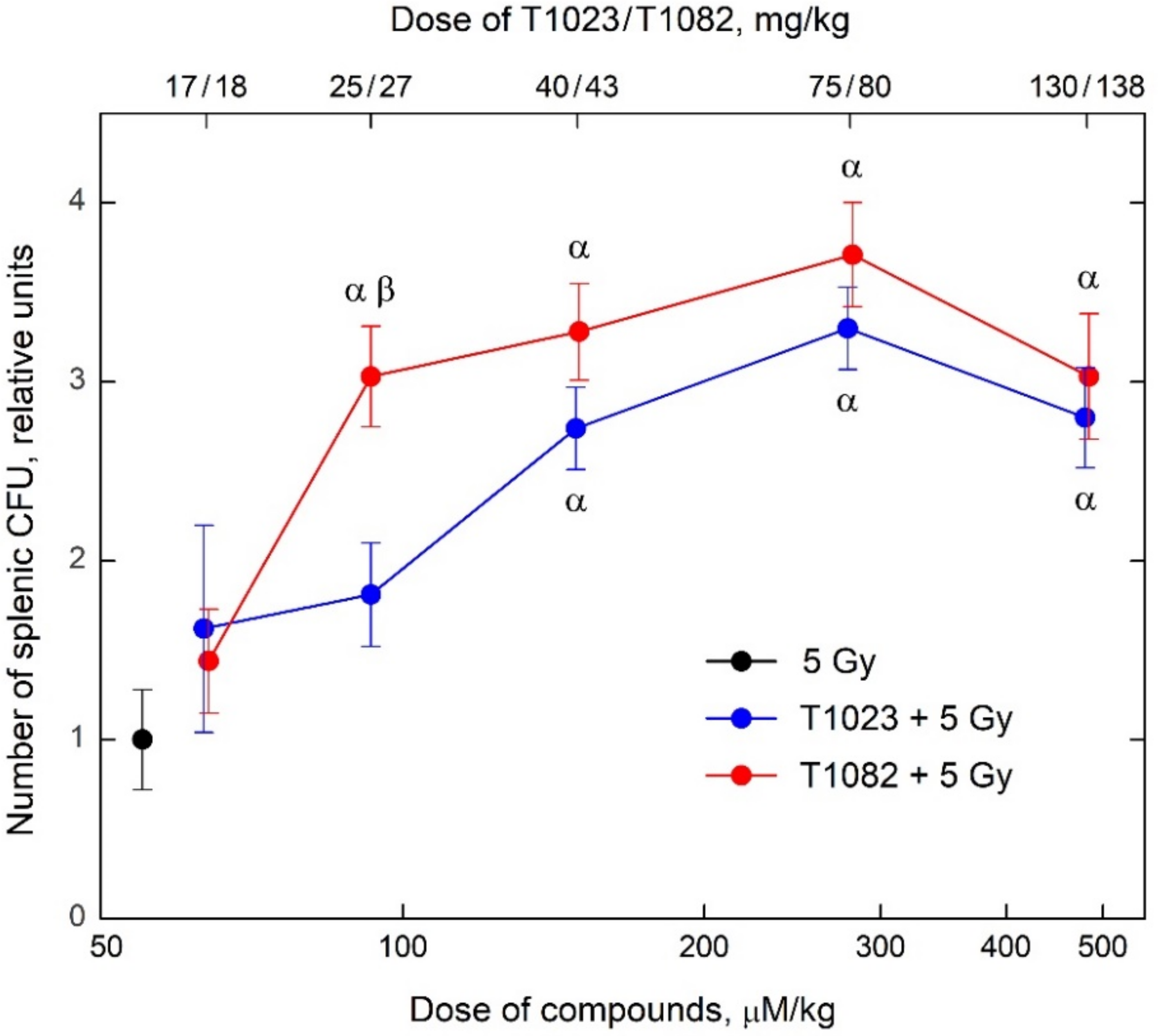
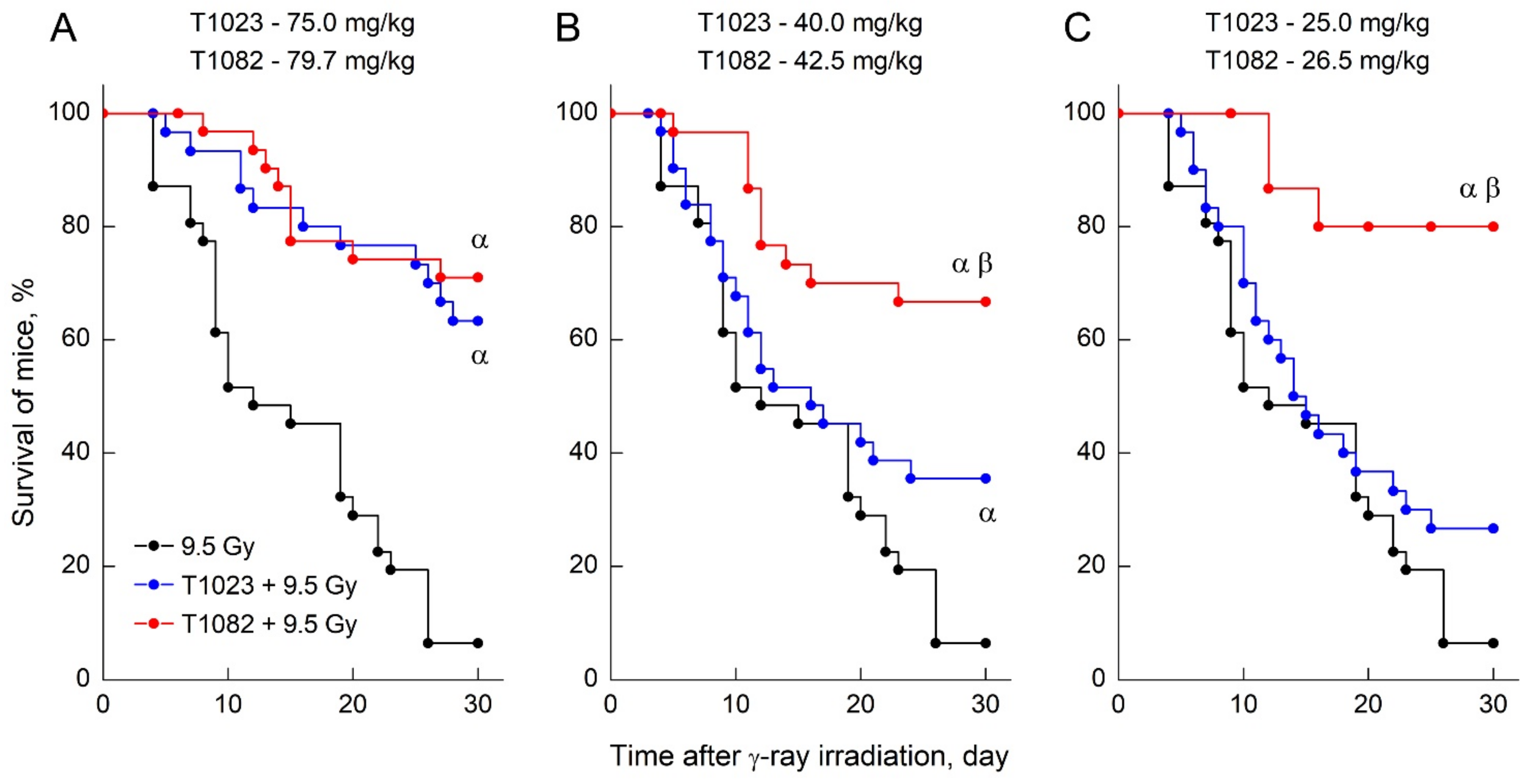
2.2. Changing the Salt-Forming Acid from HBr to H3PO4 Allows the Active Substance to Realize a Significant Effect at Low Doses and Concentrations
2.3. T1082: Perhaps the Safest Emergency Preventive Radioprotector
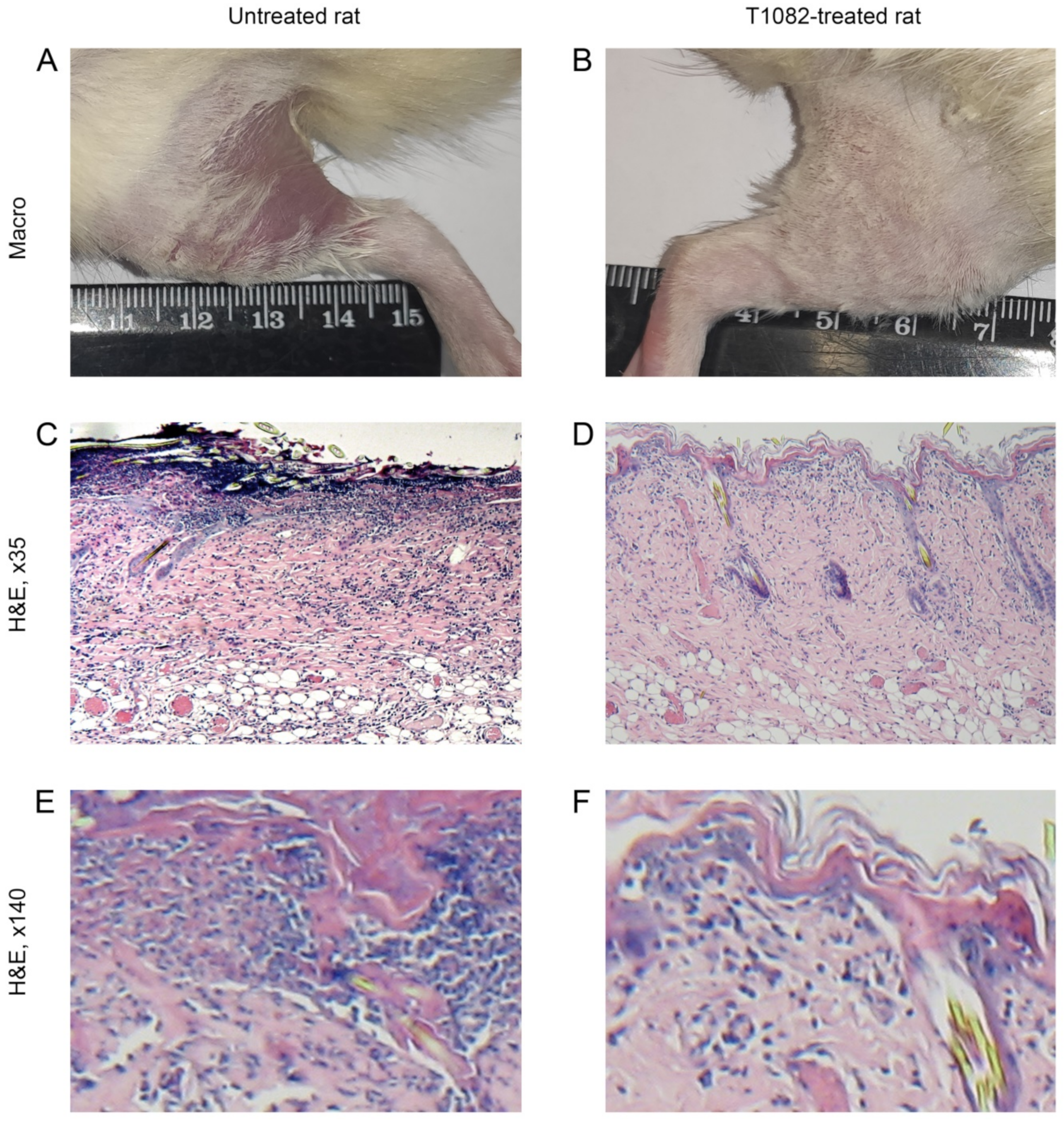
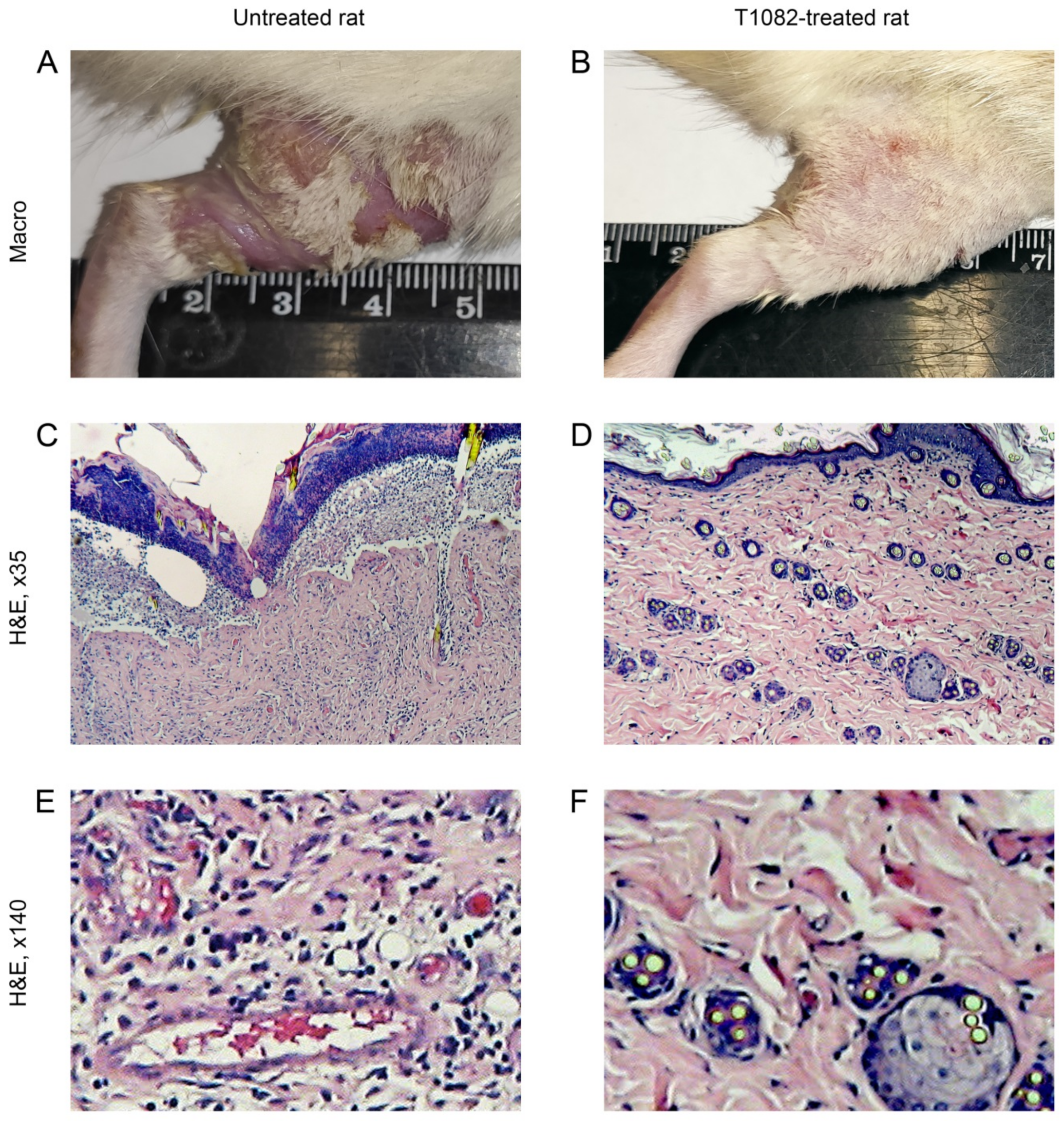
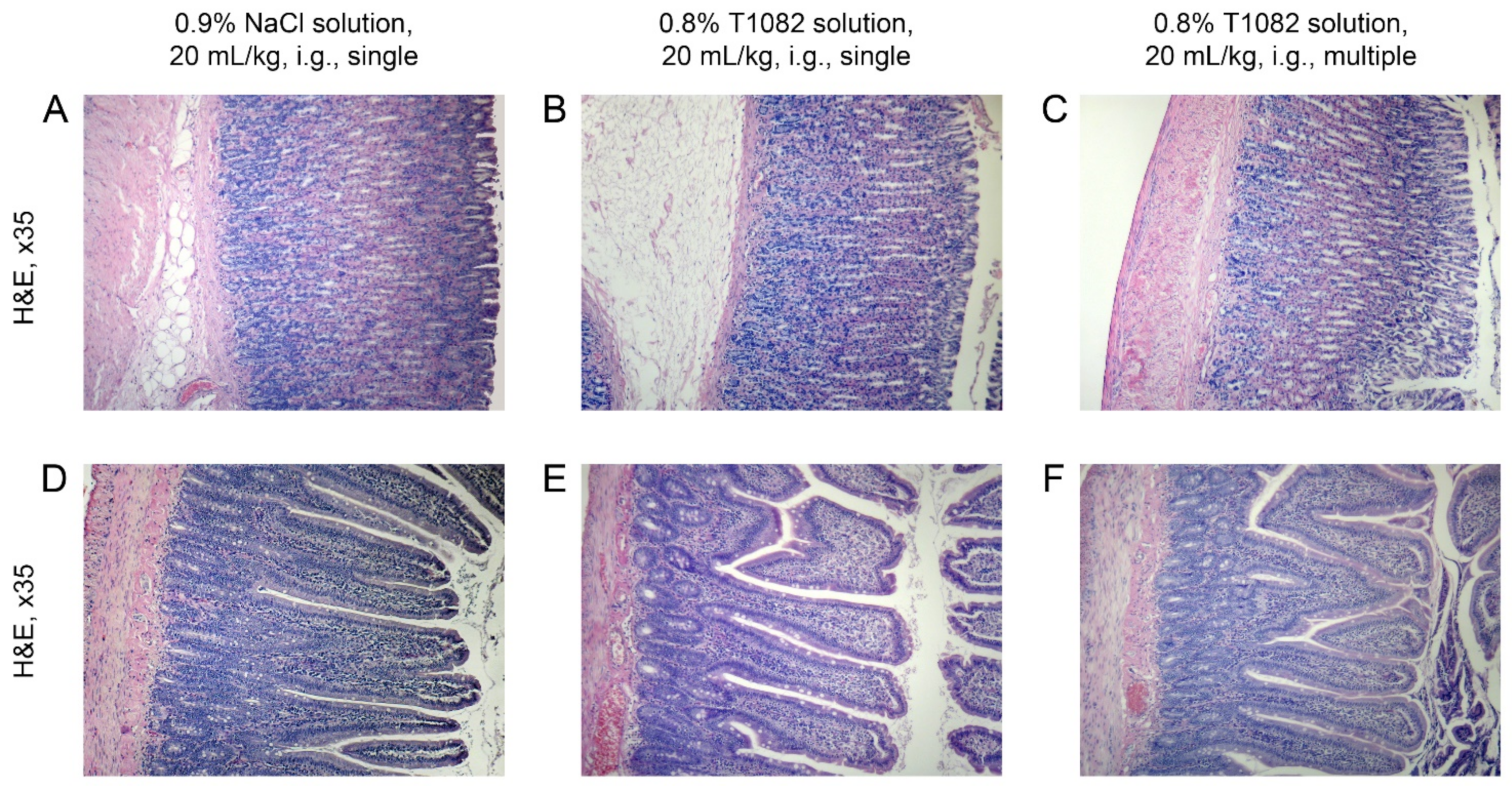
2.4. T1082: An Effective and Safe Means for the Prevention of Radiotherapy Complications
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Toxicology
4.4. Radiation Exposure
4.5. Schemes of Radiobiological Experiments in Mice and the Evaluation of Their Effects
4.6. Radiation-Induced Skin Reactions in Rats
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaprin, A.D.; Mardinskiy, Y.S.; Smirnov, V.P.; Ivanov, S.A.; Kostin, A.A.; Polikhov, S.A.; Reshetov, I.V.; Fatianova, A.S.; Denisenko, M.V.; Epatova, T.V.; et al. The history of radiation therapy (part I). Biomed. Photonics 2019, 8, 52–62. [Google Scholar] [CrossRef]
- Lushnikov, E.F.; Abrosimov, A.Y. Modern Radiation Pathology of Human: Problems of Methodology, Etiology, Pathogenesis and Classification; Medical Radiological Research Center of the Ministry of Health of the Russian Federation: Obninsk, Russia, 2012; p. 235. (In Russian) [Google Scholar]
- Joye, I.; Haustermans, K. Early and late toxicity of radiotherapy of rectal cancer. Recent Results Cancer Res. 2014, 203, 189–201. [Google Scholar] [CrossRef]
- Meattini, I.; Guenzi, M.; Fozza, A.; Vidali, C.; Rovea, P.; Meacci, F.; Livi, L. Overview on cardiac, pulmonary and cutaneous toxicity in patients treated with adjuvant radiotherapy for breast cancer. Breast Cancer 2017, 24, 52–62. [Google Scholar] [CrossRef]
- Strojan, P.; Hutcheson, K.A.; Eisbruch, A.; Beitler, J.J.; Langendijk, J.A.; Lee, A.W.M.; Corry, J.; Mendenhall, W.M.; Smee, R.; Rinaldo, A.; et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat. Rev. 2017, 59, 79–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satyamitra, M.M.; DiCarlo, A.L.; Taliaferro, L. Understanding the pathophysiology and challenges of development of medical countermeasures for radiation-induced vascular/endothelial cell injuries: Report of a NIAID workshop, August 20, 2015. Radiat. Res. 2016, 186, 99–111. [Google Scholar] [CrossRef]
- Fliedner, T.M.; Dorr, D.H.; Meineke, V. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: A study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. BJR 2005, 78 (Suppl. 27), 1–8. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Meziani, L.; Yakkada, C.; Vozenin, M. Expanding the therapeutic index of radiation therapy by normal tissue protection. Br. J. Radiol. 2019, 92, 20180008. [Google Scholar] [CrossRef]
- Moding, E.J.; Kastan, M.B.; Kirsch, D.G. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat. Rev. Drug Discov. 2013, 12, 526–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proskuryakov, S.Y.; Kucherenko, N.G.; Trishkina, A.I.; Filimonova, M.V.; Shevchuk, A.S.; Shtein, L.V.; Verkhovsky, Y.G.; Konoplyannikov, A.G.; Mandrugin, A.A.; Fedoseev, V.M.; et al. NO-inhibiting and vasotropic activity of some compounds with thioamidine group. Bull. Exp. Biol. Med. 2002, 134, 338–341. [Google Scholar] [CrossRef]
- Proskurjakov, S.Y.; Filimonova, M.V.; Borovaya, O.N.; Kucherenko, N.G.; Trishkina, A.I.; Shteyn, L.V.; Skvortsov, V.G.; Ulyanova, L.P.; Shevchenko, L.I.; Verkhovsky, Y.G. Effect of NO inhibitors on hypovolemic shock-induced hypotension. Bull. Exp. Biol. Med. 2010, 150, 18–22. [Google Scholar] [CrossRef]
- Filimonova, M.V.; Trofimova, T.P.; Borisova, G.S.; Mandrugin, A.A. Antihypotensive activity of 2-acetylamino-5,6-dihydro-4H-1,3-thiazine for an endotoxic shock model in rats. Pharm. Chem. J. 2012, 46, 210–212. [Google Scholar] [CrossRef]
- Filimonova, M.V.; Proskuryakov, S.Y.; Shevchenko, L.I.; Shevchuk, A.S.; Lushnikova, G.A.; Makarchuk, V.M.; Arzamastsev, E.V.; Laba, V.I.; Malinovskaya, K.I.; Levitskaya, E.L. Radioprotective properties of isothiourea derivatives with NO-inhibitory mechanism of action. Radiat. Biol. Radioecol. 2012, 52, 593–601. (In Russian) [Google Scholar]
- Filimonova, M.V.; Shevchenko, L.I.; Trofimova, T.P.; Makarchuk, V.M.; Shevchuk, A.S.; Lushnikova, G.A. On the mechanism of radioprotective effect of NO-synthase inhibitors. Radiat. Biol. Radioecol. 2014, 54, 500–506. (In Russian) [Google Scholar] [CrossRef]
- Makarchuk, V.M.; Filimonova, M.V.; Filimonov, A.S.; Shevchenko, L.I.; Izmestieva, O.S.; Saburova, A.S. Lactatemia as a possible pharmacological marker of NOS-inhibitor T1023 induced radioresistance. Radiat. Risk 2020, 29, 45–56. [Google Scholar] [CrossRef]
- Filimonova, M.V.; Shevchenko, L.I.; Makarchuk, V.M.; Chesnakova, E.A.; Izmest’eva, O.S.; Korneeva, T.S.; Filimonov, A.S. Radioprotective properties of NO-synthase inhibitor T1023: I. indicators of radioprotective activity and interaction with other radioprotectors. Radiat. Biol. Radioecol. 2015, 55, 250–259. (In Russian) [Google Scholar] [CrossRef]
- Filimonova, M.V.; Makarchuk, V.M.; Shevchenko, L.I.; Saburova, A.S.; Surinova, V.I.; Izmestieva, O.S.; Lychagin, A.A.; Saburov, V.O.; Shegay, P.V.; Kaprin, A.D.; et al. Radioprotective activity of nitric oxide synthase inhibitor T1023. Toxicological and biochemical properties, cardiovascular and radioprotective effects. Radiat. Res. 2020, 194, 532–543. [Google Scholar] [CrossRef]
- Filimonova, M.V.; Samsonova, A.S.; Korneeva, T.S.; Shevchenko, L.I.; Saburov, O.V.; Filimonov, A.S. The radioprotective effects of nitric oxide synthase inhibitor T1023 on normal and malignant tissues. Radiat. Risk 2018, 27, 155–169. [Google Scholar] [CrossRef]
- Saburova, A.S.; Filimonova, M.V.; Yuzhakov, V.V.; Shevchenko, L.I.; Yakovleva, N.D.; Bandurko, L.N.; Koretskaya, A.E.; Fomina, N.K.; Saburov, V.O.; Filimonov, A.S. The influence of nitric oxide synthases inhibitor T1023 on the development of radiation pneumofibrosis in rats. Radiat. Hyg. 2020, 13, 60–67. (In Russian) [Google Scholar] [CrossRef]
- Filimonova, M.; Saburova, A.; Makarchuk, V.; Shevchenko, L.; Surinova, V.; Yuzhakov, V.; Yakovleva, N.; Sevankaeva, L.; Saburov, V.; Koryakin, S.; et al. The ability of the nitric oxide synthases inhibitor T1023 to selectively protect the non-malignant tissues. Int. J. Mol. Sci. 2021, 22, 9340. [Google Scholar] [CrossRef]
- Curry, S.H.; Whelpton, R. Drug Disposition and Pharmacokinetics: From Principles to Applications, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2010; p. 388. [Google Scholar] [CrossRef]
- Filimonova, M.V.; Saburova, A.S.; Shevchenko, L.I.; Makarchuk, V.M.; Lychagin, A.A.; Filimonov, A.S. Influence of the type of salt-forming acids on the antiradiation activity of T1023 analogs—Salts of N-isobutanoyl-S-isopropylisothiourea. Radiatsionnaya Gyg. 2021, 14, 68–74. [Google Scholar] [CrossRef]
- Berezovskaya, I.V. Classification of substances with respect to acute toxicity for parenteral administration. Pharm. Chem. J. 2003, 37, 139–141. [Google Scholar] [CrossRef]
- Vasin, M.V.; Ushakov, I.B. Comparative efficacy and the window of radioprotection for adrenergic and serotoninergic agents and aminothiols in experiments with small and large animals. J. Radiat. Res. 2015, 56, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization of research and treatment of cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Wells, M.; MacBride, S. Radiation skin reactions. In Supportive Care in Radiotherapy; Churchill Livingstone: Edinburgh, UK, 2003; pp. 135–159. [Google Scholar]
- DiCarlo, A.L.; Bandremer, A.C.; Hollingsworth, B.A.; Kasim, S.; Laniyonu, A.; Todd, N.F.; Wang, S.-J.; Wertheimer, E.R.; Rios, C. Cutaneous radiation injuries: Models, assessment and treatments. Radiat. Res. 2020, 194, 315–344. [Google Scholar] [CrossRef]
- Singh, V.K.; Garcia, M.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part II. countermeasures for limited indications, internalized radionuclides, emesis, late effects, and agents demonstrating efficacy in large animals with or without FDA IND status. Int. J. Radiat. Biol. 2017, 93, 870–884. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Hanlon, B.K.; Santiago, P.T.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part III. countermeasures under early stages of development along with ‘standard of care’ medicinal and procedures not requiring regulatory approval for use. Int. J. Radiat. Biol. 2017, 93, 885–906. [Google Scholar] [CrossRef]
- King, M.; Joseph, S.; Albert, A.; Thomas, T.V.; Nittala, M.R.; Woods, W.C.; Vijayakumar, S.; Packianathan, S. Use of amifostine for cytoprotection during radiation therapy: A review. Oncology 2020, 98, 61–80. [Google Scholar] [CrossRef]
- Vasin, M.V.; Ushakov, I.B.; Suvorov, N.N. Radioprotective effectiveness of indralin in local gamma irradiation of skin. Radiat. Biol. Radioecol. 1998, 38, 42–54. (In Russian) [Google Scholar]
- Cho, Y.J.; Yi, C.O.; Jeon, B.T.; Jeong, Y.Y.; Kang, G.M.; Lee, J.E.; Roh, G.S.; Lee, J.D. Curcumin attenuates radiation-induced inflammation and fibrosis in rat lungs. Korean J. Physiol. Pharmacol. 2013, 17, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guven, B.; Can, M.; Piskin, O.; Aydin, B.G.; Karakaya, K.; Elmas, O.; Acikgoz, B. Flavonoids protect colon against radiation induced colitis. Regul. Toxicol. Pharmacol. 2019, 104, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Sonis, S.T.; Lee, C.M.; Adkins, D.; Allen, B.G.; Sun, W.; Agarwala, S.S.; Venigalla, M.L.; Chen, Y.; Zhen, W.; et al. Phase 1b/2a trial of the superoxide dismutase mimetic GC4419 to reduce chemoradiotherapy-induced oral mucositis in patients with oral cavity or oropharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Rosen, E.M.; Day, R.; Singh, V.K. New approaches to radiation protection. Front. Oncol. 2015, 4, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filimonova, M.V.; Shevchenko, L.I.; Filimonov, A.S.; Saburova, A.S.; Makarchuk, V.M.; Shitova, A.A.; Soldatova, O.V.; Shegay, P.V.; Ivanov, S.A.; Kaprin, A.D. National Medical Research Radiological Center of the Ministry of Health of the Russian Federation. Radioprotective Pharmacological Agent. Russian Federation Patent RU 2,733,883, 7 October 2020. (In Russian). [Google Scholar]
- Bunyatyan, N.D.; Vasiliev, A.N.; Verstakova, O.L.; Zhuravleva, M.V.; Korobov, N.V.; Merkulov, V.A.; Orekhov, S.N.; Sakaeva, I.V.; Uteshev, D.B.; Yavorsky, A.N. Guidelines for Preclinical Drug Research. Part 1; Mironov, A.N., Ed.; Grif & Co.: Moscow, Russia, 2012; p. 944. (In Russian) [Google Scholar]
- McCulloch, E.A.; Till, J.E. The sensitivity of cells from normal mouse bone marrow to gamma radiation in vitro and in vivo. Radiat. Res. 1962, 16, 822–832. [Google Scholar] [CrossRef]
- Greaves, P. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation, 4th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2011; p. 1248. [Google Scholar]
- McInnes, E.F. Background Lesions in Laboratory Animals. A Color Atlas; Saunders Elsevier: Edinburg, UK, 2012; p. 130. [Google Scholar]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]


| Compound, Route of Administration | LD10/15 | LD16/15 | LD50/15 | LD84/15 | ||||
|---|---|---|---|---|---|---|---|---|
| mg/kg | mM | mg/kg | mM | mg/kg | mM | mg/kg | mM | |
| T1023, i.p. * | 317 | 1.2 | 333 | 1.2 | 410 | 1.5 | 488 | 1.8 |
| T1082, i.p. | 321 | 1.1 | 338 | 1.2 | 403 | 1.4 | 481 | 1.7 |
| T1082, i.g. | 2290 | 8.0 | 2364 | 8.3 | 2638 | 9.2 | 2944 | 10.3 |
| Compound, Route of Administration | LD10/ED16 | LD50/ED50 | LD10/ED50 | LD10/EDopt |
|---|---|---|---|---|
| T1082, i.g. | 42.1 | 30.1 | 26.1 | 10.2–16.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filimonova, M.; Saburova, A.; Shevchenko, L.; Makarchuk, V.; Shitova, A.; Soldatova, O.; Rybachuk, V.; Kosachenko, A.; Nikolaev, K.; Demyashkin, G.; et al. 1-Isobutanoil-2-isopropylisothiourea Phosphate, T1082: A Safe and Effective Prevention of Radiotherapy Complications in Oncology. Int. J. Mol. Sci. 2022, 23, 2697. https://doi.org/10.3390/ijms23052697
Filimonova M, Saburova A, Shevchenko L, Makarchuk V, Shitova A, Soldatova O, Rybachuk V, Kosachenko A, Nikolaev K, Demyashkin G, et al. 1-Isobutanoil-2-isopropylisothiourea Phosphate, T1082: A Safe and Effective Prevention of Radiotherapy Complications in Oncology. International Journal of Molecular Sciences. 2022; 23(5):2697. https://doi.org/10.3390/ijms23052697
Chicago/Turabian StyleFilimonova, Marina, Alina Saburova, Ljudmila Shevchenko, Victoria Makarchuk, Anna Shitova, Olga Soldatova, Vitaly Rybachuk, Alexander Kosachenko, Kirill Nikolaev, Grigory Demyashkin, and et al. 2022. "1-Isobutanoil-2-isopropylisothiourea Phosphate, T1082: A Safe and Effective Prevention of Radiotherapy Complications in Oncology" International Journal of Molecular Sciences 23, no. 5: 2697. https://doi.org/10.3390/ijms23052697
APA StyleFilimonova, M., Saburova, A., Shevchenko, L., Makarchuk, V., Shitova, A., Soldatova, O., Rybachuk, V., Kosachenko, A., Nikolaev, K., Demyashkin, G., Saburov, V., Koryakin, S., Shegay, P., Kaprin, A., Ivanov, S., & Filimonov, A. (2022). 1-Isobutanoil-2-isopropylisothiourea Phosphate, T1082: A Safe and Effective Prevention of Radiotherapy Complications in Oncology. International Journal of Molecular Sciences, 23(5), 2697. https://doi.org/10.3390/ijms23052697







