Blood Thiol Redox State in Chronic Kidney Disease
Abstract
:1. Introduction
2. Protein Thiols and S-Thiolated Proteins in Plasma and RBCs
3. P-SH and S-Thiolated Proteins in the Moderate-to-Severe Stages of CKD
4. P-SH and S-Thiolated Proteins in HD Patients Compared to Healthy Subjects
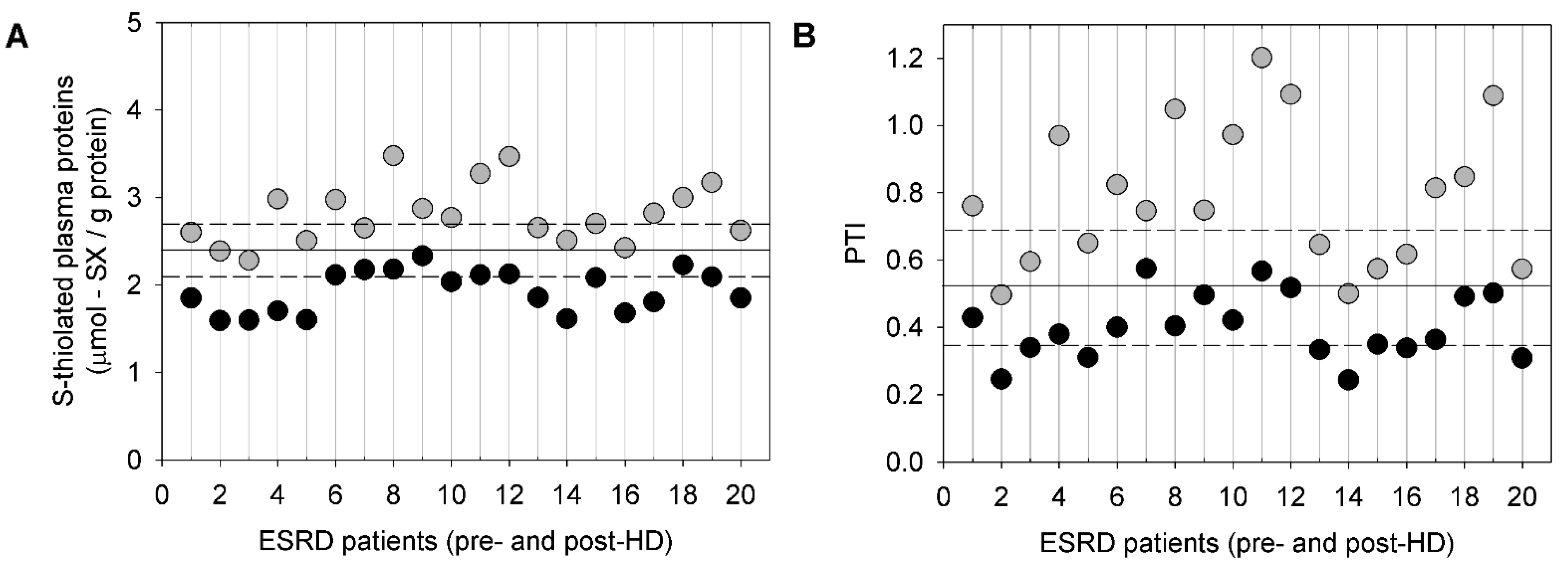
5. P-SH and S-Thiolated Proteins in HD Patients before and after an HD Session
6. P-SH and S-Thiolated Proteins in PD
7. Conclusions and Perspectives
Funding
Conflicts of Interest
Abbreviations
| Alb-SH | reduced albumin |
| CKD | chronic kidney disease |
| CVD | cardiovascular disease |
| CysGlySH | cysteinylglycine |
| CySS | cystine |
| DTNB | 5,5-dithiobis(2-nitrobenzoic acid) |
| eGFR | estimated glomerular filtration rate |
| ESRD | end-stage renal disease |
| GSH | glutathione |
| HbSSCy | cysteinylated haemoglobin |
| HbSSG | glutathionylated haemoglobin |
| HcySH | homocysteine |
| HD | haemodialysis |
| LDL | low-density lipoprotein |
| LMM-SH | low molecular mass thiols |
| PD | peritoneal dialysis |
| PSH | protein thiols |
| PTI | protein thiolation index |
| RBCs | red blood cells |
| ROS | reactive oxygen species |
References
- Bello, A.K.; Levin, A.; Tonelli, M.; Okpechi, I.G.; Feehally, J.; Harris, D.; Jindal, K.; Salako, B.L.; Rateb, A.; Osman, M.A.; et al. Assessment of global kidney health care status. JAMA 2017, 317, 1864–1881. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.A.; Cherney, D.Z.I.; Bello, A.K. Preventing CKD in developed countries. Kidney Int. Rep. 2019, 5, 263–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- KDIGO CKD Workgrop. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 16269–16288. [Google Scholar] [CrossRef]
- Purnell, T.S.; Auguste, P.; Crews, D.C.; Lamprea-Montealegre, J.; Olufade, T.; Greer, R.; Ephraim, P.; Sheu, J.; Kostecki, D.; Powe, N.R.; et al. Comparison of life participation activities among adults treated by hemodialysis, peritoneal dialysis, and kidney transplantation: A systematic review. Am. J. Kidney Dis. 2013, 62, 953–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: Untangling Ariadne’s thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative stress in the pathophysiology of kidney disease: Implications for noninvasive monitoring and identification of biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, R.; Gryp, T.; Glorieux, G. Urea and chronic kidney disease: The comeback of the century? (in uraemia research). Nephrol. Dial. Transpl. 2018, 33, 4–12. [Google Scholar] [CrossRef] [Green Version]
- van den Brand, J.A.; Mutsaers, H.A.; van Zuilen, A.D.; Blankestijn, P.J.; van den Broek, P.H.; Russel, F.G.; Masereeuw, R.; Wetzels, J.F. Uremic solutes in chronic kidney disease and their role in progression. PLoS ONE 2016, 11, e0168117. [Google Scholar] [CrossRef]
- Poulianiti, K.P.; Kaltsatou, A.; Mitrou, G.I.; Jamurtas, A.Z.; Koutedakis, Y.; Maridaki, M.; Stefanidis, I.; Sakkas, G.K.; Karatzaferi, C. Systemic redox imbalance in chronic kidney disease: A systematic review. Oxid. Med. Cell. Longev. 2016, 2016, 8598253. [Google Scholar] [CrossRef] [Green Version]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.; McMonagle, E.; Morrow, J.; Ikizler, T.A.; Himmelfarb, J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. Suppl. 2008, 74, S4–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic toxins in the progression of chronic kidney disease and cardiovascular disease: Mechanisms and therapeutic targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; Krebber, M.M.; Yang, Y.; Ahmed, S.; Lozovanu, V.; Andreeva, D.; Verhaar, M.C.; Masereeuw, R. Protein-bound uremic toxins induce reactive oxygen species-dependent and inflammasome-mediated IL-1β production in kidney proximal tubule cells. Biomedicines 2021, 9, 1326. [Google Scholar] [CrossRef]
- Pieniazek, A.; Bernasinska-Slomczewska, J.; Gwozdzinski, L. Uremic toxins and their relation with oxidative stress induced in patients with CKD. Int. J. Mol. Sci. 2021, 22, 6196. [Google Scholar] [CrossRef]
- Borges Bonan, N.; Schepers, E.; Pecoits-Filho, R.; Dhondt, A.; Pletinck, A.; De Somer, F.; Vanholder, R.; Van Biesen, W.; Moreno-Amaral, A.; Glorieux, G. Contribution of the uremic milieu to an increased pro-inflammatory monocytic phenotype in chronic kidney disease. Sci. Rep. 2019, 9, 10236. [Google Scholar] [CrossRef] [Green Version]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P. Inflammation in end-stage renal disease—What have we learned in 10 years? Semin. Dial. 2010, 23, 498–509. [Google Scholar] [CrossRef]
- Kao, M.P.; Ang, D.S.; Pall, A.; Struthers, A.D. Oxidative stress in renal dysfunction: Mechanisms, clinical sequelae and therapeutic options. J. Hum. Hypertens. 2010, 24, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Foley, R.N. Infectious complications in chronic dialysis patients. Perit. Dial. Int. 2008, 28, S167–S171. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Obesity, oxidative stress, and fibrosis in chronic kidney disease. Kidney Int. Suppl. 2011, 2014, 4113–4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrino, D.; La Russa, D.; Marrone, A. Oxidative imbalance and kidney damage: New study perspectives from animal models to hospitalized patients. Antioxidants 2019, 8, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Morimoto, S.; Okigaki, M.; Seo, M.; Someya, K.; Morita, T.; Matsubara, H.; Sugiura, T.; Iwasaka, T. Decreased plasma level of vitamin C in chronic kidney disease: Comparison between diabetic and non-diabetic patients. Nephrol. Dial. Transpl. 2011, 26, 1252–1257. [Google Scholar] [CrossRef] [Green Version]
- Clase, C.M.; Ki, V.; Holden, R.M. Water-soluble vitamins in people with low glomerular filtration rate or on dialysis: A review. Semin. Dial. 2013, 26, 546–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stępniewska, J.; Dołęgowska, B.; Popińska, M.; Sałata, D.; Budkowska, M.; Gołembiewska, E.; Myślak, M.; Domański, M.; Marchelek-Myśliwiec, M.; Ciechanowski, K. Prooxidative-antioxidative balance of cells in different types of renal replacement therapy. Blood Purif. 2014, 37, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Vostálová, J.; Galandáková, A.; Svobodová, A.R.; Orolinová, E.; Kajabová, M.; Schneiderka, P.; Zapletalová, J.; Strebl, P.; Zadražil, J. Time-course evaluation of oxidative stress-related biomarkers after renal transplantation. Ren. Fail. 2012, 34, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Navarro-García, J.A.; Rodríguez-Sánchez, E.; Aceves-Ripoll, J.; Abarca-Zabalía, J.; Susmozas-Sánchez, A.; González Lafuente, L.; Bada-Bosch, T.; Hernández, E.; Mérida-Herrero, E.; Praga, M.; et al. Oxidative status before and after renal replacement therapy: Differences between conventional high flux hemodialysis and on-line hemodiafiltration. Nutrients 2019, 11, 2809. [Google Scholar] [CrossRef] [Green Version]
- FDA-NIH Biomarker Working Group. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326791/ (accessed on 30 December 2021).
- Bachi, A.; Dalle-Donne, I.; Scaloni, A. Redox proteomics: Chemical principles, methodological approaches and biological/biomedical promises. Chem. Rev. 2013, 113, 596–698. [Google Scholar] [CrossRef]
- Colombo, G.; Reggiani, F.; Cucchiari, D.; Portinaro, N.M.; Giustarini, D.; Rossi, R.; Garavaglia, M.L.; Saino, N.; Milzani, A.; Badalamenti, S.; et al. Plasma protein-bound di-tyrosines as biomarkers of oxidative stress in end stage renal disease patients on maintenance haemodialysis. BBA Clin. 2017, 7, 55–63. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Altomare, A.; Rusconi, F.; Giustarini, D.; Portinaro, N.; Garavaglia, M.L.; Rossi, R.; Dalle-Donne, I.; Milzani, A. Thiol oxidation and di-tyrosine formation in human plasma proteins induced by inflammatory concentrations of hypochlorous acid. J. Proteom. 2017, 152, 22–32. [Google Scholar] [CrossRef]
- Colombo, G.; Reggiani, F.; Cucchiari, D.; Astori, E.; Garavaglia, M.L.; Portinaro, N.M.; Saino, N.; Finazzi, S.; Milzani, A.; Badalamenti, S.; et al. Plasma protein carbonylation in haemodialysed patients: Focus on diabetes and gender. Oxid. Med. Cell. Longev. 2018, 2018, 4149681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, G.; Reggiani, F.; Astori, E.; Finazzi, S.; Garavaglia, M.L.; Angelini, C.; Milzani, A.; Badalamenti, S.; Dalle-Donne, I. Advanced oxidation protein products in non-diabetic end stage renal disease patients on maintenance haemodialysis. Free Radic. Res. 2019, 53, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Reggiani, F.; Angelini, C.; Finazzi, S.; Astori, E.; Garavaglia, M.L.; Landoni, L.; Portinaro, N.M.; Giustarini, D.; Rossi, R.; et al. Plasma protein carbonyls as biomarkers of oxidative stress in chronic kidney disease, dialysis, and transplantation. Oxid. Med. Cell. Longev. 2020, 2020, 2975256. [Google Scholar] [CrossRef] [PubMed]
- Zavadskiy, S.; Sologova, S.; Moldogazieva, N. Oxidative distress in aging and age-related diseases: Spatiotemporal dysregulation of protein oxidation and degradation. Biochimie 2021. online ahead of print, S0300-9084(21)00273-X. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Braconi, D.; Santucci, A.; Rossi, R. Membrane skeletal protein S-glutathionylation in human red blood cells as index of oxidative stress. Chem. Res. Toxicol. 2019, 32, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in stress related diseases/disorders: Diagnostic, prognostic, and therapeutic values. Front. Mol. Biosci. 2019, 6, 91. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Milzani, A.; Gagliano, N.; Colombo, R.; Giustarini, D.; Rossi, R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid. Redox Signal. 2008, 10, 446–473. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, G.; Giustarini, D.; Milzani, A. Protein S-glutathionylation: A regulatory device from bacteria to humans. Trends Biochem. Sci. 2009, 34, 85–96. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Glushchenko, A.V.; Jacobsen, D.W. Molecular targeting of proteins by L-homocysteine: Mechanistic implications for vascular disease. Antioxid. Redox Signal. 2007, 9, 1883–1898. [Google Scholar] [CrossRef] [Green Version]
- Pastore, A.; Piemonte, F. Protein glutathionylation in cardiovascular diseases. Int. J. Mol. Sci. 2013, 14, 20845–20876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinellu, A.; Fois, A.G.; Sotgia, S.; Zinellu, E.; Bifulco, F.; Pintus, G.; Mangoni, A.A.; Carru, C.; Pirina, P. Plasma protein thiols: An early marker of oxidative stress in asthma and chronic obstructive pulmonary disease. Eur. J. Clin. Investig. 2016, 46, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Braconi, D.; Giustarini, D.; Marzocchi, B.; Peruzzi, L.; Margollicci, M.; Rossi, R.; Bernardini, G.; Millucci, L.; Gallagher, J.A.; Le Quan Sang, K.H.; et al. Inflammatory and oxidative stress biomarkers in alkaptonuria: Data from the DevelopAKUre project. Osteoarthr. Cartil. 2018, 26, 1078–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giustarini, D.; Dalle-Donne, I.; Lorenzini, S.; Selvi, E.; Colombo, G.; Milzani, A.; Fanti, P.; Rossi, R. Protein thiolation index (PTI) as a biomarker of oxidative stress. Free Radic. Biol. Med. 2012, 53, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, K.; Pieniazek, A.; Gwozdzinski, L. Reactive oxygen species and their involvement in red blood cell damage in chronic kidney disease. Oxid. Med. Cell. Longev. 2021, 2021, 6639199. [Google Scholar] [CrossRef]
- Ravarotto, V.; Bertoldi, G.; Innico, G.; Gobbi, L.; Calò, L.A. The pivotal role of oxidative stress in the pathophysiology of cardiovascular-renal remodeling in kidney disease. Antioxidants 2021, 10, 1041. [Google Scholar] [CrossRef]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P.G. Inflammation and oxidative stress in chronic kidney disease and dialysis patients. Antioxid. Redox Signal. 2021, 35, 1426–1448. [Google Scholar] [CrossRef]
- Rossi, R.; Giustarini, D.; Milzani, A.; Dalle-Donne, I. Cysteinylation and homocysteinylation of plasma protein thiols during ageing of healthy human beings. J. Cell. Mol. Med. 2009, 13, 3131–3140. [Google Scholar] [CrossRef] [Green Version]
- Aebi, S.; Assereto, R.; Lauterburg, B.H. High-dose intravenous glutathione in man. Pharmacokinetics and effects on cyst(e)ine in plasma and urine. Eur. J. Clin. Investig. 1991, 21, 103–110. [Google Scholar] [CrossRef]
- Oliveira, P.V.S.; Laurindo, F.R.M. Implications of plasma thiol redox in disease. Clin. Sci. 2018, 132, 1257–1280. [Google Scholar] [CrossRef]
- Di Minno, M.N.; Tremoli, E.; Coppola, A.; Lupoli, R.; Di Minno, G. Homocysteine and arterial thrombosis: Challenge and opportunity. Thromb. Haemost. 2010, 103, 942–961. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Homocysteine and the pathogenesis of atherosclerosis. Expert Rev. Clin. Pharmacol. 2015, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Nigwekar, S.U.; Kang, A.; Zoungas, S.; Cass, A.; Gallagher, M.P.; Kulshrestha, S.; Navaneethan, S.D.; Perkovic, V.; Strippoli, G.F.; Jardine, M.J. Interventions for lowering plasma homocysteine levels in dialysis patients. Cochrane Database Syst. Rev. 2016, 2016, CD004683. [Google Scholar] [CrossRef] [PubMed]
- Balint, B.; Jepchumba, V.K.; Guéant, J.L.; Guéant-Rodriguez, R.M. Mechanisms of homocysteine-induced damage to the endothelial, medial and adventitial layers of the arterial wall. Biochimie 2020, 173, 100–106. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, L.; Si, X.; Tian, J.-L.; Zhang, Y.; Gui, H.-L.; Li, B.; Tan, D.-H. Current progress on the mechanisms of hyperhomocysteinemia-induced vascular injury and use of natural polyphenol compounds. Eur. J. Pharmacol. 2021, 905, 174168. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Selvi, E.; Lorenzini, S.; Bisogno, S.; Galeazzi, M.; Laghi Pasini, F. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun. Rev. 2007, 6, 503–509. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Fusco, R.; Cuzzocrea, S.; Di Paola, R.; Impellizzeri, D. Involvements of hyperhomocysteinemia in neurological disorders. Metabolites 2021, 11, 37. [Google Scholar] [CrossRef]
- Park, J.H.; Song, J.S.; Choi, S.T. Increased carotid intima-media thickness (IMT) in hyperuricemic individuals may be explained by hyperhomocysteinemia associated with renal dysfunction: A cross-sectional study. J. Korean Med. Sci. 2019, 34, e237. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Bourgonje, M.F.; Binnenmars, S.H.; Gordijn, S.J.; Bulthuis, M.L.C.; la Bastide-van Gemert, S.; Kieneker, L.M.; Gansevoort, R.T.; Bakker, S.J.L.; et al. Serum free sulfhydryl status associates with new-onset chronic kidney disease in the general population. Redox Biol. 2021, 48, 102211. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A.; Tabibzadeh, S. Homocysteine in chronic kidney disease. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Academic Press: Burlington, MA, USA, 2015; Volume 72, pp. 77–106. [Google Scholar]
- Jakubowski, H. The pathophysiological hypothesis of homocysteine thiolactone-mediated vascular disease. J. Physiol. Pharmacol. 2008, 59, 155–167. [Google Scholar]
- Giustarini, D.; Milzani, A.; Dalle-Donne, I.; Rossi, R. Red blood cells as a physiological source of glutathione for extracellular fluids. Blood Cells Mol. Dis. 2008, 40, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W. Oxygen and life. In Medical Biochemistry; Baynes, J.W., Domoniczak, M.H., Eds.; Elsevier: Philadelphia, PA, USA, 2005; pp. 497–506. [Google Scholar]
- Khazim, K.; Giustarini, D.; Rossi, R.; Verkaik, D.; Cornell, J.E.; Cunningham, S.E.; Mohammad, M.; Trochta, K.; Lorenzo, C.; Folli, F.; et al. Glutathione redox potential is low and glutathionylated and cysteinylated hemoglobin levels are elevated in maintenance hemodialysis patients. Transl. Res. 2013, 162, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaziri, N.D. Oxidative stress in uremia: Nature, mechanisms, and potential consequences. Semin. Nephrol. 2004, 24, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Picot, I.; Witko-Sarsat, V.; Merad-Boudia, M.; Nguyen, A.T.; Thévenin, M.; Jaudon, M.C.; Zingraff, J.; Verger, C.; Jungers, P.; Descamps-Latscha, B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic. Biol. Med. 1996, 21, 845–853. [Google Scholar] [CrossRef]
- Colombo, G.; Dalle-Donne, I.; Giustarini, D.; Gagliano, N.; Portinaro, N.; Colombo, R.; Rossi, R.; Milzani, A. Cellular redox potential and hemoglobin S-glutathionylation in human and rat erythrocytes: A comparative study. Blood Cells Mol. Dis. 2010, 44, 133–139. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Lorenzini, S.; Milzani, A.; Rossi, R. Age-related influence on thiol, disulfide, and protein-mixed disulfide levels in human plasma. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1030–1038. [Google Scholar] [CrossRef] [Green Version]
- Colombo, G.; Clerici, M.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Redox albuminomics: Oxidized albumin in human diseases. Antioxid. Redox Signal. 2012, 17, 1515–1527. [Google Scholar] [CrossRef]
- Taverna, M.; Marie, A.L.; Mira, J.P.; Guidet, B. Specific antioxidant properties of human serum albumin. Ann. Intensive Care 2013, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Imafuku, T.; Otagiri, M.; Maruyama, T. Clinical implications associated with the posttranslational modification-induced functional impairment of albumin in oxidative stress-related diseases. J. Pharm. Sci. 2017, 106, 2195–2203. [Google Scholar] [CrossRef] [Green Version]
- Bonanata, J.; Turell, L.; Antmann, L.; Ferrer-Sueta, G.; Botasini, S.; Méndez, E.; Alvarez, B.; Coitiño, E.L. The thiol of human serum albumin: Acidity, microenvironment and mechanistic insights on its oxidation to sulfenic acid. Free Radic. Biol. Med. 2017, 108, 952–962. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Jenkins, R.O.; Goncharov, N.V. Serum albumin in health and disease: Esterase, antioxidant, transporting and signaling properties. Int. J. Mol. Sci. 2021, 22, 10318. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Stauber, R.E. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br. J. Pharmacol. 2007, 151, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; McMonagle, E.; McMenamin, E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000, 58, 2571–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, M.; Upadhya, S.; Prabhu, R. Protein thiol oxidation and lipid peroxidation in patients with uraemia. Scand. J. Clin. Lab. Investig. 2004, 64, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.F.; Shintani, A.; Ikizler, T.A.; Himmelfarb, J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J. Am. Soc. Nephrol. 2008, 19, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Prakash, M.; Shetty, J.K.; Rao, L.; Sharma, S.; Rodrigues, A.; Prabhu, R. Serum paraoxonase activity and protein thiols in chronic renal failure patients. Indian J. Nephrol. 2008, 18, 13–16. [Google Scholar] [CrossRef]
- Pieniazek, A.; Gwozdzinski, L.; Zbrog, Z.; Gwozdzinski, K. Alterations in conformational state of albumin in plasma in chronic hemodialyzed patients. PLoS ONE 2018, 13, e0192268. [Google Scholar] [CrossRef]
- Zinellu, A.; Sotgia, S.; Mangoni, A.A.; Sotgiu, E.; Ena, S.; Arru, D.; Assaretti, S.; Baralla, A.; Satta, A.E.; Carru, C. Effects of ramipril and telmisartan on plasma concentrations of low molecular weight and protein thiols and carotid intima media thickness in patients with chronic kidney disease. Dis. Markers 2016, 2016, 1821596. [Google Scholar] [CrossRef]
- Aveles, P.R.; Criminácio, C.R.; Gonçalves, S.; Bignelli, A.T.; Claro, L.M.; Siqueira, S.S.; Nakao, L.S.; Pecoits-Filho, R. Association between biomarkers of carbonyl stress with increased systemic inflammatory response in different stages of chronic kidney disease and after renal transplantation. Nephron Clin. Pract. 2010, 116, c294–c299. [Google Scholar] [CrossRef]
- Terawaki, H.; Yoshimura, K.; Hasegawa, T.; Matsuyama, Y.; Negawa, T.; Yamada, K.; Matsushima, M.; Nakayama, M.; Hosoya, T.; Era, S. Oxidative stress is enhanced in correlation with renal dysfunction: Examination with the redox state of albumin. Kidney Int. 2004, 66, 1988–1993. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, Y.; Terawaki, H.; Terada, T.; Era, S. Albumin thiol oxidation and serum protein carbonyl formation are progressively enhanced with advancing stages of chronic kidney disease. Clin. Exp. Nephrol. 2009, 13, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Loriga, G.; Scanu, B.; Pisanu, E.; Sanna, M.; Deiana, L.; Satta, A.E.; Carru, C. Increased low-density lipoprotein S-homocysteinylation in chronic kidney disease. Am. J. Nephrol. 2010, 32, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.V.; Srinivasa Rao, P.V.; Sivakumar, V.; Satish, P.; Shalini, P.; Suchitra, M.M.; Kiranmayi, V.S. Study of oxidant and anti-oxidant status in patients with chronic kidney disease. J. Clin. Sci. Res. 2018, 7, 124–130. [Google Scholar] [CrossRef]
- Suzuki, Y.; Suda, K.; Matsuyama, Y.; Era, S.; Soejima, A. Close relationship between redox state of human serum albumin and serum cysteine levels in non-diabetic CKD patients with various degrees of renal function. Clin. Nephrol. 2014, 82, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Cakirca, C.; Nas, C.; Yilmaz, K.; Guzelcicek, A.; Erel, O. Evaluation of dynamic thiol-disulfide balance in children with stage 3–5 chronic kidney disease. Ann. Med. Res. 2019, 26, 1565–1568. [Google Scholar] [CrossRef]
- Solak, I.; Guney, I.; Mercan, S.; Erel, O.; Neselioglu, S.; Cetinkaya, C.; Eryılmaz, M. Changes in thiol/disulfide homeostasis in patients with chronic kidney disease. Med. Sci. 2020, 9, 201–204. [Google Scholar] [CrossRef]
- Ates, I.; Özkayar, N.; Meriç Yılmaz, F.; Bayrakçı, N.; Neşelioğlu, S.; Erel, O.; Coşkun Yenigün, E.; Dede, F. Oxidative stress level in patients with chronic kidney disease. Ortadogu Med. J. 2018, 10, 45–50. [Google Scholar] [CrossRef]
- Sogut, I.; Aydın, A.S.; Gokmen, E.S.; Atak, P.G.; Erel, O.; Degrigo, U.G. Evaluation of oxidative stress and thiol/ disulfide parameters according to the body mass index in adult individuals. Erciyes Med. J. 2018, 40, 155–161. [Google Scholar] [CrossRef]
- Nakatani, S.; Yasukawa, K.; Ishimura, E.; Nakatani, A.; Toi, N.; Uedono, H.; Tsuda, A.; Yamada, S.; Ikeda, H.; Mori, K.; et al. Non-mercaptalbumin, oxidized form of serum albumin, significantly associated with renal function and anemia in chronic kidney disease patients. Sci. Rep. 2018, 8, 16796. [Google Scholar] [CrossRef]
- Terawaki, H.; Hayashi, T.; Murase, T.; Iijima, R.; Waki, K.; Tani, Y.; Nakamura, T.; Yoshimura, K.; Uchida, S.; Kazama, J.J. Relationship between xanthine oxidoreductase redox and oxidative stress among chronic kidney disease patients. Oxid. Med. Cell. Longev. 2018, 2018, 9714710. [Google Scholar] [CrossRef]
- Kruglova, M.P.; Ivanov, A.V.; Virus, E.D.; Bulgakova, P.O.; Samokhin, A.S.; Fedoseev, A.N.; Grachev, S.V.; Kubatiev, A.A. Urine S-adenosylmethionine are related to degree of renal insufficiency in patients with chronic kidney disease. Lab. Med. 2021, 52, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Przemysław, W.; Piotr, K.; Grażyna, C.; Danuta, K.P.; Małgorzata, I.; Bernadeta, M.; Małgorzata, S.; Witold, S. Total, free, and protein-bound thiols in plasma of peritoneal dialysis and predialysis patients. Int. Urol. Nephrol. 2011, 43, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Danielski, M.; Ikizler, T.A.; McMonagle, E.; Kane, J.C.; Pupim, L.; Morrow, J.; Himmelfarb, J. Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy. Am. J. Kidney Dis. 2003, 42, 286–294. [Google Scholar] [CrossRef]
- Himmelfarb, J.; McMonagle, E.; Freedman, S.; Klenzak, J.; McMenamin, E.; Le, P.; Pupim, L.B.; Ikizler, T.A.; The PICARD Group. Oxidative stress is increased in critically ill patients with acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 2449–2456. [Google Scholar] [CrossRef]
- Prakash, M.; Upadhya, S.; Prabhu, R. Serum non-transferrin bound iron in hemodialysis patients not receiving intravenous iron. Clin. Chim. Acta 2005, 360, 194–198. [Google Scholar] [CrossRef]
- Koca, T.; Berber, A.; Koca, H.B.; Demir, T.A.; Koken, T. Effects of hemodialysis period on levels of blood trace elements and oxidative stress. Clin. Exp. Nephrol. 2010, 14, 463–468. [Google Scholar] [CrossRef]
- Colombo, G.; Reggiani, F.; Podestà, M.A.; Garavaglia, M.L.; Portinaro, N.M.; Milzani, A.; Badalamenti, S.; Dalle-Donne, I. Plasma protein thiolation index (PTI) as a biomarker of thiol-specific oxidative stress in haemodialyzed patients. Free Radic. Biol. Med. 2015, 89, 443–451. [Google Scholar] [CrossRef]
- Anraku, M.; Kitamura, K.; Shinohara, A.; Adachi, M.; Suenga, A.; Maruyama, T.; Miyanaka, K.; Miyoshi, T.; Shiraishi, N.; Nonoguchi, H.; et al. Intravenous iron administration induces oxidation of serum albumin in hemodialysis patients. Kidney Int. 2004, 66, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Mera, K.; Anraku, M.; Kitamura, K.; Nakajou, K.; Maruyama, T.; Otagiri, M. The structure and function of oxidized albumin in hemodialysis patients: Its role in elevated oxidative stress via neutrophil burst. Biochem. Biophys. Res. Commun. 2005, 334, 1322–1328. [Google Scholar] [CrossRef]
- Perna, A.F.; Satta, E.; Acanfora, F.; Lombardi, C.; Ingrosso, D.; De Santo, N.G. Increased plasma protein homocysteinylation in hemodialysis patients. Kidney Int. 2006, 69, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Fanti, P.; Giustarini, D.; Rossi, R.; Cunningham, S.E.; Folli, F.; Khazim, K.; Cornell, J.; Matteucci, E.; Bansal, S. Dietary intake of proteins and calories is inversely associated with the oxidation state of plasma thiols in end-stage renal disease patients. J. Ren. Nutr. 2015, 25, 494–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zumbrennen-Bullough, K.; Babitt, J.L. The iron cycle in chronic kidney disease (CKD): From genetics and experimental models to CKD patients. Nephrol. Dial. Transpl. 2014, 29, 263–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Vecchio, L.; Longhi, S.; Locatelli, F. Safety concerns about intravenous iron therapy in patients with chronic kidney disease. Clin. Kidney J. 2016, 9, 260–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giustarini, D.; Galvagni, F.; Colombo, G.; Dalle-Donne, I.; Milzani, A.; Aloisi, A.M.; Rossi, R. Measurement of Protein Thiolation Index (PTI) as a biomarker of oxidative stress in serum: Effect of delayed sample separation/stabilization. Anal. Biochem. 2017, 538, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Grekas, D.; Economou, H.; Makedou, A.; Destanis, E.; Theodoridou, A.; Avdelidou, A.; Demitriadis, A.; Tourkantonis, A. Association between hyperhomocysteinemia and ultrasonographic atherosclerotic indices of carotid arteries in chronic hemodialysis patients. Nephron Clin. Pract. 2005, 101, c180–c186. [Google Scholar] [CrossRef]
- Zoccali, C. Traditional and emerging cardiovascular and renal risk factors: An epidemiologic perspective. Kidney Int. 2006, 70, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerardi, G.; Usberti, M.; Martini, G.; Albertini, A.; Sugherini, L.; Pompella, A.; Di, L.D. Plasma total antioxidant capacity in hemodialyzed patients and its relationships to other biomarkers of oxidative stress and lipid peroxidation. Clin. Chem. Lab. Med. 2002, 40, 104–110. [Google Scholar] [CrossRef]
- Ward, R.A.; Ouseph, R.; McLeish, K.R. Effects of high-flux hemodialysis on oxidant stress. Kidney Int. 2003, 63, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Palleschi, S.; De Angelis, S.; Rossi, B.; Diana, L.; Papa, V.; Severini, G.; Splendiani, G. Homocysteinemia correlates with plasma thiol redox status in patients with end-stage renal disease. Nephron Clin. Pract. 2008, 108, c106–c112. [Google Scholar] [CrossRef]
- Pieniazek, A.; Brzeszczynska, J.; Kruszynska, I.; Gwozdzinski, K. Investigation of albumin properties in patients with chronic renal failure. Free Radic. Res. 2009, 43, 1008–1018. [Google Scholar] [CrossRef]
- Wlodek, P.J.; Iciek, M.B.; Milkowski, A.; Smolenski, O.B. Various forms of plasma cysteine and its metabolites in patients undergoing hemodialysis. Clin. Chim. Acta 2001, 304, 9–18. [Google Scholar] [CrossRef]
- Regazzoni, L.; Del Vecchio, L.; Altomare, A.; Yeum, K.J.; Cusi, D.; Locatelli, F.; Carini, M.; Aldini, G. Human serum albumin cysteinylation is increased in end stage renal disease patients and reduced by hemodialysis: Mass spectrometry studies. Free Radic. Res. 2013, 47, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Bocedi, A.; Cattani, G.; Stella, L.; Massoud, R.; Ricci, G. Thiol disulfide exchange reactions in human serum albumin: The apparent paradox of the redox transitions of Cys34. FEBS J. 2018, 285, 3225–3237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capusa, C.; Stoian, I.; Rus, E.; Lixandru, D.; Barbulescu, C.; Mircescu, G. Does dialysis modality influence the oxidative stress of uremic patients? Kidney Blood Press. Res. 2012, 35, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [Green Version]
- Kok, M.B.; Tegelaers, F.P.W.; van Dam, B.; van Rijn, J.L.M.L.; van Pelt, J. Carbamylation of albumin is a cause for discrepancies between albumin assays. Clin. Chim. Acta 2014, 434, 6–10. [Google Scholar] [CrossRef]
- Mitrogianni, Z.; Barbouti, A.; Galaris, D.; Siamopoulos, K.C. Oxidative modification of albumin in predialysis, hemodialysis, and peritoneal dialysis patients. Nephron Clin. Pract. 2009, 113, c234–c240. [Google Scholar] [CrossRef]
- Pavone, B.; Sirolli, V.; Bucci, S.; Libardi, F.; Felaco, P.; Amoroso, L.; Sacchetta, P.; Urbani, A.; Bonomini, M. Adsorption and carbonylation of plasma proteins by dialyser membrane material: In vitro and in vivo proteomics investigations. Blood Transfus. 2010, 8, s113–s119. [Google Scholar] [CrossRef]
- Kalim, S.; Tamez, H.; Wenger, J.; Ankers, E.; Trottier, C.A.; Deferio, J.J.; Berg, A.H.; Karumanchi, S.A.; Thadhani, R.I. Carbamylation of serum albumin and erythropoietin resistance in end stage kidney disease. Clin. J. Am. Soc. Nephrol. 2013, 8, 1927–1934. [Google Scholar] [CrossRef] [Green Version]
- Drechsler, C.; Kalim, S.; Wenger, J.B.; Suntharalingam, P.; Hod, T.; Thadhani, R.I.; Karumanchi, S.A.; Wanner, C.; Berg, A.H. Protein carbamylation is associated with heart failure and mortality in diabetic patients with end-stage renal disease. Kidney Int. 2015, 87, 1201–1208. [Google Scholar] [CrossRef] [Green Version]
- Kalim, S.; Trottier, C.A.; Wenger, J.B.; Wibecan, J.; Ahmed, R.; Ankers, E.; Karumanchi, S.A.; Thadhani, R.; Berg, A.H. Longitudinal changes in protein carbamylation and mortality risk after initiation of hemodialysis. Clin. J. Am. Soc. Nephrol. 2016, 11, 1809–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajjala, P.R.; Fliser, D.; Speer, T.; Jankowski, V.; Jankowski, J. Emerging role of post-translational modifications in chronic kidney disease and cardiovascular disease. Nephrol. Dial. Transpl. 2015, 30, 1814–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labriola, L.; Wallemacq, P.; Gulbis, B.; Jadoul, M. The impact of the assay for measuring albumin on corrected (‘adjusted’) calcium concentrations. Nephrol. Dial. Transpl. 2009, 24, 1834–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, A.; Takita, T.; Furuhashi, M.; Fujimoto, T.; Suzuki, H.; Hakamada, M.; Maruyama, Y. Influence of the assay for measuring serum albumin on corrected total calcium in chronic hemodialysis patients. Ther. Apher. Dial. 2011, 15, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Tazza, L. Wishful thinking: The surprisingly sparse evidence for a relationship between oxidative stress and cardiovascular disease in hemodialysis patients. Semin. Dial. 2015, 28, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Liakopoulos, V.; Rapsomanikis, K.P.; Dounousi, E. Chronic kidney disease and disproportionally increased cardiovascular damage: Does oxidative stress explain the burden? Oxid. Med. Cell. Longev. 2017, 2017, 9036450. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; Lindholm, B.; Goldsmith, D.; Fliser, D.; Wiecek, A.; Suleymanlar, G.; Ortiz, A.; Massy, Z.; Martinez-Castelao, A.; Agarwal, R.; et al. Cardiovascular and non-cardiovascular mortality in dialysis patients: Where is the link? Kidney Int. Suppl. 2011, 1, 21–23. [Google Scholar] [CrossRef] [Green Version]
- van der Velde, M.; Matsushita, K.; Coresh, J.; Astor, B.C.; Woodward, M.; Levey, A.; de Jong, P.; Gansevoort, R.T.; Chronic Kidney Disease Prognosis Consortium; van der Velde, M.; et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011, 79, 1341–1352. [Google Scholar] [CrossRef] [Green Version]
- Terawaki, H.; Takada, Y.; Era, S.; Funakoshi, Y.; Nakayama, K.; Nakayama, M.; Ogura, M.; Ito, S.; Hosoya, T. The redox state of albumin and serious cardiovascular incidence in hemodialysis patients. Ther. Apher. Dial. 2010, 14, 465–471. [Google Scholar] [CrossRef]
- Terawaki, H.; Era, S.; Nakayama, M.; Hosoya, T. Decrease in reduced-form albumin among chronic kidney disease patients: New insights in cardiovascular complications. Ther. Apher. Dial. 2011, 15, 156–160. [Google Scholar] [CrossRef]
- Terawaki, H.; Matsuyama, Y.; Matsuo, N.; Ogura, M.; Mitome, J.; Hamaguchi, A.; Terada, T.; Era, S.; Hosoya, T. A lower level of reduced albumin induces serious cardiovascular incidence among peritoneal dialysis patients. Clin. Exp. Nephrol. 2012, 16, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.S.; Chen, H.P.; Chen, C.H.; Wu, M.Y.; Wu, C.Y.; Wu, T.K. Association between redox status of serum albumin and peritoneal membrane transport properties in patients on peritoneal dialysis. Blood Purif. 2015, 40, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Pavone, B.; Sirolli, V.; Giardinelli, A.; Bucci, S.; Forlì, F.; Di Cesare, M.; Sacchetta, P.; Di Pietro, N.; Pandolfi, A.; Urbani, A.; et al. Plasma protein carbonylation in chronic uremia. J. Nephrol. 2011, 24, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Albarello, K.; dos Santos, G.A.; Bochi, G.V.; Sangoi, M.B.; Almeida, T.C.; Paz da Silva, J.E.; Garcia, S.C.; Moresco, R.N. Ischemia modified albumin and carbonyl protein as potential biomarkers of protein oxidation in hemodialysis. Clin. Biochem. 2012, 45, 450–454. [Google Scholar] [CrossRef]
- Caimi, G.; Carollo, C.; Hopps, E.; Montana, M.; Lo Presti, R. Protein oxidation in chronic kidney disease. Clin. Hemorheol. Microcirc. 2013, 54, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Luo, J.F.; Jiang, Y.; Ma, Y.J.; Ji, Y.Q.; Zhu, G.L.; Zhou, C.; Chu, H.W.; Zhang, H.D. Red blood cell lifespan shortening in patients with early-stage chronic kidney disease. Kidney Blood Press. Res. 2019, 44, 1158–1165. [Google Scholar] [CrossRef]
- Naito, C.; Kajita, M.; Niwa, T. Determination of glutathionyl hemoglobin in hemodialysis patients using electrospray ionization liquid chromatography-mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1999, 731, 121–124. [Google Scholar] [CrossRef]
- Takayama, F.; Tsutsui, S.; Horie, M.; Shimokata, K.; Niwa, T. Glutathionyl hemoglobin in uremic patients undergoing hemodialysis and continuous ambulatory peritoneal dialysis. Kidney Int. Suppl. 2001, 78, S155–S158. [Google Scholar] [CrossRef] [Green Version]
- Pieniazek, A.; Gwozdzinski, K. Changes in the conformational state of hemoglobin in hemodialysed patients with chronic renal failure. Oxid. Med. Cell. Longev. 2015, 2015, 783073. [Google Scholar] [CrossRef] [Green Version]
- Włodek, P.; Marcykiewicz, B.; Iciek, M.; Suliga, M.; Smoleński, O.; Kowalczyk-Pachel, D. Thiol levels, protein carbonylation and anaerobic sulfur metabolism in erythrocytes of peritoneal dialysis and predialysis patients. Nephrology 2010, 15, 755–761. [Google Scholar] [CrossRef]
- Ozden, M.; Maral, H.; Akaydin, D.; Cetinalp, P.; Kalender, B. Erythrocyte glutathione peroxidase activity, plasma malondialdehyde and erythrocyte glutathione levels in hemodialysis and CAPD patients. Clin. Biochem. 2002, 35, 269–273. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Rossi, R. Detection of glutathione in whole blood after stabilization with N-ethylmaleimide. Anal. Biochem. 2011, 415, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Fanti, P.; Rossi, R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat. Protoc. 2013, 8, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Rudyk, O.; Eaton, P. Biochemical methods for monitoring protein thiol redox states in biological systems. Redox Biol. 2014, 2, 803–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giustarini, D.; Galvagni, F.; Tesei, A.; Farolfi, A.; Zanoni, M.; Pignatta, S.; Milzani, A.; Marone, I.M.; Dalle-Donne, I.; Nassini, R.; et al. Glutathione, glutathione disulfide, and S-glutathionylated proteins in cell cultures. Free Radic. Biol. Med. 2015, 89, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Galvagni, F.; Orlandini, M.; Fanti, P.; Rossi, R. Immediate stabilization of human blood for delayed quantification of endogenous thiols and disulfides. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1019, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Giustarini, D.; Colombo, G.; Garavaglia, M.L.; Astori, E.; Portinaro, N.M.; Reggiani, F.; Badalamenti, S.; Aloisi, A.M.; Santucci, A.; Rossi, R.; et al. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic. Biol. Med. 2017, 112, 360–375. [Google Scholar] [CrossRef]


| Concentration | Albumin Concentration mg/dL | Additional Information | Reference | ||
|---|---|---|---|---|---|
| CKD Patients | Control Group | CKD Patients | Control Group | ||
| 202 ± 20 μM (n = 10) | 279 ± 12 μM (n = 10) | - | - | CKD patients not receiving renal replacement therapy | [77] |
| 182.3 ± 15.9 μg/L (n = 24) *** | 286.4 ± 21.8 μg/L (n = 20) | - | - | albumin significantly lower in CKD patients compared to healthy controls (p < 0.0001) Analyses performed in serum | [78] |
| 304.0 ± 55.2 μM (n = 184) *** | 328.4 ± 33.3 μM (n = 43) | 4.3 ± 0.38 | 4.4 ± 0.22 | CKD patients at stages 3, 4 | [79] |
| 191.26 ± 16.75 μM (n = 41) ** | 342.34 ± 43.43 μM (n = 41) | 2.1 ± 1.0 | 4.4 ± 1.3 | CKD patients on conservative treatment Analyses performed in serum | [80] |
| 6.3 ± 0.9 μmol/g protein (n = 16) * | 7.3 ± 0.8 μmol/g protein (n = 13) | - | - | Non dialysis CKD patients | [81] |
| 3.59 (3.31–4.80) μmol/g protein (a) (n = 24) | - | - | - | CKD hypertensive patients stages 3, 4 | [82] |
| stage 2: 9.8 ± 3.5 nmol/mg albumin stage 3: 9.9 ± 2.6 nmol/mg albumin stages 5: 7.9 ± 2.5 nmol/mg albumin (n = 68) | - | - | - | - | [83] |
| 69.44 ± 7.26% (n = 55) | - | 4.1 ± 0.4 | - | Predialysis patients with CKD | [84] |
| 28.59 ± 6.93% | S-thiolated albumin | ||||
| stages 1, 2 77.2 ± 3.4% (n = 7) stage 3a 75.5 ± 3.8% (n = 7) stage 3b 71.5 ± 3.3% (n = 6) stages 4, 5 66.2 ± 4.1% (n = 12) | - | stages 1, 2 4.0 ± 0.1 stage 3a 4.0 ± 0.1 stage 3b 4.1 ± 0.3 stages 4, 5 4.1 ± 0.3 | - | [85] | |
| stages 1, 2 21.0 ± 3.4% (n = 7) stage 3a 22.4 ± 4.1% (n = 7) stage 3b 26.2 ± 3.1% (n = 6) stages 4, 5 31.1 ± 4.1% (n = 12) | S-thiolated albumin | ||||
| 633 ± 248 nM | 430 ± 153 nM | S-cysteinylated + S-homocysteinylated LDL Analyses carried out by capillary electrophoresis laser-induced fluorescence detection | [86] | ||
| Concentration | Albumin Concentration g/dL | Additional Information | Reference | ||
|---|---|---|---|---|---|
| HD Patients | Control Group | HD Patients | Control Group | ||
| 178 ± 18 μM (n = 10) *** | 279 ± 12 μM (n = 10) | - | - | - | [77] |
| normoalbuminemic patients 299.28 ± 8.64 μM (n = 18) *** hypoalbuminemic patients 229.00 ± 9.43 μM (n = 18) *** | 428.39 ± 8.61 μM (n = 18) | normoalbuminemic patients 4.2 ± 0.02 hypoalbuminemic patients 2.9 ± 0.03 | - | - | [97] |
| 174.9 ± 10.5 μg/L (n = 32) *** | 286.4 ± 21.8 μg/L (n = 20) | albumin significantly lower in CKD patients compared to healthy controls (p < 0.0001) | - | Dialysis vintage 16 ± 4 months Analyses performed in serum | [78] |
| 280 ± 11 μM (n = 28) | 416 ± 6 μM (n = 49) | 3.78 ± 0.6 | - | - | [98] |
| 170.79 ± 12.31 μM (n = 22) *** | 286.4 ± 21.8 μM (n = 20) | - | - | Dialysis vintage 16 ± 4 months Analyses performed in serum | [99] |
| 164.12 ± 13.54 μM (n = 48) ** | 342.34 ± 43.43 μM (n = 41) | 2.8 ± 0.2 | 4.4 ± 1.3 | Dialysis vintage 16 ± 4 months Analyses performed in serum | [80] |
| Figure 3 (n = §) *** | Figure 3 (n = 24) | - | - | - | [100] |
| 3.72 ± 0.66 μmol/g protein (n = 20) *** | 4.73 ± 0.71 μmol/g protein (n = 20) | 7.37 ± 0.49 (a) | 7.61 ± 0.386 | Dialysis vintage 4.4 ± 1.9 months | [101] |
| 36.0 ± 6.03% (n = 22) | 64.6 ± 0.4% (n = 11) | - | - | Dialysis vintage 1–9 years | [102] |
| 29.7 ± 0.5% | 29.7 ± 0.5% | S-cysteinylated albumin | |||
| 40.4 ± 8.7% (n = 20) ** | 53.6 ± 6.4% (n = 10) | - | - | Dialysis vintage 1–10 years | [103] |
| 49.7 ± 8.0%) ** | 38.7 ± 6.3% | - | - | S-cysteinylated albumin | |
| 825.53 ± 121.08 *** pmol/mg (n = 28) | 139.52 ± 15.43 pmol/mg (n = 14) | - | - | Dialysis vintage > 6 months S-homocysteinylated proteins | [104] |
| mean 0.76 (min–max 0.61–0.88) (b) (n = 71) *** | mean0.43 (min–max 0.40–0.54) (b) (n = 24) | 3.49 ± 0.38 | 4.23 ± 0.31 | PTI | [105] |
| 19.3 ± 4.80 pmol/mg Hb (n = 33) *** | 13.2 ± 2.79 pmol/mg (n = 21) | HbSSG | [66] | ||
| 11.5 (9.6–17.2) pmol/mg Hb (c) *** | 38.3 (29.0–63.3) pmol/mg Hb (c) | HbSSCy | [66] | ||
| Concentration | Albumin Concentration g/dL | Additional Information | Reference | ||
|---|---|---|---|---|---|
| HD Patients | Control Group | HD Patients | Control Group | ||
| pre-HD 268 ± 22 μM post-HD 425 ± 15 μM (n = 11) | 438 ± 16 μM (n = 17) | - | - | Polysulfone membrane Dialysis vintage 49 ± 11 months | [112] |
| pre-HD 265 ± 19 μM post-HD 408 ± 23 μM (n = 11) | 438 ± 16 μM (n = 17) | - | - | Cellulose triacetate membrane Dialysis vintage 49 ± 11 months | [112] |
| pre-HD 312.4 ± 60.5 μM post-HD 435.6 ± 78.9 μM (n = 55) *** | 451.1 ± 90.1 μM (n = 25) | 7.1 ± 0.4 g/L (a) | 6.9 ± 0.6 (a) | Dialysis vintage 86.6 ± 75.1 months | [111] |
| pre-HD 339 (54) μM (b) post-HD 493 (94) μM (b) (n = 19) | 512 (58) μM (a) (n = 17) | - | - | standard bicarbonate hemodialysis | [113] |
| pre-HDF 343 (48) μM (b) post-HDF 529 (110) μM (b) (n = 25) | 512 (58) μM (a) (n = 17) | - | - | hemodiafiltration | [113] |
| pre-HD 9.56 ± 2.5 mol/mg protein post-HD 11.17 ± 2.2 mol/mg protein (n = 10) * | 8.59 ± 0.7 mol/mg protein (n = 9) | - | - | - | [114] |
| pre-HD 254.62 ± 62.1 μM post-HD 258.32 ± 68.2 μM (n = 21) | 213.21 ± 42.0 μM (n = 15) | - | - | S-cysteinylated albumin Dialysis vintage 1–9 years | [115] |
| pre-HD 58 ± 7% post-HD 37 ± 7% (n = 8) ** | 27 ± 4% (n = 10) | 3.77 ± 0.36 | 4.12 ± 0.57 | S-thiolated albumin Dialysis vintage 38.6 ± 71.8 months | [116] |
| pre-HD 0.79 ± 0.21 after-HD from 23.0 to 61.5% decrease (mean 48.17 ± 9.17) (n = 20) | 0.52 ± 0.17 (n = 20) | pre-HD 7.37 ± 0.49 (a) post-HD 7.89 ± 0.72 (a) | 7.61 ± 0.386 (a) | PTI Dialysis vintage 4.4 ± 19 months | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garavaglia, M.L.; Giustarini, D.; Colombo, G.; Reggiani, F.; Finazzi, S.; Calatroni, M.; Landoni, L.; Portinaro, N.M.; Milzani, A.; Badalamenti, S.; et al. Blood Thiol Redox State in Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 2853. https://doi.org/10.3390/ijms23052853
Garavaglia ML, Giustarini D, Colombo G, Reggiani F, Finazzi S, Calatroni M, Landoni L, Portinaro NM, Milzani A, Badalamenti S, et al. Blood Thiol Redox State in Chronic Kidney Disease. International Journal of Molecular Sciences. 2022; 23(5):2853. https://doi.org/10.3390/ijms23052853
Chicago/Turabian StyleGaravaglia, Maria Lisa, Daniela Giustarini, Graziano Colombo, Francesco Reggiani, Silvia Finazzi, Marta Calatroni, Lucia Landoni, Nicola Marcello Portinaro, Aldo Milzani, Salvatore Badalamenti, and et al. 2022. "Blood Thiol Redox State in Chronic Kidney Disease" International Journal of Molecular Sciences 23, no. 5: 2853. https://doi.org/10.3390/ijms23052853
APA StyleGaravaglia, M. L., Giustarini, D., Colombo, G., Reggiani, F., Finazzi, S., Calatroni, M., Landoni, L., Portinaro, N. M., Milzani, A., Badalamenti, S., Rossi, R., & Dalle-Donne, I. (2022). Blood Thiol Redox State in Chronic Kidney Disease. International Journal of Molecular Sciences, 23(5), 2853. https://doi.org/10.3390/ijms23052853









