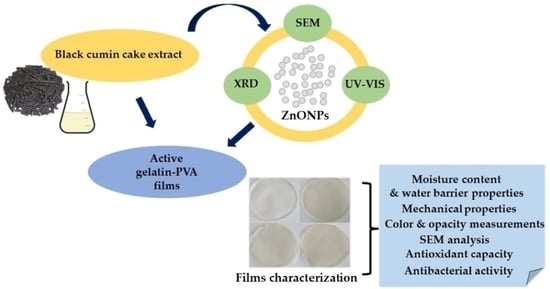
Functional Properties of Gelatin/Polyvinyl Alcohol Films Containing Black Cumin Cake Extract and Zinc Oxide Nanoparticles Produced via Casting Technique
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Zinc Oxide Nanoparticles
2.2. Moisture Content in Films
2.3. Water Vapor Transmission Rate and Water Vapor Permeability of Films
2.4. Mechanical Properties of Films
2.5. Surface Color and Optical Properties of Films
2.6. Microstructure of Films
2.7. Antioxidant Properties of Films
2.8. Antimicrobial Properties of Films
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of Black Cumin Cake Extract
3.3. Green Concept of Receiving Zinc Oxide Nanoparticles
3.4. Characterization of Zinc Oxide Nanoparticles
3.4.1. UV–Vis Spectrophotometry
3.4.2. X-ray Diffraction Analysis
3.4.3. Scanning Electron Microscopy Analysis
3.5. Preparation of Active Films
3.6. Physicochemical, Optical, and Morphological Properties of Films
3.6.1. Moisture Content
3.6.2. Water Vapor Permeability
3.6.3. Mechanical Properties
3.6.4. Surface Color and Opacity Measurement
3.6.5. Microstructure of Films
3.7. Antioxidant Capacity of Films
3.7.1. QUENCHERDPPH Procedure
3.7.2. QUENCHERABTS Procedure
3.7.3. QUENCHERCUPRAC Procedure
3.8. Antibacterial Activity of Films
3.8.1. Bacterial Strains and Inoculum Preparation
3.8.2. Antibacterial Activity by Disk Diffusion Method
3.8.3. Antibacterial Activity by Microtiter Plate Method
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables-A Review: Fruit and Vegetable Edible Films. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; González-Martínez, C.; Chiralt, A. Biodegradable Antimicrobial Films for Food Packaging: Effect of Antimicrobials on Degradation. Foods 2021, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.-C.; Ficai, A.; Ene, V.-L.; Vasile, B.-S.; Andronescu, E.; Holban, A.-M. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and Antimicrobial Properties of Starch-PVA Blend Films as Affected by the Incorporation of Natural Antimicrobial Agents. Foods 2015, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-Acorn Starch-Eugenol Edible Film: Physico-Chemical, Barrier, Antimicrobial, Antioxidant and Structural Properties. Int. J. Biol. Macromol. 2019, 135, 344–352. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-Based Composite Films and Their Application in Food Packaging: A Review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- De Barros Vinhal, G.L.R.R.; Silva-Pereira, M.C.; Teixeira, J.A.; Barcia, M.T.; Pertuzatti, P.B.; Stefani, R. Gelatine/PVA Copolymer Film Incorporated with Quercetin as a Prototype to Active Antioxidant Packaging. J. Food Sci. Technol. 2021, 58, 3924–3932. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, X.; Qin, Y.-R.; Zhang, Y.-H.; Wang, X.-P.; Wang, J.-Y.; Ning, Z.-X.; Ruan, Q.-J.; Zhang, Y.-S. Preparation and Characterization of a Novel Colorimetric Indicator Film Based on Gelatin/Polyvinyl Alcohol Incorporating Mulberry Anthocyanin Extracts for Monitoring Fish Freshness. Food Res. Int. 2019, 126, 108604. [Google Scholar] [CrossRef]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable Use of Fruit and Vegetable By-Products to Enhance Food Packaging Performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Effect of Date Fruit Waste Extract as an Antioxidant Additive on the Properties of Active Gelatin Films. Food Chem. 2021, 355, 129631. [Google Scholar] [CrossRef]
- Jridi, M.; Boughriba, S.; Abdelhedi, O.; Nciri, H.; Nasri, R.; Kchaou, H.; Kaya, M.; Sebai, H.; Zouari, N.; Nasri, M. Investigation of Physicochemical and Antioxidant Properties of Gelatin Edible Film Mixed with Blood Orange (Citrus sinensis) Peel Extract. Food Packag. Shelf Life 2019, 21, 100342. [Google Scholar] [CrossRef]
- Albertos, I.; Avena-Bustillos, R.J.; Martín-Diana, A.B.; Du, W.-X.; Rico, D.; McHugh, T.H. Antimicrobial Olive Leaf Gelatin Films for Enhancing the Quality of Cold-Smoked Salmon. Food Packag. Shelf Life 2017, 13, 49–55. [Google Scholar] [CrossRef]
- Mariod, A.A.; Ibrahim, R.M.; Ismail, M.; Ismail, N. Antioxidant Activity and Phenolic Content of Phenolic Rich Fractions Obtained from Black Cumin (Nigella sativa) Seedcake. Food Chem. 2009, 116, 306–312. [Google Scholar] [CrossRef]
- Ekramian, S.; Abbaspour, H.; Roudi, B.; Amjad, L.; Nafchi, A.M. Influence of Nigella sativa L. Extract on Physico–Mechanical and Antimicrobial Properties of Sago Starch Film. J. Polym. Environ. 2021, 29, 201–208. [Google Scholar] [CrossRef]
- Sabbah, M.; Altamimi, M.; Di Pierro, P.; Schiraldi, C.; Cammarota, M.; Porta, R. Black Edible Films from Protein-Containing Defatted Cake of Nigella sativa Seeds. Int. J. Mol. Sci. 2020, 21, 832. [Google Scholar] [CrossRef] [Green Version]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial Polyphenol-Rich Extracts: Applications and Limitations in the Food Industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef]
- Meshram, J.V.; Koli, V.B.; Phadatare, M.R.; Pawar, S.H. Anti-Microbial Surfaces: An Approach for Deposition of ZnO Nanoparticles on PVA-Gelatin Composite Film by Screen Printing Technique. Mater. Sci. Eng. C 2017, 73, 257–266. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Alizadeh-Sani, M.; Divband, B.; Ehsani, A.; McClements, D.J. Nanocomposite Films Consisting of Functional Nanoparticles (TiO2 and ZnO) Embedded in 4A-Zeolite and Mixed Polymer Matrices (Gelatin and Polyvinyl Alcohol). Food Res. Int. 2020, 137, 109716. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Juszczak, L.; Kawecka, A.; Bytesnikova, Z.; Milosavljevic, V.; Makarewicz, M. Development of Furcellaran-Gelatin Films with Se-AgNPs as an Active Packaging System for Extension of Mini Kiwi Shelf Life. Food Packag. Shelf Life 2019, 21, 100339. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2020, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green Synthesis of Zinc Oxide Nanoparticles: A Review of the Synthesis Methodology and Mechanism of Formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Ahmed, B.; Solanki, B.; Zaidi, A.; Khan, M.S.; Musarrat, J. Bacterial Toxicity of Biomimetic Green Zinc Oxide Nanoantibiotic: Insights into ZnONP Uptake and Nanocolloid–Bacteria Interface. Toxicol. Res. 2019, 8, 246–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-Said, M.S.; Alkhedhairy, A.A.; Musarrat, J. Aloe Vera Extract Functionalized Zinc Oxide Nanoparticles as Nanoantibiotics against Multi-Drug Resistant Clinical Bacterial Isolates. J. Colloid Sci. 2016, 472, 145–156. [Google Scholar] [CrossRef]
- Agarwal, H.; Shanmugam, V. A Review on Anti-Inflammatory Activity of Green Synthesized Zinc Oxide Nanoparticle: Mechanism-Based Approach. Bioorg. Chem. 2020, 94, 103423. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kim, H.C.; Panicker, P.S.; Rhim, J.-W.; Kim, J. Cellulose Nanofiber-Based Nanocomposite Films Reinforced with Zinc Oxide Nanorods and Grapefruit Seed Extract. Nanomaterials 2021, 11, 877. [Google Scholar] [CrossRef]
- Vinay, S.P.; Chandrasekhar, N. Structural and Biological Investigation of Green Synthesized Silver and Zinc Oxide Nanoparticles. J. Inorg. Organomet. Polym. 2021, 31, 552–558. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green Route to Synthesize Zinc Oxide Nanoparticles Using Leaf Extracts of Cassia fistula and Melia azadarach and Their Antibacterial Potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Bharti, A.; Singh, J.P.; Chae, K.H.; Goyal, N.; Gautam, S. Structural and Electronic Investigation of ZnO Nanostructures Synthesized under Different Environments. Heliyon 2018, 4, e00594. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, W.; Ullah, N.; Haroon, M.; Abbasi, B.H. Optical, Morphological and Biological Analysis of Zinc Oxide Nanoparticles (ZnO NPs) Using Papaver somniferum L. RSC Adv. 2019, 9, 29541–29548. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Teng, X.; Li, G.; Rhim, J.-W. Preparation, Characterization, and Antimicrobial Activity of Gelatin/ZnO Nanocomposite Films. Food Hydrocoll. 2015, 45, 264–271. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Properties and Characterization of Bionanocomposite Films Prepared with Various Biopolymers and ZnO Nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Kim, S.-M.; Rhim, J.-W. Carboxymethyl Cellulose-Based Multifunctional Film Combined with Zinc Oxide Nanoparticles and Grape Seed Extract for the Preservation of High-Fat Meat Products. Sustain. Mater. Technol. 2021, 29, e00325. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Properties and Antimicrobial Activity of Fish Protein Isolate/Fish Skin Gelatin Film Containing Basil Leaf Essential Oil and Zinc Oxide Nanoparticles. Food Hydrocoll. 2014, 41, 265–273. [Google Scholar] [CrossRef]
- Shahvalizadeh, R.; Ahmadi, R.; Davandeh, I.; Pezeshki, A.; Seyed Moslemi, S.A.; Karimi, S.; Rahimi, M.; Hamishehkar, H.; Mohammadi, M. Antimicrobial Bio-Nanocomposite Films Based on Gelatin, Tragacanth, and Zinc Oxide Nanoparticles—Microstructural, Mechanical, Thermo-Physical, and Barrier Properties. Food Chem. 2021, 354, 129492. [Google Scholar] [CrossRef]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.-W.; Bagheri, R. Gelatin-Based Functional Films Integrated with Grapefruit Seed Extract and TiO2 for Active Food Packaging Applications. Food Hydrocoll. 2020, 112, 106314. [Google Scholar] [CrossRef]
- Kumar, S.; Mudai, A.; Roy, B.; Basumatary, I.B.; Mukherjee, A.; Dutta, J. Biodegradable Hybrid Nanocomposite of Chitosan/Gelatin and Green Synthesized Zinc Oxide Nanoparticles for Food Packaging. Foods 2020, 9, 1143. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Sarbon, N.M. A Comparative Study: Physical, Mechanical and Antibacterial Properties of Bio-Composite Gelatin Films as Influenced by Chitosan and Zinc Oxide Nanoparticles Incorporation. Food Biosci. 2021, 43, 101250. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Davudian, S.H.; Pourmohammad, S.; Hamishehkar, H.; Roufegarinejad, L. Preparation and Characterization of Gelatin-Based Nanocomposite Containing Chitosan Nanofiber and ZnO Nanoparticles. Carbohydr. Polym. 2019, 216, 376–384. [Google Scholar] [CrossRef]
- Hedayatnia, S.; Tan, C.P.; Joanne Kam, W.-L.; Tan, T.B.; Mirhosseini, H. Modification of Physicochemical and Mechanical Properties of a New Bio-Based Gelatin Composite Films through Composition Adjustment and Instantizing Process. LWT Food Sci. Technol. 2019, 116, 108575. [Google Scholar] [CrossRef]
- Haghighi, H.; Gullo, M.; La China, S.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Characterization of Bio-Nanocomposite Films Based on Gelatin/Polyvinyl Alcohol Blend Reinforced with Bacterial Cellulose Nanowhiskers for Food Packaging Applications. Food Hydrocoll. 2021, 113, 106454. [Google Scholar] [CrossRef]
- Villasante, J.; Martin-Lujano, A.; Almajano, M.P. Characterization and Application of Gelatin Films with Pecan Walnut and Shell Extract (Carya illinoiensis). Polymers 2020, 12, 1424. [Google Scholar] [CrossRef] [PubMed]
- Javidi, S.; Mohammadi Nafchi, A.; Moghadam, H.H. Synergistic Effect of Nano-ZnO and Mentha piperita Essential Oil on the Moisture Sorption Isotherm, Antibacterial Activity, Physicochemical, Mechanical, and Barrier Properties of Gelatin Film. J. Food Meas. Charact. 2021. [Google Scholar] [CrossRef]
- Sahraee, S.; Ghanbarzadeh, B.; Milani, J.M.; Hamishehkar, H. Development of Gelatin Bionanocomposite Films Containing Chitin and ZnO Nanoparticles. Food Bioprocess Technol. 2017, 10, 1441–1453. [Google Scholar] [CrossRef]
- Vejdan, A.; Ojagh, S.M.; Adeli, A.; Abdollahi, M. Effect of TiO2 Nanoparticles on the Physico-Mechanical and Ultraviolet Light Barrier Properties of Fish Gelatin/Agar Bilayer Film. LWT Food Sci. Technol. 2016, 71, 88–95. [Google Scholar] [CrossRef]
- Choudhary, S.; Sengwa, R.J. ZnO Nanoparticles Dispersed PVA–PVP Blend Matrix Based High Performance Flexible Nanodielectrics for Multifunctional Microelectronic Devices. Curr. Appl. Phys. 2018, 18, 1041–1058. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Fernandes, E.G.; Kenawy, E.-R.S.; Lazzeri, A. Gelatin-Based Blends and Composites. Morphological and Thermal Mechanical Characterization. Biomacromolecules 2001, 2, 806–811. [Google Scholar] [CrossRef]
- Kavoosi, G.; Bordbar, Z.; Dadfar, S.M.; Dadfar, S.M.M. Preparation and Characterization of a Novel Gelatin-Poly(Vinyl Alcohol) Hydrogel Film Loaded with Zataria multiflora Essential Oil for Antibacterial-Antioxidant Wound-Dressing Applications. J. Appl. Polym. Sci. 2017, 134, 45351. [Google Scholar] [CrossRef]
- Tymczewska, A.; Furtado, B.U.; Nowaczyk, J.; Hrynkiewicz, K.; Szydłowska-Czerniak, A. Development and Characterization of Active Gelatin Films Loaded with Rapeseed Meal Extracts. Materials 2021, 14, 2869. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of Pomegranate (Punica granatum L.) Peel Powder on the Antioxidant and Antimicrobial Properties of Fish Gelatin Films as Active Packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Das, D.; Nath, B.C.; Phukon, P.; Kalita, A.; Dolui, S.K. Synthesis of ZnO Nanoparticles and Evaluation of Antioxidant and Cytotoxic Activity. Colloids Surf. B 2013, 111, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective Toxicity of ZnO Nanoparticles toward Gram-Positive Bacteria and Cancer Cells by Apoptosis through Lipid Peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial Activity of Metal Oxide Nanoparticles against Gram-Positive and Gram-Negative Bacteria: A Comparative Study. Int. J. Nanomed. 2012, 7, 6003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seltmann, G.; Holst, O. The Bacterial Cell Wall, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 31–65. [Google Scholar]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [Green Version]
- Lallo da Silva, B.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship Between Structure And Antimicrobial Activity Of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [Green Version]
- ASTM E96-95; Standard Test Methods for Water Vapour Transmission of Material. American Society for Testing and Materials (ASTM): Philadelphia, PA, USA, 1993.
- ISO 527-3; Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. ISO: Geneva, Switzerland, 2018.
- Wang, W.; Liu, Y.; Jia, H.; Liu, Y.; Zhang, H.; He, Z.; Ni, Y. Effects of Cellulose Nanofibers Filling and Palmitic Acid Emulsions Coating on the Physical Properties of Fish Gelatin Films. Food Biophys. 2017, 12, 23–32. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tułodziecka, A. Antioxidant Capacity of Rapeseed Extracts Obtained by Conventional and Ultrasound-Assisted Extraction. J. Am. Oil Chem. Soc. 2014, 91, 2011–2019. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and Characterization of Chitosan Film Incorporated with Thinned Young Apple Polyphenols as an Active Packaging Material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Approved Standard M2-A6 (M100-S7); Performance Standard for Antimicrobial Disc Susceptibility Test. 6th ed. NCCLS (National Committe For Clinical Laboratory Standards): Wayne, PA, USA, 1997.



| Film Type | MC * ± SD (%) | WVTR * ± SD (g·mm−2·h−1) | WVP * ± SD (g·mm·Pa−1·h−1·mm−2) | TS ** ± SD (MPa) | EAB **± SD (%) | YM ** ± SD (MPa) |
|---|---|---|---|---|---|---|
| G/PVA | 18.09 ± 0.30 a | 2.45 × 10−5 ± 2.08 × 10−6 b | 1.59 × 10−9 ± 3.93 × 10−10 a,b | 2.97 ± 0.18 d | 137.03 ± 6.73 c | 11.14 ± 0.92 c |
| G/PVA/BCCE | 19.84 ± 0.08 b | 2.72 × 10−5 ± 2.34 × 10−6 b | 1.93 × 10−9 ± 1.28 × 10−10 b | 2.31 ± 0.08 c | 125.16 ± 7.13 b | 8.19 ± 0.97 b |
| G/PVA/ZnONPs | 25.63 ± 0.45 d | 2.65 × 10−5 ± 1.98 × 10−6 b | 1.92 × 10−9 ± 1.71 × 10−10 b | 1.69 ± 0.1 a | 106.46 ± 7.03 a | 8.51 ± 0.33 b |
| G/PVA/BCCE/ZnONPs | 24.21 ± 0.12 c | 1.82 × 10−5 ± 2.79 × 10−6 a | 1.14 × 10−9 ± 1.78 × 10−10 a | 1.84 ± 0.1 b | 141.45 ± 7.55 c | 6.64 ± 0.35 a |
| Film Type | L * ± SD | a * ± SD | b * ± SD | ΔE * ± SD | Opacity * (mm−1) |
|---|---|---|---|---|---|
| G/PVA | 88.9 ± 0.1 c | 1.0 ± 0.1 d | −7.5 ± 0.2 a | - | 2.60 ± 0.02 a |
| G/PVA/BCCE | 87.5 ± 0.2 b | 0.7 ± 0.1 c | −4.4 ± 0.3 b | 3.5 ± 0.4 a | 2.95 ± 0.04 b |
| G/PVA/ZnONPs | 87.5 ± 0.2 b | 0.3 ± 0.1 b | −4.1 ± 0.3 b | 3.8 ± 0.5 a | 3.29 ± 0.04 c |
| G/PVA/BCCE/ZnONPs | 85.3 ± 0.3 a | −0.1 ± 0.1 a | −0.6 ± 0.1 c | 7.9 ± 0.3 b | 3.41 ± 0.07 d |
| Film Type | AC * ± SD (μmol Trolox (TE)/100 g) | ||
|---|---|---|---|
| QUENCHERDPPH | QUENCHERABTS | QUENCHERCUPRAC | |
| G/PVA | 70.12 ± 1.65 a | 207.08 ± 6.11 a | 482.57 ± 15.23 a |
| G/PVA/BCCE | 171.09 ± 4.60 d | 342.51 ± 5.20 b | 756.53 ± 1.55 c |
| G/PVA/ZnONPs | 100.50 ± 4.53 b | 366.49 ± 10.91 c | 518.97 ± 18.19 b |
| G/PVA/BCCE/ZnONPs | 116.15 ± 2.50 c | 391.84 ± 4.14 d | 733.88 ± 15.75 c |
| Film Type | Bacterial Strain * | |||||
|---|---|---|---|---|---|---|
| E. coli | K. pneumoniae | S. enterica | M. luteus | L. monocytogenes | S. aureus | |
| G/PVA | No ZOI | No ZOI | No ZOI | No ZOI | No ZOI | No ZOI |
| G/PVA/BCCE | No ZOI | No ZOI | No ZOI | No ZOI | No ZOI | No ZOI |
| G/PVA/ZnONPs | No ZOI | No ZOI | No ZOI | 19.3 ± 0.7 c | 13.2 ± 0.7 b | 8.3 ± 0.8 a |
| G/PVA/BCCE/ZnONPs | No ZOI | No ZOI | No ZOI | 18.0 ± 0.7 c | 9.9 ± 0.3 b | 6.7 ± 0.3 a |
| Film Type | Gelatin (c = 5% w/v) (mL) | PVA (c = 5% w/v) (mL) | Glycerol (mL) | BCCE (mL) | ZnONPs (g) | Water (mL) |
|---|---|---|---|---|---|---|
| G/PVA | 100 | 60 | 10 | - | - | 20 |
| G/PVA/BCCE | 100 | 60 | 10 | 10 | - | 10 |
| G/PVA/ZnONPs | 100 | 60 | 10 | - | 0.1 | 20 |
| G/PVA/BCCE/ZnONPs | 100 | 60 | 10 | 10 | 0.1 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tymczewska, A.; Furtado, B.U.; Nowaczyk, J.; Hrynkiewicz, K.; Szydłowska-Czerniak, A. Functional Properties of Gelatin/Polyvinyl Alcohol Films Containing Black Cumin Cake Extract and Zinc Oxide Nanoparticles Produced via Casting Technique. Int. J. Mol. Sci. 2022, 23, 2734. https://doi.org/10.3390/ijms23052734
Tymczewska A, Furtado BU, Nowaczyk J, Hrynkiewicz K, Szydłowska-Czerniak A. Functional Properties of Gelatin/Polyvinyl Alcohol Films Containing Black Cumin Cake Extract and Zinc Oxide Nanoparticles Produced via Casting Technique. International Journal of Molecular Sciences. 2022; 23(5):2734. https://doi.org/10.3390/ijms23052734
Chicago/Turabian StyleTymczewska, Alicja, Bliss Ursula Furtado, Jacek Nowaczyk, Katarzyna Hrynkiewicz, and Aleksandra Szydłowska-Czerniak. 2022. "Functional Properties of Gelatin/Polyvinyl Alcohol Films Containing Black Cumin Cake Extract and Zinc Oxide Nanoparticles Produced via Casting Technique" International Journal of Molecular Sciences 23, no. 5: 2734. https://doi.org/10.3390/ijms23052734
APA StyleTymczewska, A., Furtado, B. U., Nowaczyk, J., Hrynkiewicz, K., & Szydłowska-Czerniak, A. (2022). Functional Properties of Gelatin/Polyvinyl Alcohol Films Containing Black Cumin Cake Extract and Zinc Oxide Nanoparticles Produced via Casting Technique. International Journal of Molecular Sciences, 23(5), 2734. https://doi.org/10.3390/ijms23052734