Identification of Kukoamine A, Zeaxanthin, and Clexane as New Furin Inhibitors
Abstract
:1. Introduction
2. Results
2.1. Virtual Screening
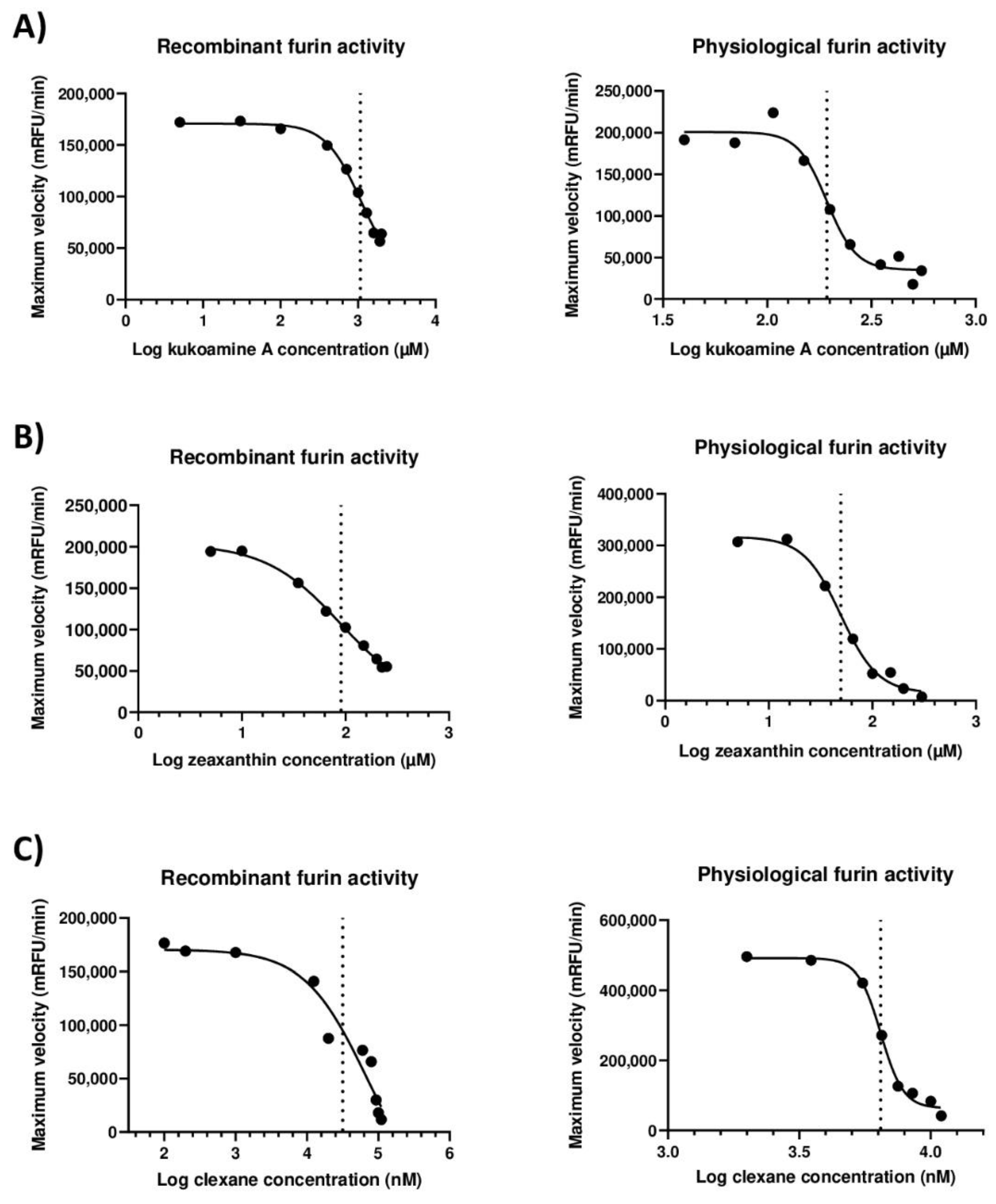
2.2. Inhibitors of Furin
2.3. Adjuvant Effect of Selected Compounds in the Inhibition of Furin
2.4. Cell Viability in Presence of Zeaxanthin and Kukoamine A
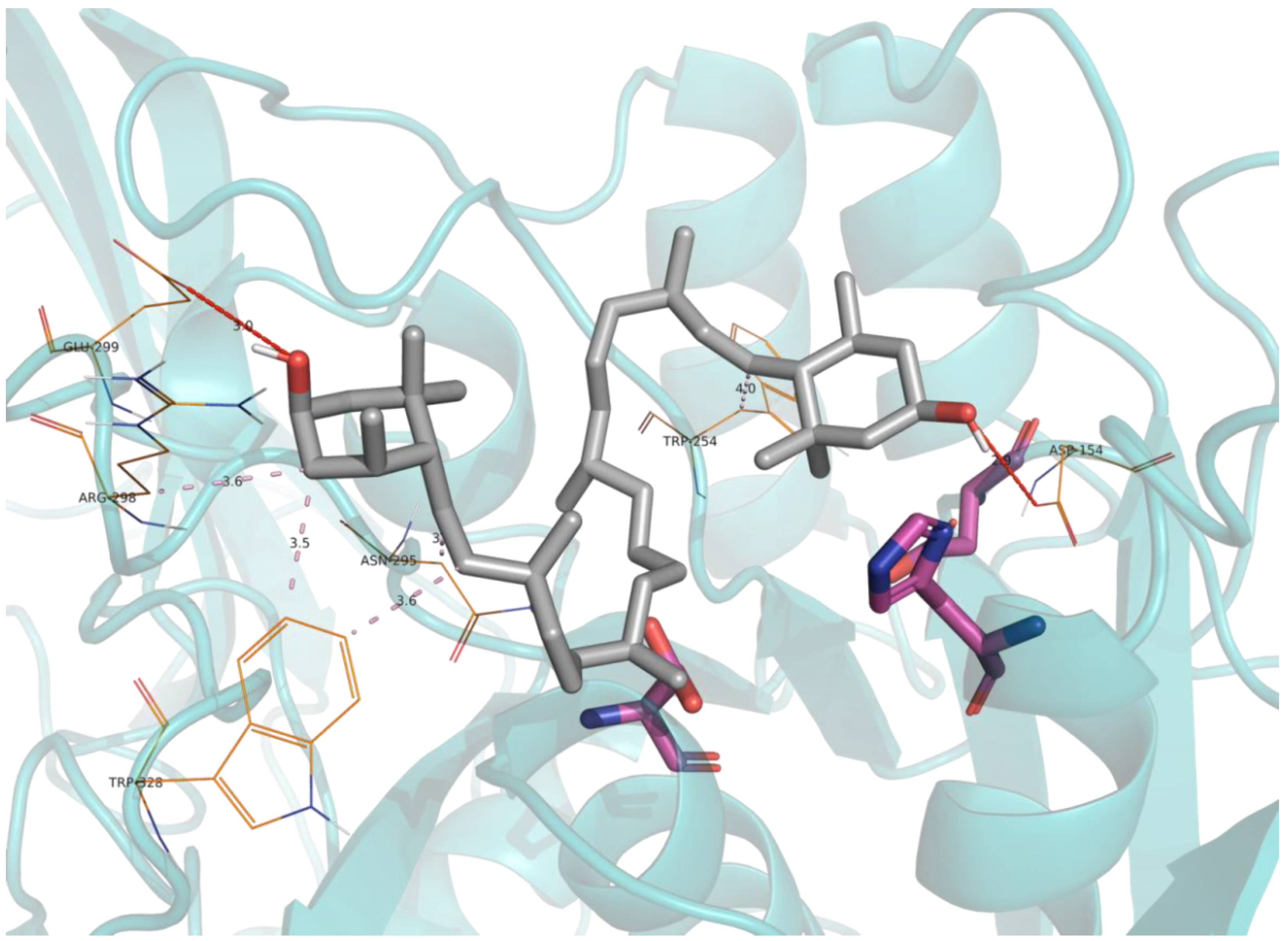
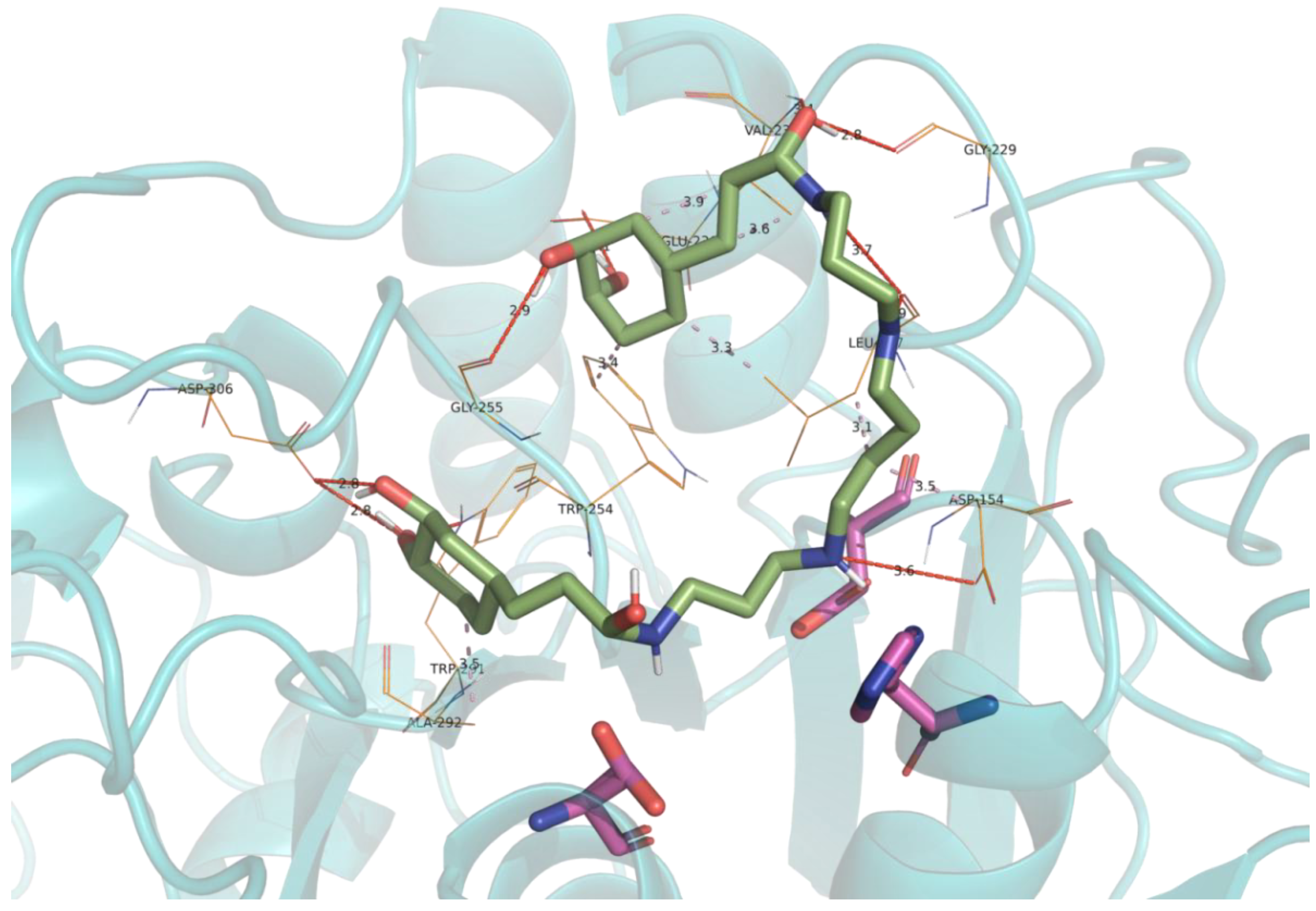
2.5. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Virtual Screening
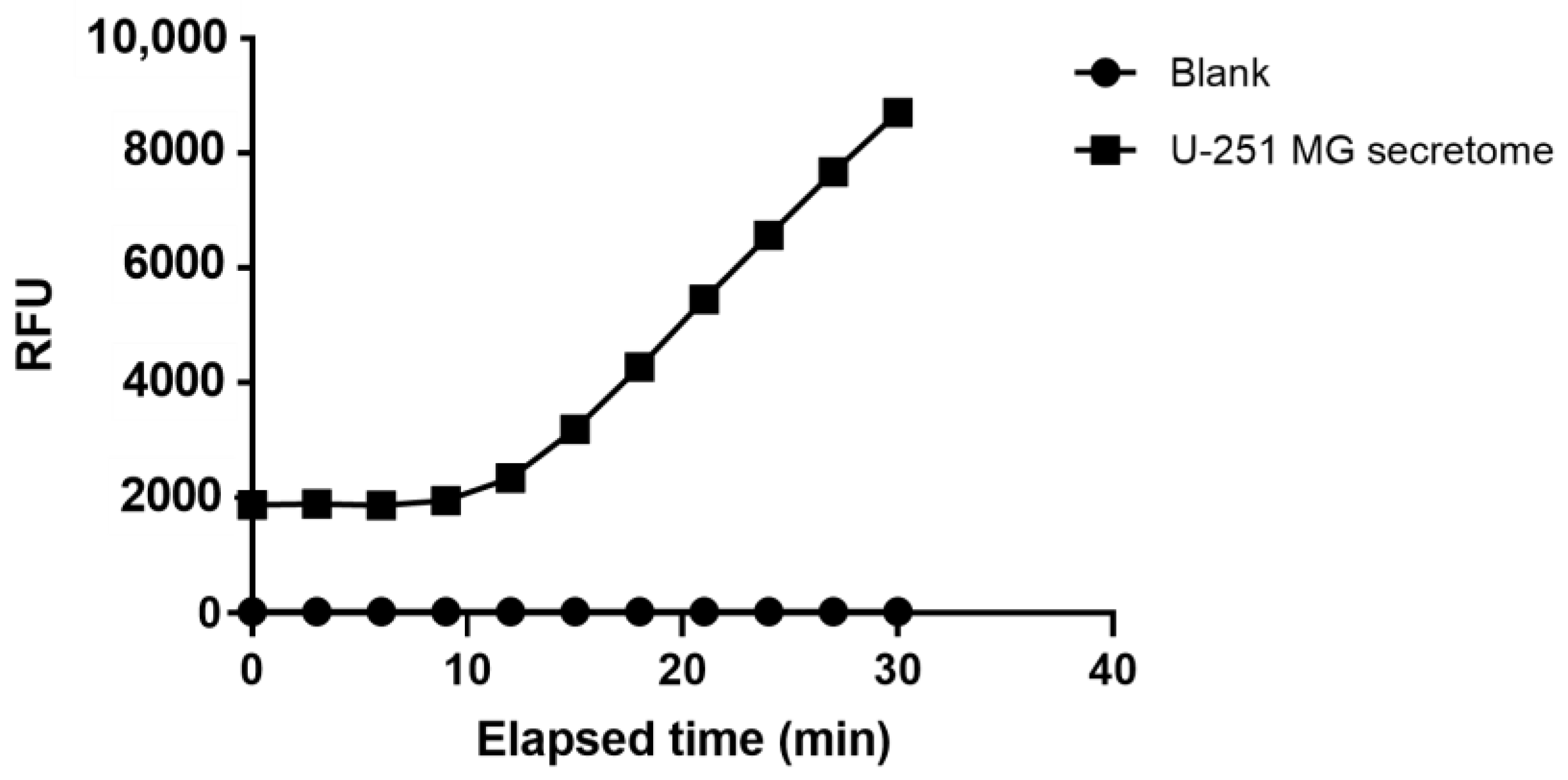
4.2. Secretome Collection from U-251 MG Cells
4.3. Furin Inhibition Assay
4.4. IC50 Calculation
4.5. Furin Inhibition Assay in Coadjuvants
4.6. Cell Viability in Presence of Selected Furin Inhibitors
4.7. Molecular Docking
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Braun, E.; Sauter, D. Furin-mediated protein processing in infectious diseases and cancer. Clin. Transl. Immunol. 2019, 8, e1073. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zheng, M.; Yang, Y.; Gu, X.; Yang, K.; Li, M.; Liu, Y.; Zhang, Q.; Zhang, P.; Wang, Y.; et al. Furin: A Potential Therapeutic Target for COVID-19. iScience 2020, 23, 101642. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Huang, Q.; Fang, Y.; Wu, J. FurinDB: A database of 20-residue furin cleavage site motifs, substrates and their associated drugs. Int. J. Mol. Sci. 2011, 12, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Louagie, E.; Taylor, N.A.; Flamez, D.; Roebroek, A.J.M.; Bright, N.A.; Meulemans, S.; Quintens, R.; Herrera, P.L.; Schuit, F.; Van de Ven, W.J.M.; et al. Role of furin in granular acidification in the endocrine pancreas: Identification of the V-ATPase subunit Ac45 as a candidate substrate. Proc. Natl. Acad. Sci. USA 2008, 105, 12319–12324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Wang, Q.; Gao, Z.; Zhou, Z.; Peng, S.; Chang, W.L.; Lin, H.Y.; Zhang, W.; Wang, H. Proprotein convertase furin regulates apoptosis and proliferation of granulosa cells in the rat ovary. PLoS ONE 2013, 8, e50479. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Shen, T.; Zhang, B.H.; Lv, X.Y.; Lin, H.Y.; Zhu, C.; Xue, L.Q.; Wang, H. The proprotein convertase furin in human trophoblast: Possible role in promoting trophoblast cell migration and invasion. Placenta 2009, 30, 929–938. [Google Scholar] [CrossRef]
- Jaaks, P.; Bernasconi, M. The proprotein convertase furin in tumour progression: The PC furin in tumour progression. Int. J. Cancer 2017, 141, 654–663. [Google Scholar] [CrossRef] [Green Version]
- Bassi, D.E.; Mahloogi, H.; Al-Saleem, L.; Lopez De Cicco, R.; Ridge, J.A.; Klein-Szanto, A.J. Elevated furin expression in aggressive human head and neck tumors and tumor cell lines. Mol. Carcinog. 2001, 31, 224–232. [Google Scholar] [CrossRef]
- Bassi, D.E.; Lopez De Cicco, R.; Mahloogi, H.; Zucker, S.; Thomas, G.; Klein-Szanto, A.J. Furin inhibition results in absent or decreased invasiveness and tumorigenicity of human cancer cells. Proc. Natl. Acad. Sci. USA 2001, 98, 10326–10331. [Google Scholar] [CrossRef] [Green Version]
- Bassi, D.E.; Mahloogi, H.; Lopez De Cicco, R.; Klein-Szanto, A. Increased furin activity enhances the malignant phenotype of human head and neck cancer cells. Am. J. Pathol. 2003, 162, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Lopez de Cicco, R.; Bassi, D.E.; Zucker, S.; Seidah, N.G.; Klein-Szanto, A.J.P. Human carcinoma cell growth and invasiveness is impaired by the propeptide of the ubiquitous proprotein convertase furin. Cancer Res. 2005, 65, 4162–4171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatib, A.M.; Siegfried, G.; Prat, A.; Luis, J.; Chrétien, M.; Metrakos, P.; Seidah, N.G. Inhibition of proprotein convertases is associated with loss of growth and tumorigenicity of HT-29 human colon carcinoma cells: Importance of insulin-like growth factor-1 (IGF-1) receptor processing in IGF-1-mediated functions. J. Biol. Chem. 2001, 276, 30686–30693. [Google Scholar] [CrossRef] [Green Version]
- Scamuffa, N.; Siegfried, G.; Bontemps, Y.; Ma, L.; Basak, A.; Cherel, G.; Calvo, F.; Seidah, N.G.; Khatib, A.M. Selective inhibition of proprotein convertases represses the metastatic potential of human colorectal tumor cells. J. Clin. Investig. 2008, 118, 352–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, V.M.; Leppla, S.H. Proteolytic activation of bacterial toxins: Role of bacterial and host cell proteases. Infect. Immun. 1994, 62, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Tsuneoka, M.; Nakayama, K.; Hatsuzawa, K.; Komada, M.; Kitamura, N.; Mekada, E. Evidence for involvement of furin in cleavage and activation of diphtheria toxin. J. Biol. Chem. 1993, 268, 26461–26465. [Google Scholar] [CrossRef]
- Ogata, M.; Fryling, C.M.; Pastan, I.; FitzGerald, D.J. Cell-mediated cleavage of Pseudomonas exotoxin between Arg279 and Gly280 generates the enzymatically active fragment which translocates to the cytosol. J. Biol. Chem. 1992, 267, 25396–25401. [Google Scholar] [CrossRef]
- Spaete, R.R.; Thayer, R.M.; Probert, W.S.; Masiarz, F.R.; Chamberlain, S.H.; Rasmussen, L.; Merigan, T.C.; Pachl, C. Human cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology 1988, 167, 207–225. [Google Scholar] [CrossRef]
- Cavanagh, D.; Davis, P.J.; Pappin, D.J.; Binns, M.M.; Boursnell, M.E.; Brown, T.D. Coronavirus IBV: Partial amino terminal sequencing of spike polypeptide S2 identifies the sequence Arg-Arg-Phe-Arg-Arg at the cleavage site of the spike precursor propolypeptide of IBV strains Beaudette and M41. Virus Res. 1986, 4, 133–143. [Google Scholar] [CrossRef]
- Gonzalez-Dunia, D.; Cubitt, B.; de la Torre, J.C. Mechanism of Borna disease virus entry into cells. J. Virol. 1998, 72, 783–788. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Li, D.; Tong, W.; Shi, T.; Ning, B. Biochemical features and mutations of key proteins in SARS-CoV-2 and their impacts on RNA therapeutics. Biochem. Pharmacol. 2021, 189, 114424. [Google Scholar] [CrossRef]
- Lal, A.; Erondu, N.A.; Heymann, D.L.; Gitahi, G.; Yates, R. Fragmented health systems in COVID-19: Rectifying the misalignment between global health security and universal health coverage. Lancet 2021, 397, 61–67. [Google Scholar] [CrossRef]
- Rishi, P.; Thakur, K.; Vij, S.; Rishi, L.; Singh, A.; Kaur, I.P.; Patel, S.K.S.; Lee, J.K.; Kalia, V.C. Diet, Gut Microbiota and COVID-19. Indian J. Microbiol. 2020, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Lee, J.K.; Kalia, V.C. Deploying Biomolecules as Anti-COVID-19 Agents. Indian J. Microbiol. 2020, 60, 263–268. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Weekly Epidemiological Update on COVID-19—25 January 2022. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---25-january-2022 (accessed on 5 February 2022).
- Thakur, V.; Bhola, S.; Thakur, P.; Patel, S.K.S.; Kulshrestha, S.; Ratho, R.K.; Kumar, P. Waves and variants of SARS-CoV-2: Understanding the causes and effect of the COVID-19 catastrophe. Infection 2021, 16, 1–16. [Google Scholar] [CrossRef]
- Belen-Apak, F.B.; Sarialioglu, F. The old but new: Can unfractioned heparin and low molecular weight heparins inhibit proteolytic activation and cellular internalization of SARS-CoV2 by inhibition of host cell proteases? Med. Hypotheses 2020, 142, 109743. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chao, T.L.; Li, C.L.; Chiu, M.F.; Kao, H.C.; Wang, S.H.; Pang, Y.H.; Lin, C.H.; Tsai, Y.M.; Lee, W.H.; et al. Furin Inhibitors Block SARS-CoV-2 Spike Protein Cleavage to Suppress Virus Production and Cytopathic Effects. Cell Rep. 2020, 33, 108254. [Google Scholar] [CrossRef]
- Jean, F.; Stella, K.; Thomas, L.; Liu, G.; Xiang, Y.; Reason, A.J.; Thomas, G. Alpha1-Antitrypsin Portland, a bioengineered serpin highly selective for furin: Application as an antipathogenic agent. Proc. Natl. Acad. Sci. USA 1998, 95, 7293–7298. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J. Heparin inhibits activation of latent transforming growth factor-β1. Pharmacology 2013, 92, 238–244. [Google Scholar] [CrossRef]
- Hills, F.A.; Abrahams, V.M.; González-Timón, B.; Francis, J.; Cloke, B.; Hinkson, L.; Rai, R.; Mor, G.; Regan, L.; Sullivan, M.; et al. Heparin prevents programmed cell death in human trophoblast. Mol. Hum. Reprod. 2006, 12, 237–243. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Khatib, A.M.; Creemers, J.W.M. The proprotein convertase furin in cancer: More than an oncogene. Oncogene 2022. [Google Scholar] [CrossRef]
- Volchkov, V.E.; Feldmann, H.; Volchkova, V.A.; Klenk, H.D. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc. Natl. Acad. Sci. USA 1998, 95, 5762–5767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaoka, Y.; Nestorowicz, A.; Alexander, D.J.; Webster, R.G. Molecular analyses of the hemagglutinin genes of H5 influenza viruses: Origin of a virulent turkey strain. Virology 1987, 158, 218–227. [Google Scholar] [CrossRef]
- Aerts, L.; Hamelin, M.È.; Rhéaume, C.; Lavigne, S.; Couture, C.; Kim, W.; Susan-Resiga, D.; Prat, A.; Seidah, N.G.; Vergnolle, N.; et al. Modulation of protease activated receptor 1 influences human metapneumovirus disease severity in a mouse model. PLoS ONE 2013, 8, e72529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, M.; Munzer, J.S.; Basak, A.; Benjannet, S.; Mowla, S.J.; Decroly, E.; Chrétien, M.; Seidah, N.G. The prosegments of furin and PC7 as potent inhibitors of proprotein convertases. In vitro and ex vivo assessment of their efficacy and selectivity. J. Biol. Chem. 1999, 274, 33913–33920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapierre, M.; Siegfried, G.; Scamuffa, N.; Bontemps, Y.; Calvo, F.; Seidah, N.G.; Khatib, A.M. Opposing function of the proprotein convertases furin and PACE4 on breast cancer cells´malignant phenotypes: Role of tissue inhibitors of metalloproteinase-1. Cancer Res. 2007, 67, 9030–9034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. Vigil + Irinotecan and Temozolomide in Ewing’s Sarcoma (VITA). Available online: https://clinicaltrials.gov/ct2/show/NCT03495921 (accessed on 10 January 2022).
- Karicherla, P.; Hobden, J.A. Nona-D-arginine therapy for Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2009, 50, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2016, 174, 1290–1324. [Google Scholar] [CrossRef] [Green Version]
- Tang, A.M.; Smit, E.; Semba, R.D.; Shah, N.; Lyles, C.M.; Li, D.; Vlahov, D. Improved antioxidant status among HIV-infected injecting drug users on potent antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2000, 23, 321–326. [Google Scholar] [CrossRef]
- Arnold, C.; Jentsch, S.; Dawczynski, J.; Böhm, V. Age-related macular degeneration: Effects of a short-term intervention with an oleaginous kale extract--a pilot study. Nutrition 2013, 29, 1412–1417. [Google Scholar] [CrossRef]
- Arnold, C.; Winter, L.; Fröhlich, K.; Jentsch, S.; Dawczynski, J.; Jahreis, G.; Böhm, V. Macular xanthophylls and ω-3 long-chain polyunsaturated fatty acids in age-related macular degeneration: A randomized trial. JAMA Ophthalmol. 2013, 131, 564–572. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Sun, Z.; Dong, L.; Gao, L.; Liu, C.; Wang, X. Kukoamine A inhibits human glioblastoma cell growth and migration through apoptosis induction and epithelial-mesenchymal transition attenuation. Sci. Rep. 2016, 6, 36543. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, L.; Niu, Y.; Li, X.; Liu, W.; Jiang, X.; Liu, Y.; Zhao, Q. Kukoamine A Protects against NMDA-Induced Neurotoxicity Accompanied with Down-Regulation of GluN2B-Containing NMDA Receptors and Phosphorylation of PI3K/Akt/GSK-3β Signaling Pathway in Cultured Primary Cortical Neurons. Neurochem. Res. 2020, 45, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Z.; Wang, C.; Ma, H.; Meng, W.; Zhao, Q. Neuroprotective Effects of Kukoamine a against Radiation-induced Rat Brain Injury through Inhibition of Oxidative Stress and Neuronal Apoptosis. Neurochem. Res. 2016, 41, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, F.; Chen, Y.; Zhang, W.; Wang, N. Kukoamine A attenuates insulin resistance and fatty liver through downregulation of Srebp-1c. Biomed. Pharmacother. 2017, 89, 536–543. [Google Scholar] [CrossRef]
- Nadi, S.; Vreugdenburg, T.D.; Atukorale, Y.; Ma, N.; Maddern, G.; Rovers, M. Safety and effectiveness of aspirin and enoxaparin for venous thromboembolism prophylaxis after total hip and knee arthroplasty: A systematic review. ANZ J. Surg. 2019, 89, 1204–1210. [Google Scholar] [CrossRef]
- Wijting, I.E.A.; Lungu, C.; Rijnders, B.J.A.; van der Ende, M.E.; Pham, H.T.; Mesplede, T.; Pas, S.D.; Voermans, J.J.C.; Schuurman, R.; van de Vijver, D.A.M.C.; et al. HIV-1 Resistance Dynamics in Patients with Virologic Failure to Dolutegravir Maintenance Monotherapy. J. Infect. Dis. 2018, 218, 688–697. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.W.S.; Wong, G.L.H.; Tse, C.H.; Yuen, L.K.W.; Chan, H.Y.; Locarnini, S.A.; Chan, H.L.Y. Antiviral drug resistance testing in patients with chronic hepatitis B. Dig. Dis. Sci. 2012, 57, 221–231. [Google Scholar] [CrossRef]
- Wallis, C.L.; Aga, E.; Ribaudo, H.; Saravanan, S.; Norton, M.; Stevens, W.; Kumarasamy, N.; Bartlett, J.; Katzenstein, D.; A5230 team. Drug susceptibility and resistance mutations after first-line failure in resource limited settings. Clin. Infect. Dis. 2014, 59, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Stat Pearls [Internet]. Low Molecular Weight Heparin (LMWH). Available online: https://www.ncbi.nlm.nih.gov/books/NBK525957/ (accessed on 10 January 2022).
- Kramer, C.; Kalliokoski, T.; Gedeck, P.; Vulpetti, A. The Experimental Uncertainty of Heterogeneous Public Ki Data. J. Med. Chem. 2012, 55, 5165–5173. [Google Scholar] [CrossRef]
- Fourches, D.; Muratov, E.; Tropsha, A. Trust, but Verify: On the Importance of Chemical Structure Curation in Cheminformatics and QSAR Modeling Research. J. Chem. Inf. Model. 2010, 50, 1189–1204. [Google Scholar] [CrossRef]
- Fourches, D.; Muratov, E.; Tropsha, A. Curation of chemogenomics data. Nat. Chem. Biol. 2015, 11, 535. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 1–1257. [Google Scholar]
- Castillo González, D.; Mergny, J.L.; De Rache, A.; Pérez-Machado, G.; Cabrera-Pérez, M.A.; Nicolotti, O.; Introcaso, A.; Mangiatordi, G.F.; Guédin, A.; Bourdoncle, A.; et al. Harmonization of QSAR Best Practices and Molecular Docking Provides an Efficient Virtual Screening Tool for Discovering New G-Quadruplex Ligands. J. Chem. Inf. Model. 2015, 55, 2094–2110. [Google Scholar] [CrossRef] [PubMed]
- Melagraki, G.; Afantitis, A.; Sarimveis, H.; Igglessi Markopoulou, O.; Koutentis, P.A.; Kollias, G. In Silico Exploration for Identifying Structure-Activity Relationship of MEK Inhibition and Oral Bioavailability for Isothiazole Derivatives. Chem. Biol. Drug Des. 2010, 76, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Afantitis, A.; Melagraki, G.; Koutentis, P.A.; Sarimveis, H.; Kollias, G. Ligand-based virtual screening procedure for the prediction and the identification of novel β -amyloid aggregation inhibitors using Kohonen Maps and Counterpropagation Artificial Neural Networks. Eur. J. Med. Chem. 2011, 46, 497–508. [Google Scholar] [CrossRef]
- Golbraikh, A.; Muratov, E.; Fourches, D.; Tropsha, A. Data Set Modelability by QSAR. J. Chem. Inf. Model. 2014, 54, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Garrido, A.; Morales Helguera, A.; Borges, F.; Cordeiro, M.N.D.S.; Rivero, V.; Garrido Escudero, A. Two new parameters based on distances in a receiver operating characteristic chart for the selection of classification models. J. Chem. Inf. Model. 2011, 51, 2746–2759. [Google Scholar] [CrossRef]
- Todeschini, R.; Ballabio, D.; Consonni, V.; Manganaro, A.; Mauri, A. Canonical measure of correlation (CMC) and canonical measure of distance (CMD) between sets of data. Part 1. Theory and simple chemometric applications. Anal. Chim. Acta 2009, 648, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Leitlein, J.; Aulwurm, S.; Waltereit, R.; Naumann, U.; Wagenknecht, B.; Garten, W.; Weller, M.; Platten, M. Processing of immunosuppressive pro-TGF-beta 1,2 by human glioblastoma cells involves cytoplasmic and secreted furin-like proteases. J. Immunol. 2001, 166, 7238–7243. [Google Scholar] [CrossRef] [Green Version]
- Case, D.A.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Greene, D.; Homeyer, N.; et al. AMBER 2017; University of California: San Francisco, CA, USA, 2017. [Google Scholar]
- Halgren, T.A. Potential energy functions. COSB 1995, 5, 205–210. [Google Scholar] [CrossRef]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Stroganov, O.V.; Novikov, F.N.; Stroylov, V.S.; Kulkov, V.; Chilov, G.G. Lead finder: An approach to improve accuracy of protein-ligand docking, binding energy estimation, and virtual screening. J. Chem. Inf. Model. 2008, 48, 2371–2385. [Google Scholar] [CrossRef] [PubMed]


| Train | Test | External | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | CCR a | AUC b | CCR | AUC | ROCED | CCR | AUC | ROCED | |
| QSAR1 | 6 | 0.96 | 0.97 | 0.92 | 0.89 | 0.27 | 0.73 | 0.82 | 0.81 |
| QSAR2 | 6 | 0.96 | 0.96 | 0.92 | 0.9 | 0.27 | 0.79 | 0.8 | 0.66 |
| QSAR3 | 8 | 0.91 | 0.96 | 0.92 | 0.86 | 0.34 | 0.7 | 0.77 | 1.04 |
| QSAR4 | 9 | 0.98 | 0.97 | 0.92 | 0.85 | 0.25 | 0.73 | 0.71 | 0.79 |
| QSAR5 | 11 | 0.98 | 0.98 | 0.9 | 0.86 | 0.23 | 0.7 | 0.7 | 0.91 |
| Consensus | - | 0.98 | 1 | 0.89 | 1 | 0.35 | 0.73 | 1 | 0.79 |
| LOOcv a | Train | Test | External | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CCR b | ROCED | CCR | AUC c | CCR | AUC | ROCED | CCR | AUC | ROCED | |
| QSAR1 | 0.93 | 0.19 | 0.62 | 0.67 | 0.5 | 0.59 | 2.67 | 0.5 | 0.65 | 2.72 |
| QSAR2 | 0.89 | 0.31 | 0.63 | 0.68 | 0.5 | 0.59 | 2.68 | 0.5 | 0.65 | 2.89 |
| QSAR3 | 0.79 | 0.67 | 0.65 | 0.71 | 0.5 | 0.59 | 2.68 | 0.5 | 0.61 | 2.71 |
| QSAR4 | 0.89 | 0.24 | 0.66 | 0.72 | 0.5 | 0.6 | 2.69 | 0.5 | 0.62 | 2.77 |
| QSAR5 | 0.86 | 0.35 | 0.68 | 0.75 | 0.5 | 0.61 | 2.72 | 0.49 | 0.6 | 2.76 |
| FDB ID a | Compound | Activity Vote b | Probability Mean c | (%) in Domain d |
|---|---|---|---|---|
| FDB030264 | 15,15′-dihydroxy-β-carotene | 1 | 0.897 | 100 |
| FDB001534 | Lactucaxanthin | 1 | 0.867 | 100 |
| FDB002245 | Kukoamine A | 1 | 0.867 | 100 |
| FDB002479 | (3S,3′R,4xi)-beta,beta-Carotene-3,3′,4-triol | 1 | 0.865 | 100 |
| FDB007276 | Lutein ester | 1 | 0.865 | 100 |
| FDB014726 | Zeaxanthin | 1 | 0.863 | 100 |
| FDB015828 | 7,8-Dehydroastaxanthianthin | 1 | 0.862 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaragoza-Huesca, D.; Martínez-Cortés, C.; Banegas-Luna, A.J.; Pérez-Garrido, A.; Vegara-Meseguer, J.M.; Peñas-Martínez, J.; Rodenas, M.C.; Espín, S.; Pérez-Sánchez, H.; Martínez-Martínez, I. Identification of Kukoamine A, Zeaxanthin, and Clexane as New Furin Inhibitors. Int. J. Mol. Sci. 2022, 23, 2796. https://doi.org/10.3390/ijms23052796
Zaragoza-Huesca D, Martínez-Cortés C, Banegas-Luna AJ, Pérez-Garrido A, Vegara-Meseguer JM, Peñas-Martínez J, Rodenas MC, Espín S, Pérez-Sánchez H, Martínez-Martínez I. Identification of Kukoamine A, Zeaxanthin, and Clexane as New Furin Inhibitors. International Journal of Molecular Sciences. 2022; 23(5):2796. https://doi.org/10.3390/ijms23052796
Chicago/Turabian StyleZaragoza-Huesca, David, Carlos Martínez-Cortés, Antonio Jesús Banegas-Luna, Alfonso Pérez-Garrido, Josefina María Vegara-Meseguer, Julia Peñas-Martínez, Maria Carmen Rodenas, Salvador Espín, Horacio Pérez-Sánchez, and Irene Martínez-Martínez. 2022. "Identification of Kukoamine A, Zeaxanthin, and Clexane as New Furin Inhibitors" International Journal of Molecular Sciences 23, no. 5: 2796. https://doi.org/10.3390/ijms23052796
APA StyleZaragoza-Huesca, D., Martínez-Cortés, C., Banegas-Luna, A. J., Pérez-Garrido, A., Vegara-Meseguer, J. M., Peñas-Martínez, J., Rodenas, M. C., Espín, S., Pérez-Sánchez, H., & Martínez-Martínez, I. (2022). Identification of Kukoamine A, Zeaxanthin, and Clexane as New Furin Inhibitors. International Journal of Molecular Sciences, 23(5), 2796. https://doi.org/10.3390/ijms23052796







