Thermal Mapping of Self-Promoted Calcium Carbide Reactions for Performing Energy-Economic Processes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of the Tube-In-Tube Reactor
3D Printing of the Nylon Liner
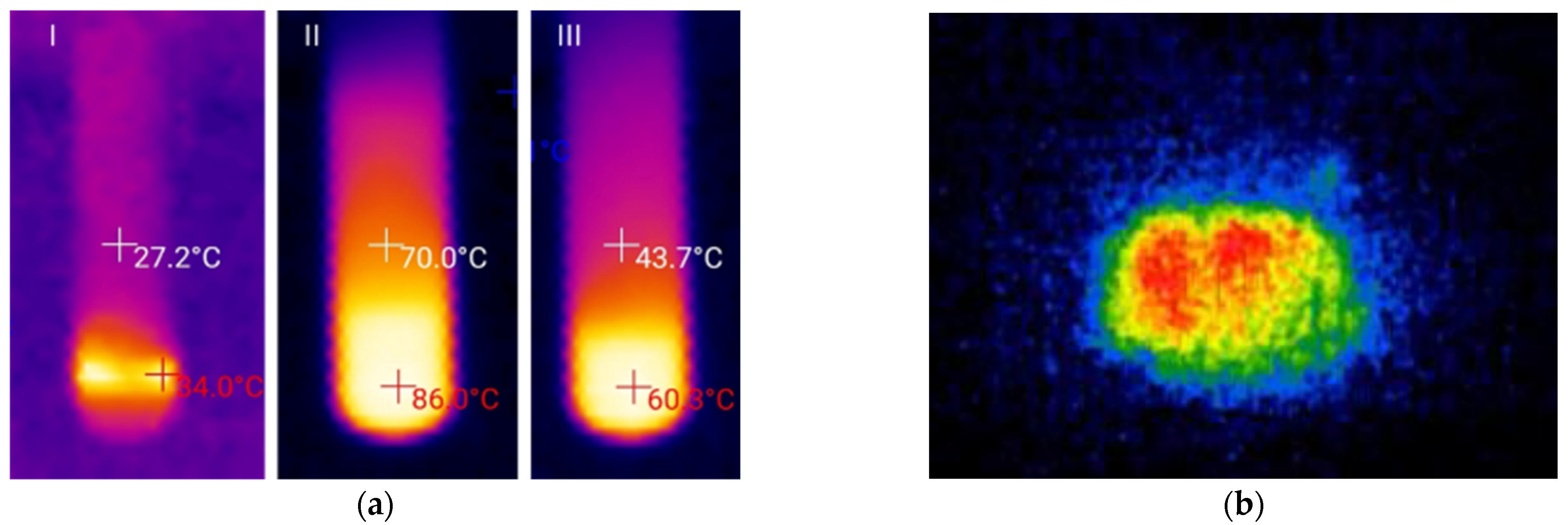
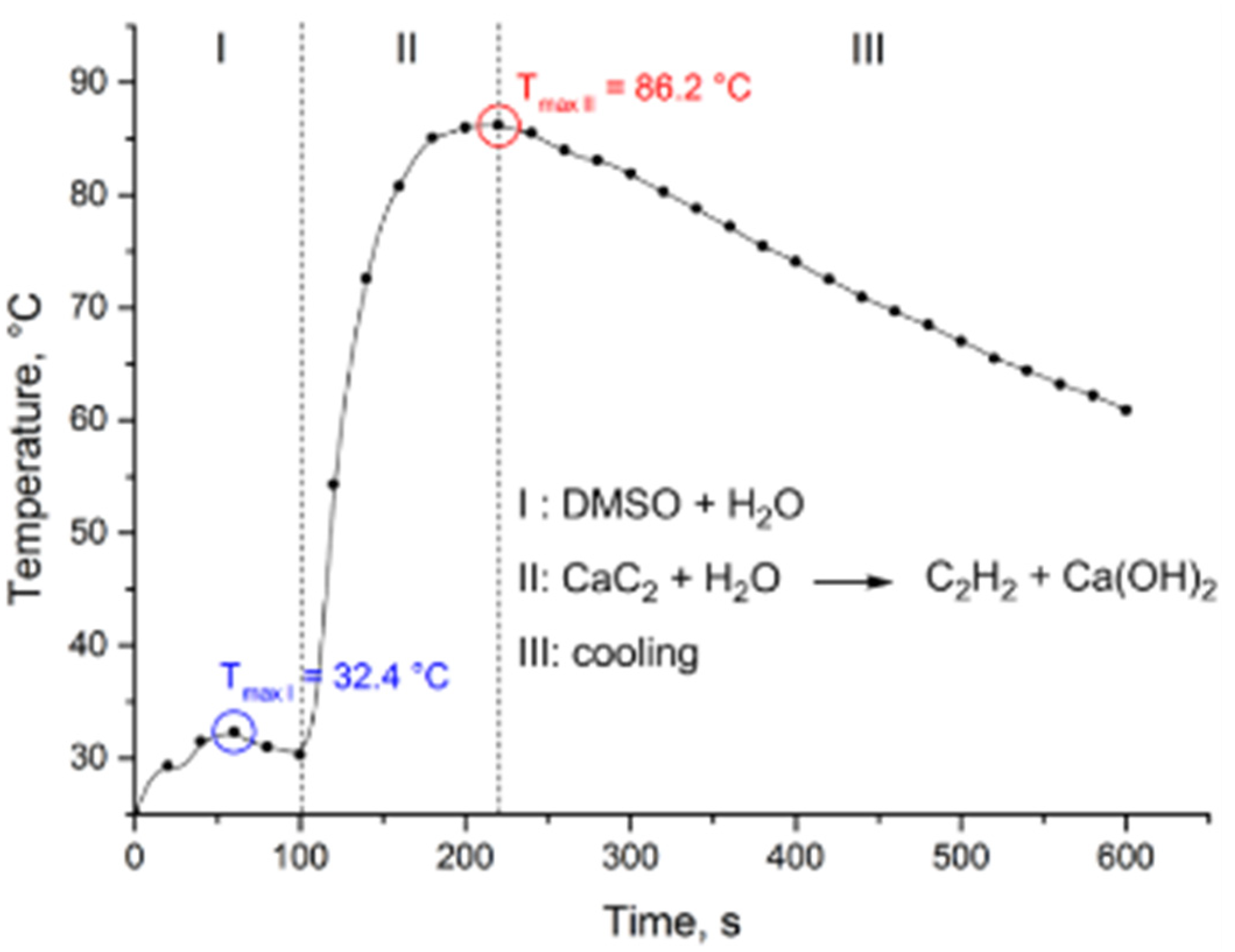
2.2. Thiovinylation Reaction Profile
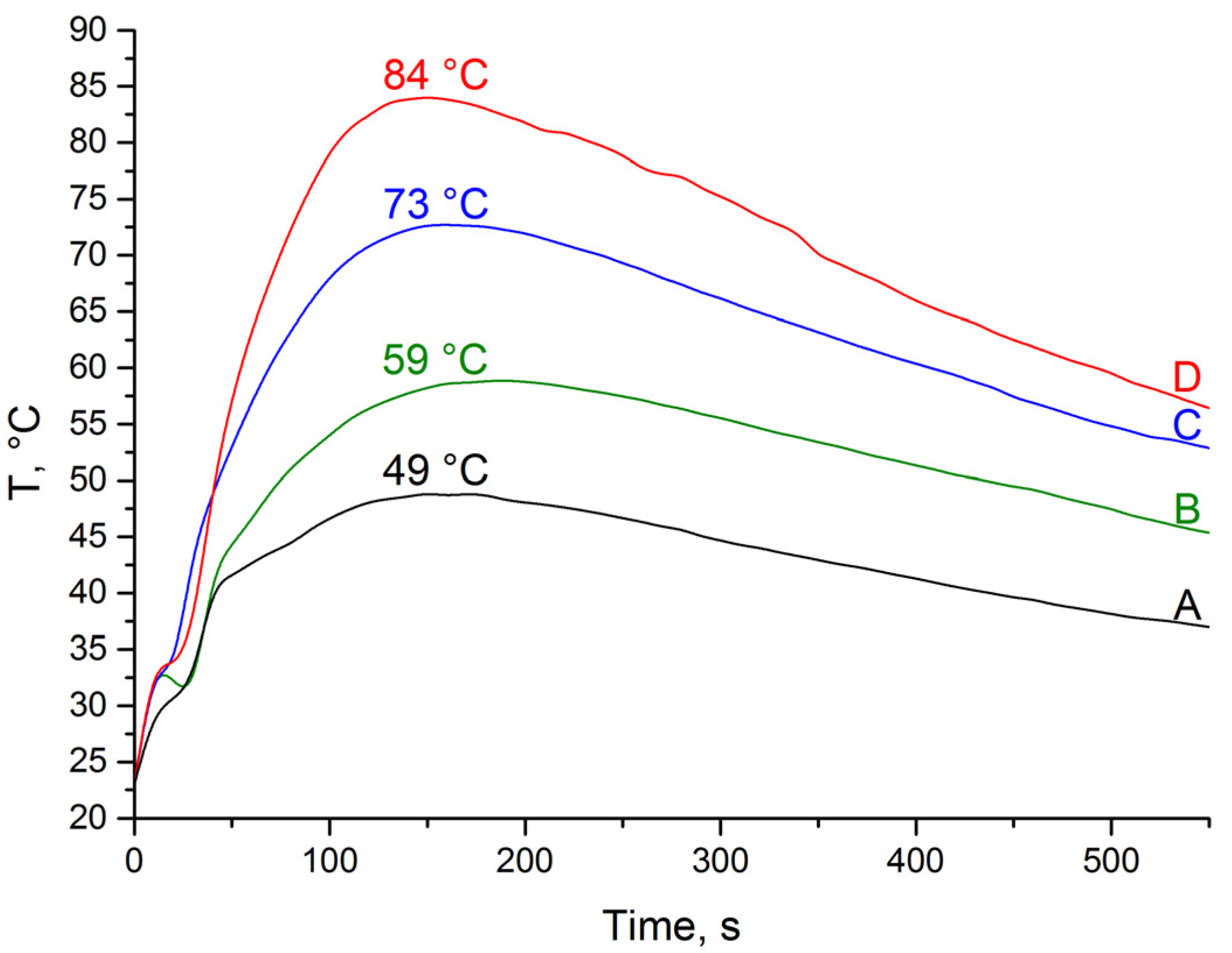
2.3. Optimization of the Heat Release Profile
2.4. Thiovinylation Reaction. Calcium Carbide as a Double Source of Acetylene and Heat
3. Materials and Methods
3.1. General Information
3.2. General Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oxtoby, D.W.; Gillis, H.P.; Campion, A. Principles of Modern Chemistry, 6th ed.; Thomson Brooks/Cole: Belmont, CA, USA, 2008. [Google Scholar]
- Bergman, T.L.; Lavine, A.S.; Incropera, F.P.; DeWitt, D.P. Fundamentals of Heat and Mass Transfer, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Brown, R.C. Process Intensification through Directly Coupled Autothermal Operation of Chemical Reactors. Joule 2020, 4, 2268–2289. [Google Scholar] [CrossRef]
- Haynes, W.M. Handbook of Chemistry and Physics, 95th ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2014–2015. [Google Scholar]
- Fu, R.; Li, Z.; Gao, L. Direct Synthesis of Organic Compounds Using Calcium Carbide as the Acetylene Source. Prog. Chem. 2019, 39, 1303–1313. (In Chinese) [Google Scholar] [CrossRef]
- Gao, L.; Li, Z. Direct Synthesis of 1-Arylprop-1-ynes with Calcium Carbide as an Acetylene Source. Synlett 2019, 30, 1580–1584. [Google Scholar] [CrossRef]
- Li, D.; Qiu, S.; Chen, Y.; Wu, L. K2CO3-Promoted Pyrazoles Synthesis from 1,3-Dipolar Cycloaddition of N-Tosylhydrazones with Acetylene Gas. ChemistrySelect 2020, 5, 12034–12037. [Google Scholar] [CrossRef]
- Teong, S.P.; Yu, D.; Sum, Y.N.; Zhang, Y. Copper catalysed alkynylation of tertiary amines with CaC2 via sp3 C-H activation. Green Chem. 2016, 18, 3499–3502. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Z.; Liu, Z.; Li, Z. One-Pot Three-Component Synthesis of 2-Methyl-3-aminobenzofurans Using Calcium Carbide as a Concise Solid Alkyne Source. Chin. J. Chem. 2021, 39, 2990–2994. [Google Scholar] [CrossRef]
- Ma, X.; Li, Z. Synthesis of Diarylethynes from Aryldiazonium Salts by Using Calcium Carbide as an Alkyne Source in a Deep Eutectic Solvent. Synlett 2021, 32, 631–635. [Google Scholar]
- Fu, R.; Lu, Y.; Yue, G.; Wu, D.; Xu, L.; Song, H.; Cao, C.; Yu, X.; Zong, Y. Direct Synthesis of 3-Coumaranones with Calcium Carbide as an Acetylene Source. Org. Lett. 2021, 23, 3141–3145. [Google Scholar] [CrossRef]
- Lu, H.; Li, Z. Synthesis of 1,2,3-Triazolyl-Based Ketoximes Using Calcium Carbide as an Acetylene Source. Eur. J. Org. Chem. 2020, 7, 845–851. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Samoylenko, D.E.; Seitkalieva, M.M.; Lotsman, K.A.; Metlyaeva, S.A.; Ananikov, V.P. Generation, regeneration, and recovery of Cu catalytic system by changing the polarity of electrodes. Green Chem. 2022, 24, 1132–1140. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Bogachenkov, A.S.; Ananikov, V.P. Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials. Molecules 2018, 23, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voronin, V.V.; Ledovskaya, M.S.; Rodygin, K.S.; Ananikov, V.P. Examining the vinyl moiety as a protecting group for hydroxyl (-OH) functionality under basic conditions. Org. Chem. Front. 2020, 7, 1334–1342. [Google Scholar] [CrossRef]
- Kirillov, E.; Rodygin, K.; Ananikov, V. Recent advances in applications of vinyl ether monomers for precise synthesis of custom-tailored polymers. Eur. Polym. J. 2020, 136, 109872. [Google Scholar] [CrossRef]
- Metlyaeva, S.A.; Rodygin, K.S.; Lotsman, K.A.; Samoylenko, D.E.; Ananikov, V.P. Biomass- and calcium carbide-based recyclable polymers. Green Chem. 2021, 23, 2487–2495. [Google Scholar] [CrossRef]
- Li, A.; Song, H.; Xu, X.; Meng, H.; Lu, Y.; Li, C. Greener production process of acetylene and calcium diglyceroxide via mechanochemical reaction of CaC2 and glycerol. ACS Sustain. Chem. Eng. 2018, 6, 9560–9565. [Google Scholar] [CrossRef]
- Liu, Q.-N.; Cheng, L.; Xu, X.-B.; Meng, H.; Lu, Y.-Z.; Li, C.-X. Greatly enhanced reactivity of CaC 2 with perchloro- hydrocarbons in a stirring ball mill for the manufacture of alkynyl carbon materials. Chem. Eng. Process. 2018, 124, 261–268. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Bolm, C.; Hernandez, J.G. Mechanosynthesis of Odd-Numbered Tetraaryl[n]cumulenes. Angew. Chem. Int. Ed. 2019, 58, 12945–12949. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Y.; He, X.; Gu, J.; Yu, M.; Li, W.; Li, C. Efficient synthesis of alkynyl carbon materials derived from CaC2 through solvent-free mechanochemical strategy for supercapacitors. SN Appl. Sci. 2019, 1, 195. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Lu, Y.; Meng, H.; Li, C. Structure and adsorptive property of carbon materials derived from thermal and mechanochemical reaction of CaC2 and chlorinated polymers. Chem. Eng. J. 2019, 372, 181–190. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Ledovskaya, M.S.; Voronin, V.V.; Lotsman, K.A.; Ananikov, V.P. Calcium Carbide: Versatile Synthetic Applications, Green Methodology and Sustainability. Eur. J. Org. Chem. 2021, 2021, 43–52. [Google Scholar] [CrossRef]
- Lebedev, A.N.; Rodygin, K.S.; Mironenko, R.M.; Saybulina, E.R.; Ananikov, V.P. Metal-Catalyzed Chemical Activation of Calcium Carbide: New Way to Hierarchical Metal/Alloy-on-Carbon Catalysts. J. Catal. 2022, 407, 281–289. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Lotsman, K.A.; Ananikov, V.P. Calcium Carbide Looping System for Acetaldehyde Manufacturing from Virtually any Carbon Source. ChemSusChem 2020, 13, 3679–3685. [Google Scholar] [CrossRef] [PubMed]
- Rodygin, K.S.; Vikenteva, Y.A.; Ananikov, V.P. Calcium-Based Sustainable Chemical Technologies for Total Carbon Recycling. ChemSusChem 2019, 12, 1483–1516. [Google Scholar] [CrossRef] [PubMed]
- Erokhin, K.S.; Gordeev, E.G.; Samoylenko, D.E.; Rodygin, K.S.; Ananikov, V.P. 3D Printing to Increase the Flexibility of the Chemical Synthesis of Biologically Active Molecules: Design of On-Demand Gas Generation Reactors. Int. J. Mol. Sci. 2021, 22, 9919. [Google Scholar] [CrossRef] [PubMed]
- Zastrow, M. 3D printing gets bigger, faster and stronger. Nature 2020, 578, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Silver, A. Five innovative ways to use 3D printing in the laboratory. Nature 2019, 565, 123–124. [Google Scholar] [CrossRef]
- Capel, A.J.; Rimington, R.P.; Lewis, M.P.; Christie, S.D.R. 3D printing for chemical, pharmaceutical and biological applications. Nat. Rev. Chem. 2018, 2, 422–436. [Google Scholar] [CrossRef]
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Polynski, M.V.; Sapova, M.D.; Ananikov, V.P. Understanding the solubilization of Ca acetylide with a new computational model for ionic pairs. Chem. Sci. 2020, 11, 13102–13112. [Google Scholar] [CrossRef]
- Friis, S.D.; Lindhardt, A.T.; Skrydstrup, T. The Development and Application of Two-Chamber Reactors and Carbon Monoxide Precursors for Safe Carbonylation Reactions. Acc. Chem. Res. 2016, 49, 594–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verschueren, R.H.; Gilles, P.; Van Mileghem, S.; De Borggraeve, W.M. Solvent-free N-Boc deprotection by ex situ generation of hydrogen chloride gas. Org. Biomol. Chem. 2021, 19, 5782–5787. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Dong, C.; Li, D.; Wang, G.H. Palladium-based bimetallic catalysts for highly selective semihydrogenation of alkynes using ex situ generated hydrogen. Mater. Today Chem. 2021, 20, 100467. [Google Scholar] [CrossRef]
- Keating, J.J.; Alam, R.M. An Expedient Approach to Pyrazolo[3,4-b]pyridine-3-carboxamides via Palladium-Catalyzed Aminocarbonylation. Synthesis 2021, 53, 4709–4722. [Google Scholar] [CrossRef]
- Danilenko, N.; Shmalyuk, V.; Khlebnikov, A. 2-(2-(Fluorosulfonyloxy)phenyl)benzoxazole. Molbank 2021, 2021, M1242. [Google Scholar] [CrossRef]
- Pedersen, S.K.; Gudmundsson, H.G.; Nielsen, D.U.; Donslund, B.S.; Hammershøj, H.C.D.; Daasbjerg, K.; Skrydstrup, T. Main element chemistry enables gas-cylinder-free hydroformylations. Nat. Catal. 2020, 3, 843–850. [Google Scholar] [CrossRef]
- Dong, C.; Yu, Q.; Ye, R.P.; Su, P.; Liu, J.; Wang, G.H. Hollow Carbon Sphere Nanoreactors Loaded with PdCu Nanoparticles: Void-Confinement Effects in Liquid-Phase Hydrogenations. Angew. Chem. Int. Ed. 2020, 59, 18374–18379. [Google Scholar] [CrossRef]
- Veryser, C.; Demaerel, J.; Bieliu Nas, V.; Gilles, P.; De Borggraeve, W.M. Ex Situ Generation of Sulfuryl Fluoride for the Synthesis of Aryl Fluorosulfates. Org. Lett. 2017, 19, 5244–5247. [Google Scholar] [CrossRef]
- Kristensen, S.K.; Eikeland, E.Z.; Taarning, E.; Lindhardt, A.T.; Skrydstrup, T. Ex situ generation of stoichiometric HCN and its application in the Pd-catalysed cyanation of aryl bromides: Evidence for a transmetallation step between two oxidative addition Pd-complexes. Chem. Sci. 2017, 8, 8094–8105. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, D.U.; Lescot, C.; Gogsig, T.M.; Lindhardt, A.T.; Skrydstrup, T. Pd-catalyzed carbonylative alpha-arylation of aryl bromides: Scope and mechanistic studies. Chemistry 2013, 19, 17926–17938. [Google Scholar] [CrossRef]
- Min, G.K.; Bjerglund, K.; Kramer, S.; Gogsig, T.M.; Lindhardt, A.T.; Skrydstrup, T. Generation of stoichiometric ethylene and isotopic derivatives and application in transition-metal-catalyzed vinylation and enyne metathesis. Chemistry 2013, 19, 17603–17607. [Google Scholar] [CrossRef] [PubMed]
- Korsager, S.; Taaning, R.H.; Lindhardt, A.T.; Skrydstrup, T. Reductive carbonylation of aryl halides employing a two-chamber reactor: A protocol for the synthesis of aryl aldehydes including 13C- and D-isotope labeling. J. Org. Chem. 2013, 78, 6112–6120. [Google Scholar] [CrossRef] [PubMed]
- Brancour, C.; Fukuyama, T.; Mukai, Y.; Skrydstrup, T.; Ryu, I. Modernized Low Pressure Carbonylation Methods in Batch and Flow Employing Common Acids as a CO Source. Org. Lett. 2013, 15, 2794–2797. [Google Scholar] [CrossRef] [PubMed]
- Demaerel, J.; Veryser, C.; De Borggraeve, W.M. Ex situ gas generation for lab scale organic synthesis. React. Chem. Eng. 2020, 5, 615–631. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodygin, K.S.; Lotsman, K.A.; Erokhin, K.S.; Korabelnikova, V.A.; Ananikov, V.P. Thermal Mapping of Self-Promoted Calcium Carbide Reactions for Performing Energy-Economic Processes. Int. J. Mol. Sci. 2022, 23, 2763. https://doi.org/10.3390/ijms23052763
Rodygin KS, Lotsman KA, Erokhin KS, Korabelnikova VA, Ananikov VP. Thermal Mapping of Self-Promoted Calcium Carbide Reactions for Performing Energy-Economic Processes. International Journal of Molecular Sciences. 2022; 23(5):2763. https://doi.org/10.3390/ijms23052763
Chicago/Turabian StyleRodygin, Konstantin S., Kristina A. Lotsman, Kirill S. Erokhin, Viktoria A. Korabelnikova, and Valentine P. Ananikov. 2022. "Thermal Mapping of Self-Promoted Calcium Carbide Reactions for Performing Energy-Economic Processes" International Journal of Molecular Sciences 23, no. 5: 2763. https://doi.org/10.3390/ijms23052763
APA StyleRodygin, K. S., Lotsman, K. A., Erokhin, K. S., Korabelnikova, V. A., & Ananikov, V. P. (2022). Thermal Mapping of Self-Promoted Calcium Carbide Reactions for Performing Energy-Economic Processes. International Journal of Molecular Sciences, 23(5), 2763. https://doi.org/10.3390/ijms23052763






