Abstract
The zinc/iron-regulated transporter-like protein (ZIP) family has a crucial role in Zn homeostasis of plants. Although the ZIP genes have been systematically studied in many plant species, the significance of this family in wild emmer wheat (Triticum turgidum ssp. dicoccoides) is not yet well understood. In this study, a genome-wide investigation of ZIPs genes based on the wild emmer reference genome was conducted, and 33 TdZIP genes were identified. Protein structure analysis revealed that TdZIP proteins had 1 to 13 transmembrane (TM) domains and most of them were predicted to be located on the plasma membrane. These TdZIPs can be classified into three clades in a phylogenetic tree. They were annotated as being involved in inorganic ion transport and metabolism. Cis-acting analysis showed that several elements were involved in hormone, stresses, grain-filling, and plant development. Expression pattern analysis indicated that TdZIP genes were highly expressed in different tissues. TdZIP genes showed different expression patterns in response to Zn deficiency and that 11 genes were significantly induced in either roots or both roots and shoots of Zn-deficient plants. Yeast complementation analysis showed that TdZIP1A-3, TdZIP6B-1, TdZIP6B-2, TdZIP7A-3, and TdZIP7B-2 have the capacity to transport Zn. Overexpression of TdZIP6B-1 in rice showed increased Zn concentration in roots compared with wild-type plants. The expression levels of TdZIP6B-1 in transgenic rice were upregulated in normal Zn concentration compared to that of no Zn. This work provides a comprehensive understanding of the ZIP gene family in wild emmer wheat and paves the way for future functional analysis and genetic improvement of Zn deficiency tolerance in wheat.
1. Introduction
Zinc (Zn) is one of the most essential micronutrients for plants and humans and plays a critical role in diverse biochemical processes [1,2]. Zn is irreplaceable for plant normal growth and development [3]. Both deficient and excess Zn has negative effects on the physiological and biochemical processes of the plant [4]. The Zn availability in agriculture soils of many parts of the world is low, which leads to yield reduction and poor nutritional quality in harvested grains [5]. It was estimated that one-third of the world’s population suffers from inadequate intake of Zn, resulting in various health problems [5,6,7]. A food-based strategy (biofortification) is considered the most cost effective and sustainable option for improvement of human health [8]. Thus, improving the Zn nutrition in crop varieties is an important goal of both public and private breeding programs to overcome the Zn deficiency problem.
To adapt low and fluctuating Zn availability in soil, plants have developed various mechanisms to equipoise the uptake, utilization, and storage of Zn ion. Three metal transporter families, namely, the Zn/iron-regulated transporter-like protein (ZIP), the cation diffusion facilitator (CDF), and the heavy metal ATPase (HMA) have been shown to be involved in Zn homeostasis of plants [9]. Among them, the ZIP family has been validated to be involved in the Zn2+ uptake from soil and the transport of this metal from extracellular or intracellular compartment into the cytoplasm [10].
Several ZIP family transporters have been identified from various plant species [2,11,12]. Most of the ZIP proteins have eight transmembrane (TM) domains and similar membrane topologies. Many members have relatively long variable regions between TM regions III and IV, which underlies the length variations (300 to 480 amino acid residues) of different ZIP proteins. This variable region is histidine-rich and resided in the cytoplasm, which is predicted to be involved in the binding and the transport of metal ions [13,14].
In plants, multiple members of the ZIP family have been shown to respond to Zn deficiency, and some of the Zn deficiency-inducible ZIP genes reported thus far have the ability to transport Zn [12,15,16,17]. For example, the AtZIP1 and AtZIP2 genes played an important role in Mn and Zn translocation from the roots to the shoots in Arabidopsis thaliana [18]. In barley, six HvZIP genes were highly induced by Zn deficiency, which is associated with enhanced uptake and root-to-shoot translocation of Zn under low Zn conditions [8]. The OsZIP1, OsZIP3, OsZIP4, OsZIP5, and OsZIP8 were shown to transport of Zn and three of them (OsZIP4, OsZIP5, and OsZIP8) were involved in response to Zn-deficiency in rice [12,16,19,20]. Evens et al. (2017) identified 14 ZIPs in hexaploid wheat and verified five members (TaZIP3, TaZIP6, TaZIP7, TaZIP9, and TaZIP13) as functional Zn transporters [21].
Wild emmer wheat (Triticum turgidum ssp. dicoccoides, 2n = 4× = 28, AABB), the tetraploid progenitor of domesticated wheat, represents an important genetic source for high-grain Zn, Fe, and protein concentrations [5,22,23,24]. Although wide variation in response to Zn deficiency has been reported in wild emmer, little is known about the ZIP family genes except for TdZIP1, a Zn transporter with higher expression under Zn deficiency, which can complement the function of the zrt1/zrt2 yeast mutant [25]. In the present study, 33 TdZIP genes were identified from the wild emmer wheat genome. The gene structure, expression profiles induced by Zn deficiency, and Zn transport capability of these TdZIP genes were systematically analyzed. The function of TdZP6B-1 was further verified by transgenic rice. The results will improve our understanding of the regulatory roles of TdZIPs in Zn accumulation and will lay a foundation for further functional study and genetic improvement of Zn deficiency tolerance in wheat.
2. Results
2.1. Phylogenetic Relationships and Comparative Analysis of the ZIP Gene Family in Wild Emmer Wheat
A total of 33 putative ZIP genes were identified and confirmed from the wild emmer reference genome. These ZIP genes were tentatively designated as TdZIP1A-1 to TdZIP7B-3 (Table 1) according to their locations on chromosomes. We found that these ZIPs were unevenly distributed on the chromosomes, with 16 and 17 genes positioned on the A and B chromosomes, respectively (Table 1, Figure S1). The first chromosome group had the largest number of TdZIPs (10 genes), followed by the second (7 genes), the sixth (5 genes), and the seventh chromosome groups (5 genes). The remaining chromosomes had one TdZIP gene each (Figure S1).

Table 1.
Information and physicochemical characteristics of the TdZIP genes.
Gene structure analysis revealed that the number of introns varied from 0 to 11 (Figure S2A), and exons ranged from 1 to 12 among the 33 TdZIP genes. The amino acid lengths of the TdZIPs varied from 68 (TdZIP2A-2) to 577 (TdZIP1A-3 and TdZIP1B-3), the PIs ranged from 5.05 (TdZIP2A-2) to 9.8 (TdZIP2A-3), and these proteins had 1 to 13 TM domains. Using the MEME tool, we identified 15 conserved motifs with length varied from 6 to 50 amino acids (Figure S2B). Most of the TdZIP proteins were predicted to be localized on the plasma membrane, except three TdZIPs (TdZIP6A-2, TdZIP6B-2, and TdZIP7B-1) were predicted to be localized on the endoplasmic reticulum (ER) (Table 1), suggesting that these three genes may be responsible for transporting Zn from the ER to the cytoplasm.
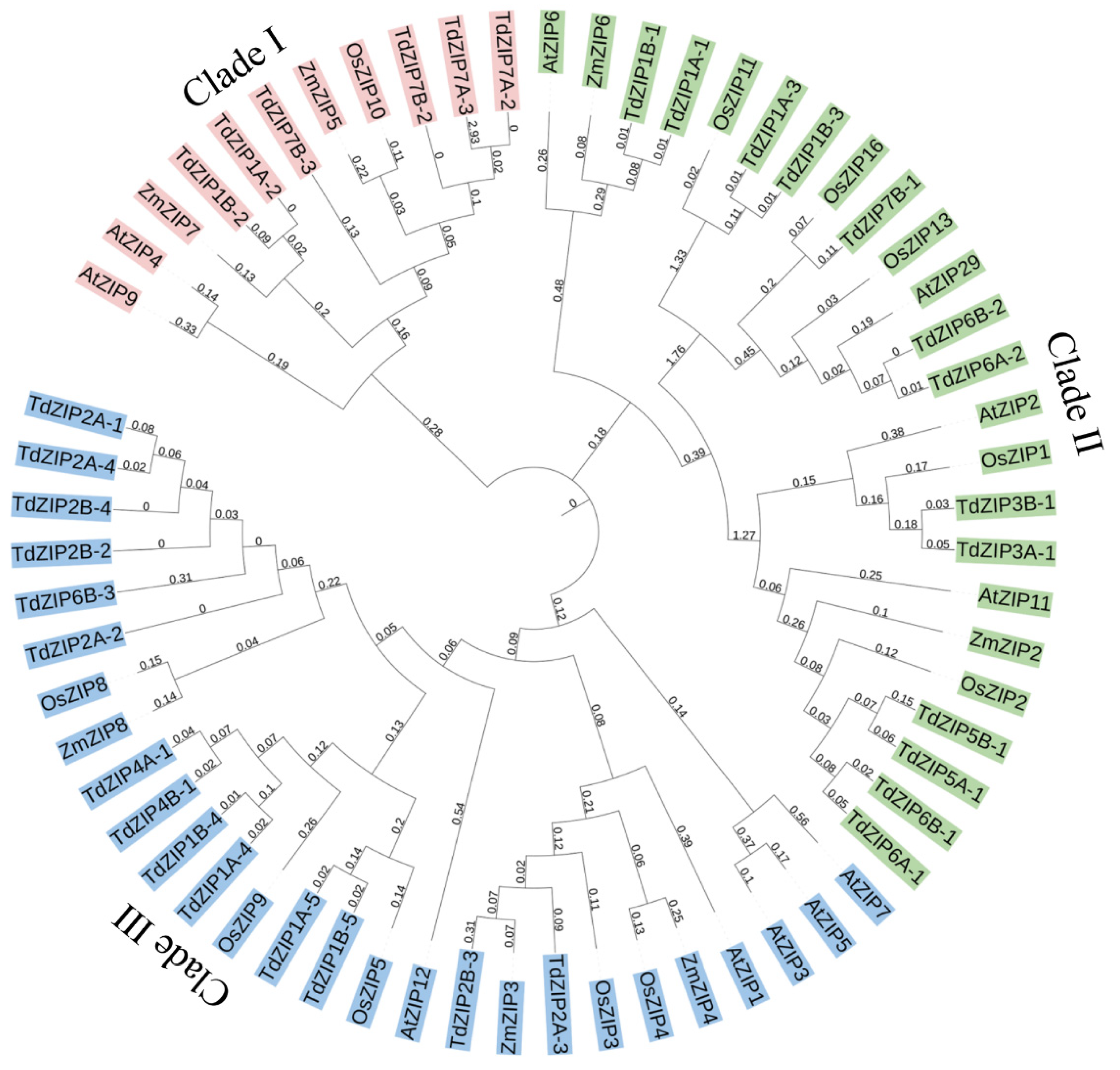
We constructed a phylogenetic tree using 33 TdZIPs and 29 ZIPs from rice, maize, and Arabidopsis to further study the phylogenetic relationships between TdZIPs and other ZIPs in plants. The result is presented in Figure 1. In the phylogenetic tree, these ZIPs were divided into three clades: clade I (1 OsZIP, 2 ZmZIPs, 2 AtZIPs, and 6 TdZIPs), clade II (5 OsZIPs, 2 ZmZIPs, 4 AtZIPs, and 13 TdZIPs), and clade III (5 OsZIPs, 3 ZmZIPs, 5 AtZIPs, and 14 TdZIPs). We further investigated the collinearity relationship of ZIP genes between wild emmer wheat and common wheat. The 33 TdZIPs genes were blasted against the genome of Chinese Spring (CS). Among them, 32 ZIPs had homologous genes/sequences in the CS genome, and 15 genes were able to be detected with 82–100% identities. One gene TdZIP5B-1 was not matched with CS genes (Figure S3, Table S1).
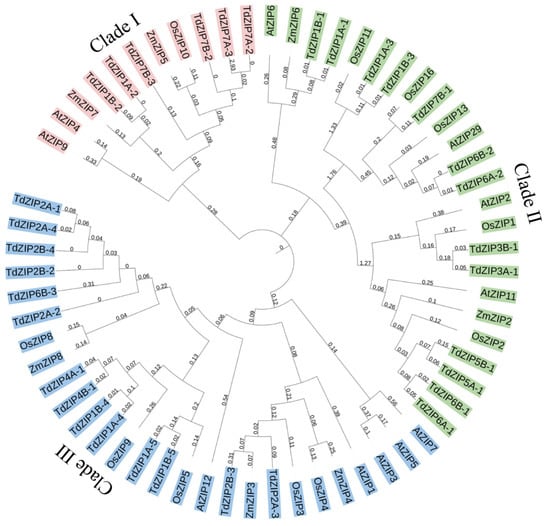
Figure 1.
Phylogenetic relationships of the 33 TdZIPs, 11 OsZIPs, 7 ZmZIPs, and 11 AtZIPs. The subgroups (clades I, II, and III) of the ZIPs were marked in different color.
2.2. Functional Annotation and Promoter Analysis of TdZIP Genes
To investigate the potential functions of ZIPs, we performed GO, KOG, and Swiss_Prot_annotation analyses for the 33 TdZIPs. GO terms for those ZIP genes can be classified into three categories: biological process (BP), cellular component (CC), and molecular function (MF). In the BP annotation, TdZIP proteins predicted their functions in cellular process (GO:0009987), single-organism process (GO:44699), location (GO:0051179), and response to stimulus (GO:0050896). In the CC annotation, TdZIP proteins percentage annotated with the membrane (GO:0016020), membrane part (GO:0044425) and cell (GO:0005623), cell part (GO:0044464), organelle (GO:0043226), and organelle part (GO:0044422). Transporter activity (GO:0005215), catalytic activity (GO:0003824), and binding (GO:0005488) were annotated in the MF annotation (Figure S4A). KOG annotation revealed that all TdZIPs were involved in inorganic ion transport and metabolism (Figure S4B). In addition, Swiss_Prot_annotation showed that most of TdZIPs (27 genes) were orthologous to OsZIP genes in rice (Table S2). The remaining TdZIPs were orthologous to AtZIP genes in Arabidopsis thaliana. These results were consistent with the phylogenetic analysis data.
To further understand the role of TdZIPs, we used the 2000 bp upstream sequence from the translation initiation site (ATG) of TdZIP genes and analyzed in the PlanCARE for cis-element prediction. The results showed that hormone, stresses, and grain-filling, and developmental responsive cis-elements were widely predicted in TdZIPs promoters. In hormone category, abscisic acid (ABRE), MeJA (CGTCA_motif), auxin (TGA_element), salicylic acid (TCA), and gibberellin (GARE_motif) responsive cis-elements were dominant (Table S3). Abscisic acid-responsive element was the most frequent in TdZIPs promoters. Category for stresses had a different type of cis-regulatory elements. For instance, LTR involved in low-temperature responses, MBS in drought-inducibility, ARE in anaerobic induction, and TC_rich_repeats in defense and stress responses (Figure S5). In grain-filling and developmental category, zein metabolism regulation (O2_site) was the most frequent elements in TdZIP promoters, followed by meristem expression (CAT_box), light responsive element (TCCC_motif), seed-specific regulation (RY_element), and endosperm expression (GCN4_motif). These results showed that the TdZIPs may undergo different types of transcriptional regulation and the potential diversity functions of TdZIPs in wild emmer.
2.3. Expression Pattern of TdZIP Genes under Zn-Deficient Stress
To understand the potential expression pattern of TdZIP genes in different tissues, we retrieved the expressions of 33 TaZIPs in different tissues sampled at different time points from public available RNA-seq data of wild emmer [26]. The log2 (TPM + 1) value was used for the heat map display (Figure S6). We found TdZIP genes showed different expression patterns. According to the expression data, 14 TdZIP genes, namely, TdZIP1A-1, TdZIP1A-2, TdZIP1A-3, TdZIP1B-3, TdZIP1B-5, TdZIP2A-2, TdZIP2A-3, TdZIP2B-3, TdZIP6A-2, TdZIP6B-1, TdZIP6B-2, TdZIP6B-3, TdZIP7A-2, and TdZIP7B-2, were highly expressed in different tissues sampled at different time points. Some genes were expressed in all sampled tissues. For example, TdZIP6B-2 had high expression in root, leaf, developing spike, lemma, glume, flower, and grain at most time points. TdZIP1A-1 was highly expressed in leaf, lemma, glume, and grain. In addition, some genes only expressed at specific developmental stages of specific tissues. For example, four genes—TdZIP1A-2, TdZIP1A-3, TdZIP1B-3, and TdZIP6B-2—were highly expressed in roots at 20 days from sowing (DFS). TdZIP2A-3 showed high expression levels at developing stages of spike length 2.5, 4.5, and 5.5 cm as well as in lemma and glume at 112 DFS. TdZIP6B-2 was extremely highly expressed in flower at 105 and 110 DFS. Seven genes (TdZIP1A-1, TdZIP1A-3, TdZIP1B-3, TdZIP2A-4, TdZIP6A-2, TdZIP6B-2, and TdZIP6B-3) showed high expression levels in grain at 123 DFS (Figure S6).
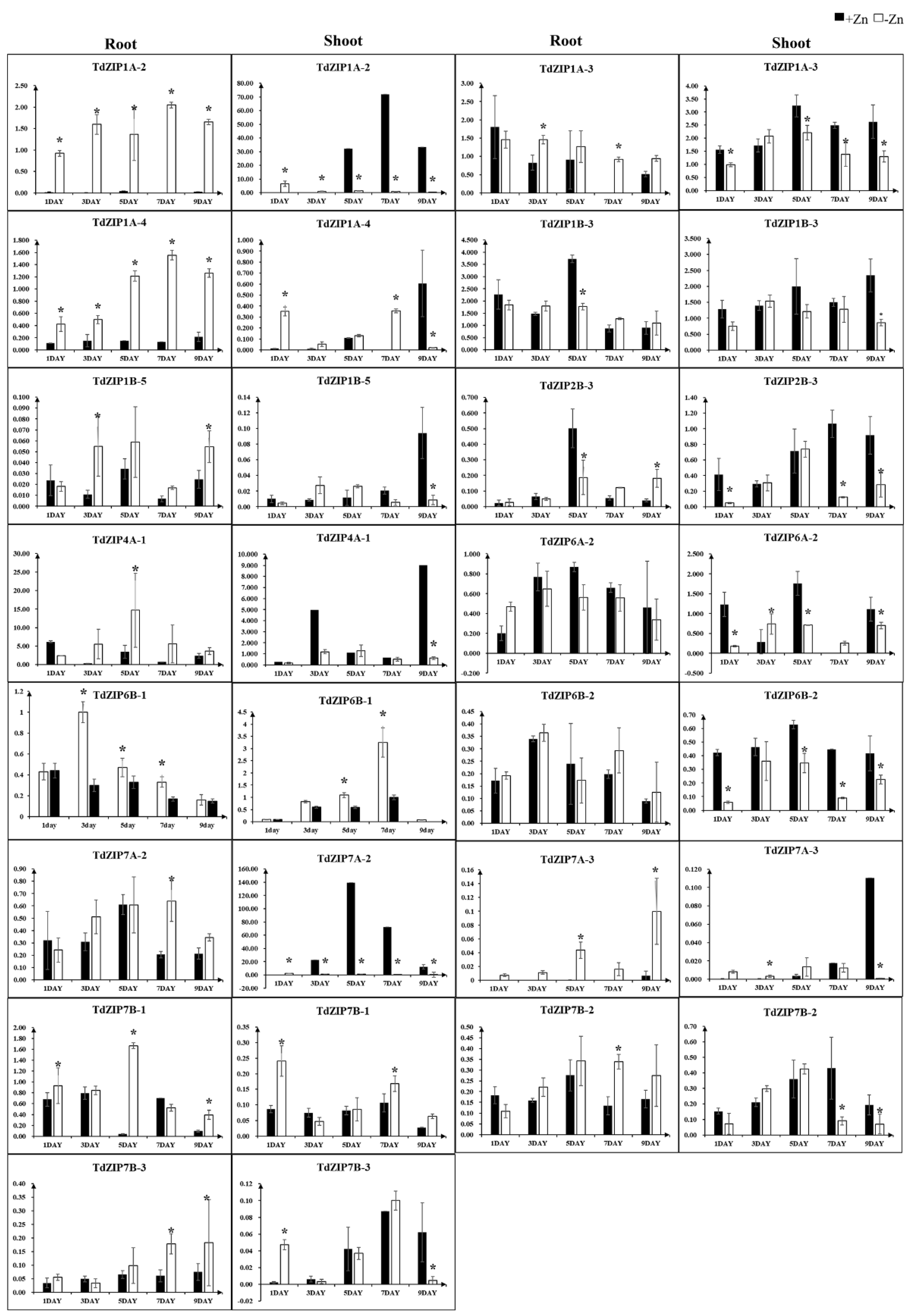
To further investigate the transcript changes of TdZIPs in response to different concentrations of ZnSO4 treatments, we selected 14 TdZIPs represent the three clades of TdZIPs in a phylogenetic tree (Figure 1), and their expression patterns in roots and leaves were analyzed by qRT-PCR under Zn-deficient and normal Zn concentrations (Table S4). Three expression patterns were observed: (i) transcript levels of nine genes (TdZIP1A-2, TdZIP1A-3, TdZIP1B-5, TdZIP2B-3, TdZIP4A-1, TdZIP7A-2, TdZIP7A-3, TdZIP7B-2, and TdZIP7B-3) were significantly upregulated in roots of Zn-deficient plants, while not in leaves; (ii) the expression levels of three genes (TdZIP1A-4, TdZIP6B-1, and TdZIP7B-1) were significantly upregulated in both roots and shoots of Zn-deficient plants; and (iii) three genes (TdZIP1B-3, TdZIP6A-2, and TdZIP6B-2) in both leaves and roots showed little or no response to Zn deficiency or normal Zn concentration (Figure 2).
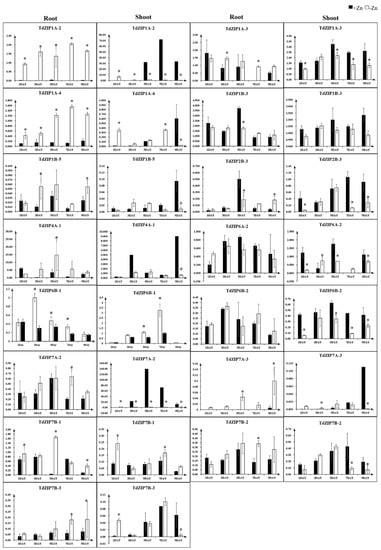
Figure 2.
Expression levels of 15 TdZIP genes in the shoots and roots in response to Zn-deficient stress. Error bars indicate the mean values between three replicates ± standard deviation (SD). * denotes the statistically differences at p < 0.05 (Student’s t-test).
2.4. Yeast Complementation Analysis
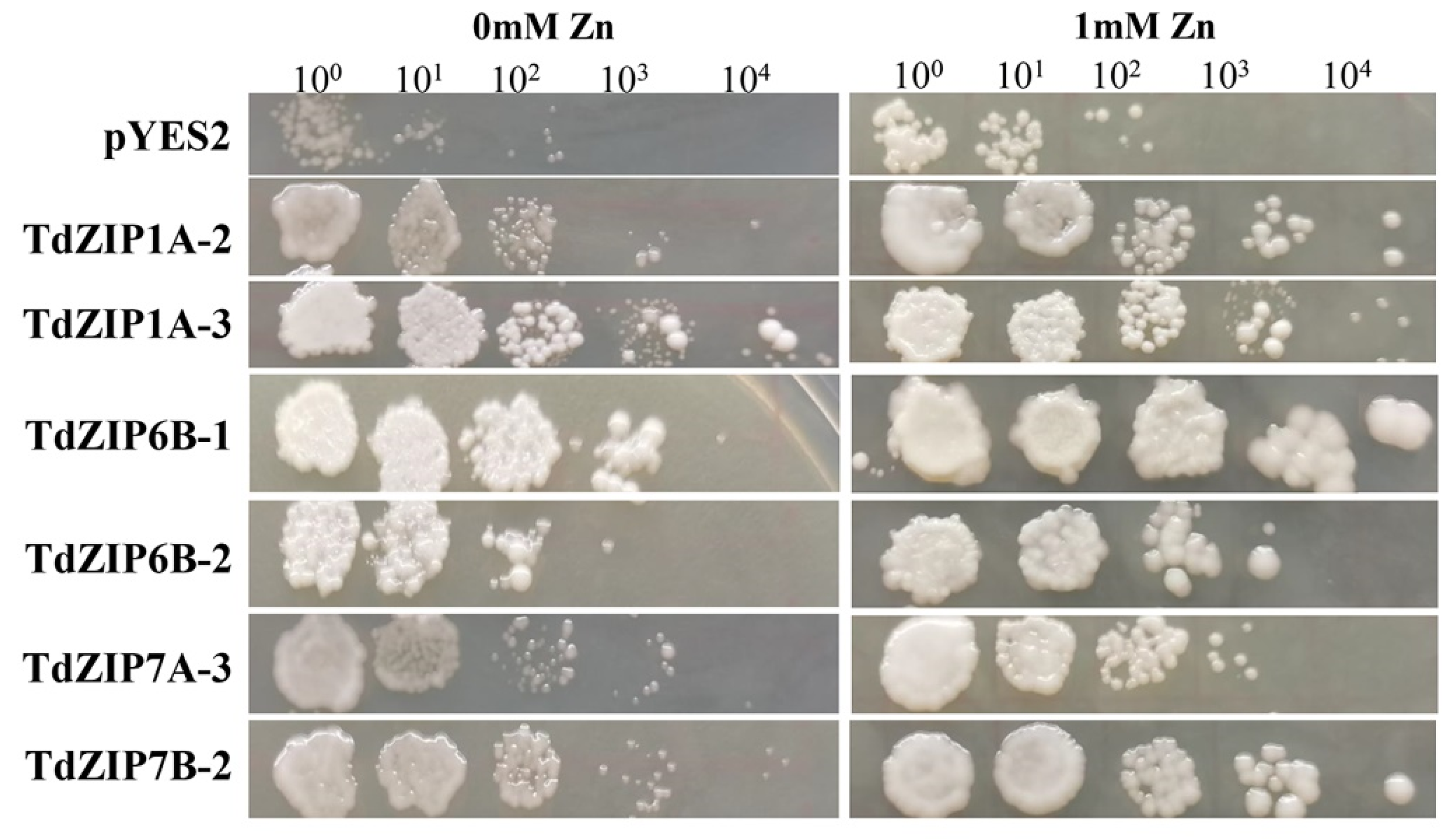
To determine the biological function of TdZIPs, we chose six TdZIP genes (TdZIP1A-2, TdZIP1A-3, TdZIP6B-1, TdZIP6B-2, TdZIP7A-3, and TdZIP7B-2) for yeast complementation analysis. The coding sequences of these genes were cloned and expressed in the wild-type yeast strain DY1457 and extracellular Zn transporter (zrt1/zrt2) double mutant. The zrt1/zrt2 mutant cells transformed with TdZIP genes were grown on SD media containing different Zn concentrations. Among them, cells transformed with TdZIP6B-1 had the strongest growth on SD media with ZnSO4. TdZIP genes TdZIP1A-2, TdZIP1A-3, TdZIP6B-1, and TdZIP7B-2 were able to fully complement and TdZIP6B-2 and TdZIP7A-3 were able to partially complement the growth phenotypes of the double zrt1/zrt2Δ yeast mutant (Figure 3). These results suggest that the six TdZIPs genes have the ability to transport Zn.
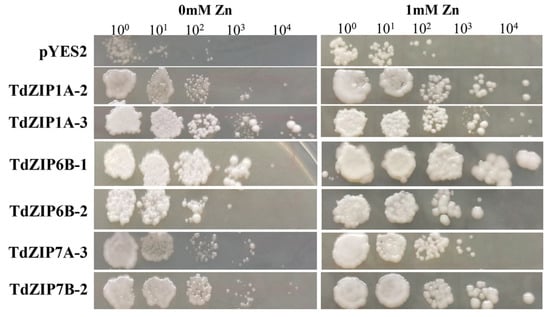
Figure 3.
Complementation of yeast Zn uptake mutant (zrt1/zrt2Δ) with TdZIP genes under different Zn concentrations. The yeast zrt1/zrt2Δ mutant transformed with the empty vector pYES2 was used as a negative control. Each spot represents a 1:10 dilution of the culture starting with an OD of 0.5 on the far left (10-, 100-, 1000-, 10,000-fold dilutions).
2.5. Analysis of Phenotypes and Metal Concentrations in TdZIP6B-1 Overexpression Rice Plants
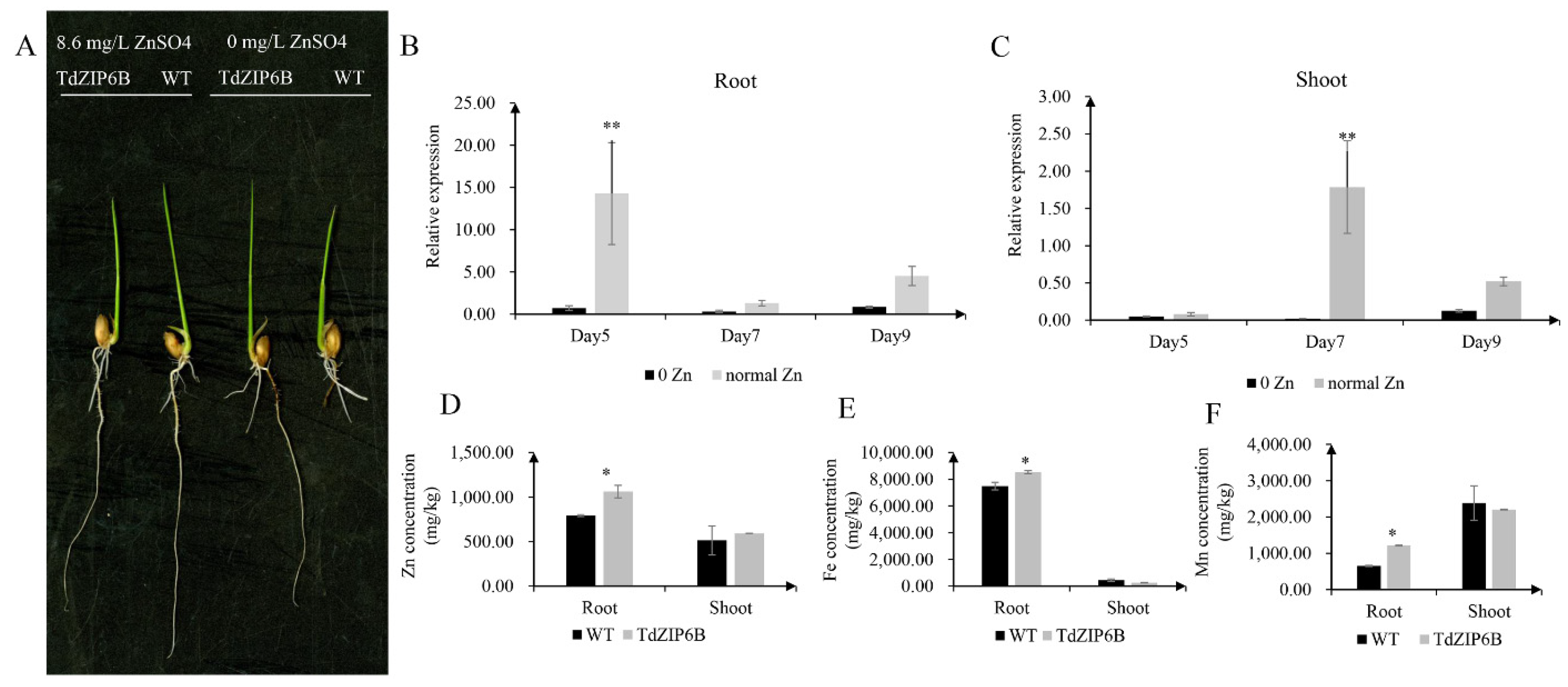
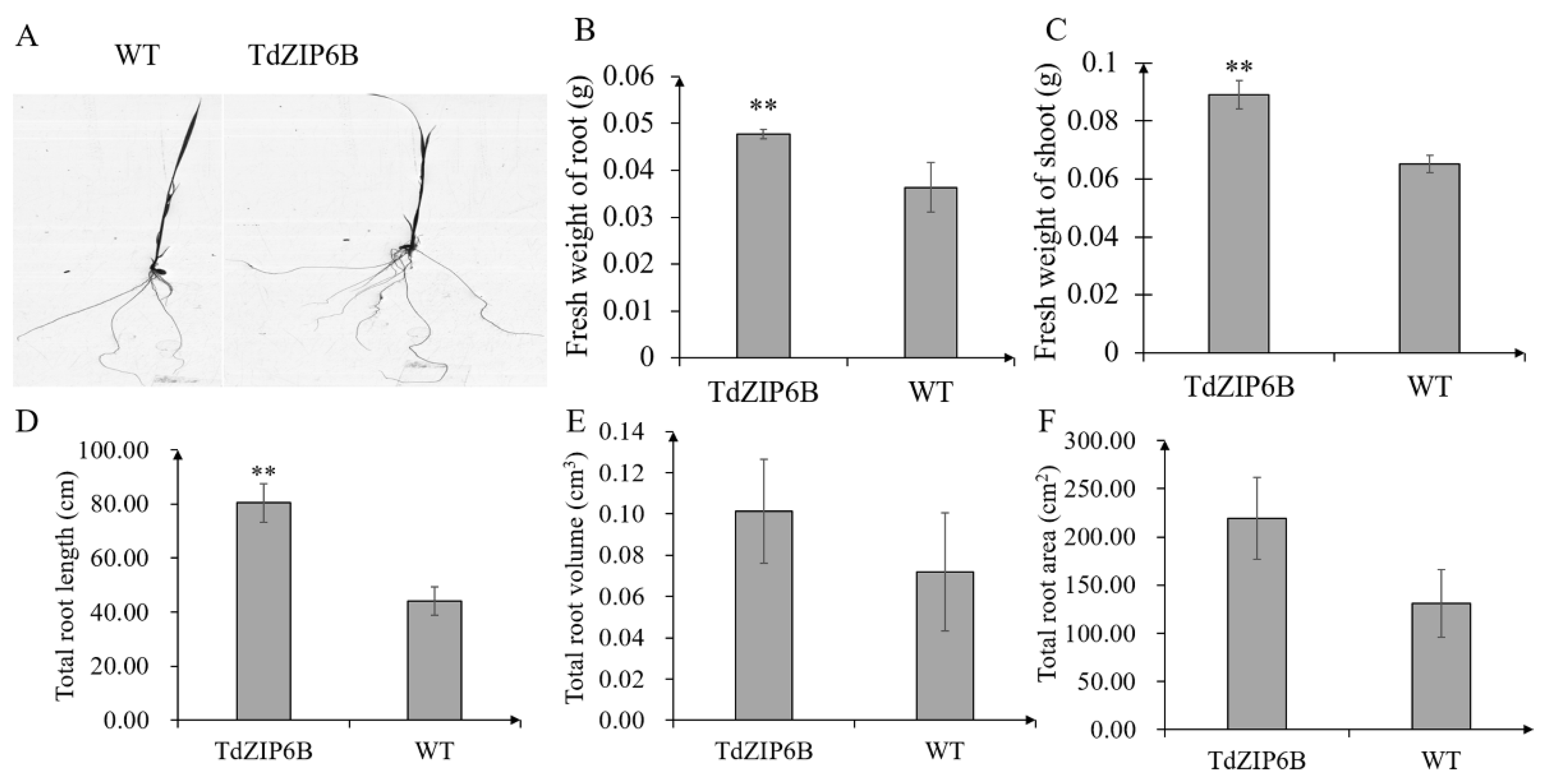
The yeast complementation experiment showed that TdZIP6B-1 has the highest ability to complement the growth phenotypes of the double zrt1/zrt2Δ yeast mutant. The cDNA sequence of TdZIP6B-1 shares 95.77% identity with that of TaZIP6B-1 in CS. TdZIP6B-1 was phylogenetically close to Arabidopsis gene AtZIP11, maize gene ZmZIP2, and rice gene OsZIP2 (Figure 1). We constructed transgenic plants overexpression TdZIP6B-1 to further verify the function of TdZIP6B-1. The TdZIP6B-1 overexpression line (TdZIP6B) together with wild-type (WT) cultivar (Oryza. Sativa L. spp. Japonica) were exposed to no Zn (0 mg/L ZnSO4) and normal Zn conditions (8.6 mg/L ZnSO4) (Figure 4A). The TdZIP6B-1 overexpression lines had higher fresh weight of roots and shoots than those of WT plants after 14 days growth under normal Zn conditions (Figure 5B, C). The total roots length of overexpression lines was significantly longer than that of WT plants (Figure 5A,D). The total roots area and volume were higher than those of WT plants, but the differences were not significant (Figure 5E,F). In roots, the expression levels of TdZIP6B-1 were significantly higher in normal Zn condition than those of in no Zn condition at 5 days post-treatment (Figure 4B). No significant differences were observed for the expression levels of TdZIP6B-1 at 7 and 9 days. In shoots, the expression levels of TdZIP6B-1 were significantly higher in normal Zn condition than those of in no Zn condition at 7 days post-treatment (Figure 4C). We further examined the Zn, Mn, and Fe concentrations in roots and shoots after 14 days of growth under normal Zn condition. The results showed that the transgenic plants had higher Zn, Fe, and Mn concentrations compared with that of WT plants in roots (Figure 4D), while those of in shoots between overexpression lines and WT plants were not significantly different (Figure 4E,F).
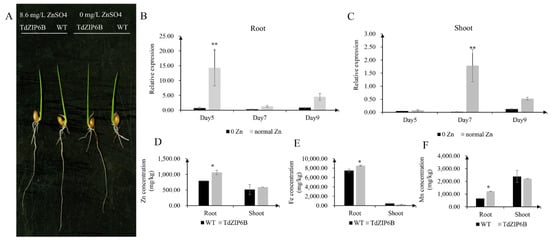
Figure 4.
Comparison of phenotypes and metal concentration between TdZIP6B-1 overexpression lines (TdZIP6B) and WT plants under no Zn (0 mg/L ZnSO4) and normal Zn (8.6 mg/L ZnSO4.7H2O) conditions. (A) Morphology of rice seedlings that were exposed to no Zn and normal Zn for 14 days. (B,C) The expression levels of TdZIP6B-1 in transgenic lines exposed to no Zn and normal Zn conditions for 5, 7, and 9 days. (D–F) The Zn, Fe, and Mn concentrations in roots and shoots of TdZIP6B and WT plants under normal Zn condition at 14 days. Error bars show SE and the symbol * and ** indicate statistical differences (p < 0.05 and p < 0.01, respectively).
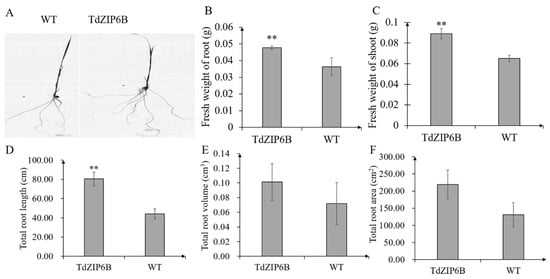
Figure 5.
Seedling phenotypes of TdZIP6B-1 overexpression lines (TdZIP6B) and WT plants exposed to normal Zn condition at 14 days. (A) Root phenotype; (B,C) fresh weight of roots and shoots; (D–F) total roots length, volume, and area. Error bars show SE, and the symbol ** indicates statistical differences (p < 0.01).
3. Discussion
Zn is a micronutrient element that is necessary for all living organisms [8,27]. Zn deficiency in major food crops could lead to yield reduction and poor nutritional quality [2]. Zn homeostasis is tightly regulated by kinds of proteins, with Zn transporter proteins being particularly important [27,28]. The ZIP transporters contribution to Zn homeostasis has been widely reported in many plant species, including common wheat [21]. To our knowledge, however, the ZIP family has not been well studied in wild emmer wheat. In the present study, we performed a genome-wide investigation of ZIP family in wild emmer and identified 33 TdZIP genes. Although most (97%) TdZIPs had homologous genes/sequences in common wheat cv. CS genome, large sequences differences in ZIP genes were detected between wild emmer and CS. These TdZIP genes in wild emmer can provide new candidate genes for improving the nutritional quality of wheat.
The phylogenetic tree of the TdZIPs genes was clustered into three clades. In clade I, six TdZIPs were closely related to OsZIP10, AtZIP4, AtZIP9, ZmZIP5, and ZmZIP7. The OsZIP10 was found to be associated with grain Zn content in rice [20]. A previous study had shown that AtZIP9 complemented a yeast Mn uptake-deficient mutant, while it did not complement the Zn uptake-deficient mutant [29]. ZmZIP5 played an important role in Zn and Fe uptake and root-to-shoot translocation in maize [30]. In clade II, 13 TdZIPs were clustered with OsZIP1, AtZIP2, and AtZIP11, which were previously shown to have ability to transport Zn and response to Zn deficiency [21,29,31]. A total of 14 TdZIPs in clade III were clustered together with OsZIP3, OsZIP4, OsZIP5, OsZIP8, OsZIP9, ZmZIP3, ZmZIP4, ZmZIP8, AtZIP1, AtZIP3, AtZIP5, AtZIP7, and AtZIP12. The OsZIP3, OsZIP4, OsZIP5, OsZIP8, AtZIP1, AtZIP3, AtZIP7, and AtZIP12 had been verified to have ability to transport Zn in plants [11,12,20,29,32,33], while the ZmZIPs could complement the transport of Zn and Fe in yeast mutants [34]. Taken together, these data indicate that the TdZIPs closely related to OsZIP, ZmZIP, and AtZIP genes are likely to have the ability to absorb and transport Zn and Mn and respond to Zn deficiency.
The cis-regulatory elements present in the promoter region have an important role in plant regulation control and in different stimulus responsive genes [35]. On the basis of cis-element analysis, many TdZIPs were predicted to have hormone, stress, grain-filling, and developmentally responsive cis-elements. We have identified different types of cis-elements such as the hormones ABRE, which participates in ABA responses [36]; CGTCA_motif, which has a role in MeJA responses [37]; and TGA_element, which participates in auxin responses [38]. The cis-element for stress responses is LTR, which is involved in low-temperature responses [39]; meanwhile, MBS functions in drought-inducibility [40], ARE is involved in anaerobic induction, and TC_rich_repeats participate in defense and stress responses [41]. For grain-filling and developmental, O2_site has a role in zein metabolism regulation [42], CAT_box functions in meristem expression [38], TCCC_motif participates in light responsiveness [43], RY_element functions in seed-specific regulation [44], and GCN4_motif is involved in endosperm expression [45]. The presence of different types of cis-elements in TdZIPs implies the different transcriptional regulatory mechanisms in which the TdZIPs genes may be involved.
The expression pattern of genes is often tightly correlated with their functions [30]. The expression data of TdZIPs in different tissues at different time points are important for understanding genes’ functions in which they participate. Previous studies have reported that ZIP genes were mainly expressed in roots and shoots of rice, Arabidopsis, and common wheat [29,34,46,47]. In this study, six TdZIPs (TdZIP1A-1, TdZIP1A-2, TdZIP1A-3, TdZIP1B-3, TdZIP6B-2, and TdZIP7B-2) were mainly expressed in root and/or leaf tissues, suggesting their functions in these tissues, which is consistent with previous studies [8,46]. We found some TdZIP genes were highly expressed in developing spike and glume (e.g., TdZIP1B-5, TdZIP2B-3, and TdZIP2A-3), flower (TdZIP6B-3), and grain (TdZIP1A-1 and TdZIP6B-2), suggesting their functions in spike developmental and grain filling processes. Interestingly, TdZIP6B-2 had high expression in most tissues sampled at different time points, implying the potential diverse functions of this gene.
Several ZIP genes related to Zn transportation were upregulated in different tissues under Zn deficiency. For example, OsZIP1 was upregulated in roots and the transcripts were not detected in shoots under Zn deficiency [11,32,46,48]. Zn deficiency induced the expression of HvZIP6 in roots, while it did not in shoots [8]. TaZIP3, TaZIP5, TaZIP6, TaZIP7, and TaZIP13 were upregulated in both roots and shoots under Zn deficiency [21]. In the present study, different expression patterns of TdZIPs in roots and shoots responding to Zn deficiency were determined. We observed that 11 TdZIPs were significantly induced in roots under Zn deficiency, of which three genes (TdZIP1A-4, TdZIP6B-1, and TdZIP7B-1) were highly induced in both roots and shoots of Zn-deficient plants, suggesting that these genes are involved in Zn deficiency responses and enhanced absorbance and root-to-shoot translocation of Zn. Three genes (TdZIP1B-3, TdZIP6A-2, and TdZIP6B-2) showed little or no sensitivity to Zn deficiency or the normal Zn concentration, suggesting that they contribute little to the uptake and root-to-shoot translocation of Zn.
High-affinity Zn transporter Zrt1 is required for yeast growth in Zn-limiting media. Low-affinity transporter Zrt2 can transport Zn under mild or abundant Zn concentrations, whereas it cannot transport Zn under more severe Zn-limiting media [49,50]. Mutations on each of them will lead to yeast growth abnormal under Zn deficiency. Therefore, the yeast zrt1/zrt2Δ mutant has been widely used to demonstrate the complement ability of Zn transporter genes in rice, maize, barely, Arabidopsis, and common wheat under Zn-deficient conditions [18,19,21,51]. In wheat, TdZIP1 and TaZIP3, -5, -6, -7, and -13 have been identified to have the ability to transport Zn by functional complementation study of the zrt1/zrt2 yeast mutant [21,25]. In this study, six genes that belong to three clades of TdZIPs (Figure 3) were randomly selected to rescue the growth defects in the zrt1/zrt2Δ mutant under Zn-deficient conditions. The results revealed that four genes TdZIP1A-2, TdZIP1A-3, TdZIP6B-1, and TdZIP7B-2 rescued the growth defects of the zrt1/zrt2Δ mutant, suggesting that these TdZIPs could transport Zn effectively.
In the present study, we further verified the Zn transport capability of TdZIP6B-1 by rice transformation. We found that the expression levels of TdZIP6B-1 in transgenic rice lines were higher under normal Zn condition than that of no Zn condition in both the roots and shoots. The Zn concentrations in roots of TdZIP6B-1 overexpression lines were higher than the WT plants. Previous reports showed that the overexpression of OsZIP4 and OsZIP5 in rice [12,52] and TaZIP13-B [53] in Arabidopsis could enhance the concentration of Zn in roots and shoots. Our preliminary results verified that the TdZIP6B-1 has the ability to uptake and probably induce root-to-shoot translocation of Zn. In addition, the accumulated Fe and Mn concentrations in roots and shoots of overexpression lines indicate that TdZIP6B-1 may have pleiotropic effects on metal ion absorption in plants.
4. Materials and Methods
4.1. Identification and Bioinformatic Analysis of TdZIP Genes
The wild emmer wheat genome sequences (Triticum_dicoccoides.WEWSeq_v.1.0.dna.toplevel.fa.gz) and annotation profiles (Triticum_dicoccoides.WEWSeq_v.1.0.50.gff3.gz) [26] were downloaded from Ensembl Plant database (ftp://ftp.ensemblgenomes.org/pub/plants/release-50/fasta/triticum_dicoccoides/, accessed on 10 January 2022). The ZIP DNA-binding domain (PF02535.24) downloaded from Pfam protein families database (http://pfam.xfam.org/, accessed on 10 January 2022) was used to identify ZIP genes from the wild emmer genome using HMMER 3.0. The TdZIP proteins and corresponding sequences were acquired from the annotation profiles. The potential TdZIPs were further examined with the Conserved Domain Search (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi/, accessed on 10 January 2022). In addition, ZIP protein sequences from rice, maize, and Arabidopsis were downloaded from Ensembl Plant (http://plants.ensembl.org/index.html/, accessed on 10 January 2022) [54]. All ZIPs were aligned with clustalW (https://www.ebi.ac.uk/Tools/msa/clustalo/, accessed on 10 January 2022) [55], and the resulting alignments were used to construct a phylogenetic tree using the neighbor-joining method with 1000 bootstrap replications and MEGAX software as well as iTOL (https://itol.embl.de/upload.cgi/, accessed on 10 January 2022) [56]. The comparative synteny analysis of ZIPs between wild emmer wheat genome and common wheat CS genome (https://urgi.versailles.inra.fr/blast_iwgsc/, accessed on 10 January 2022) was performed using TBtool software v1.087 [57]. The TdZIP genomic sequences and CDS sequences of wild emmer wheat retrieved from Ensembl Plant database were compared using the gene structure display server program (GSGD, http://gsds.gao-lab.org/, accessed on 10 January 2022) to construct the exon/intron organization of ZIP genes [58]. All the TdZIP genes were mapped to wild emmer wheat chromosomes on the basis of physical location information from the database of wild emmer genome (WEWSeq_v.1.0). Conserved protein motifs were predicted using the MEME Suite web server (https://meme-suite.org/meme/tools/meme, accessed on 10 January 2022), with the maximum number of motifs specified as 15 and the optimum width of motif sets specified as 5 to 200 amino acids [59]. The online ExPASy (https://www.expasy.org/, accessed on 10 January 2022) and SMART sever (http://smart.embl.de/smart/batch.pl, accessed on 10 January 2022) were used to predict the amino acid length, transmembrane (TM) domain, and theoretical isoelectric point (PI) of the TdZIP proteins.
4.2. Gene Annotation and Cis-Element Analysis of TdZIPs
The TdZIP genes were annotated using Gene Ontology (GO) (http://www.geneontology.org/, accessed on 10 January 2022), Clusters of Eukaryotic Groups (KOG) (ftp://ftp.ncbi.nih.gov/pub/COG/KOG, accessed on 10 January 2022), and Swiss_Prot_annotation databases (https://ftp.ebi.ac.uk/pub/databases/swissprot, accessed on 10 January 2022). Cis-element analysis on the promoter regions (2000 bp) upstream of the translation initiation site (ATG) of TdZIP genes was performed using PlantCARE [60]. Microsoft Excel and TBtools were used to conduct statistical analysis and data visualization [57].
4.3. Plant Materials and Zn-Deficient Treatments
The advanced wheat line BAd7-209 (T. aestivum CN16/T. dicoccoides D97 F12) [61] and transgenic rice (Oryza. Sativa L. spp. Japonica) were used in the present study. The seeds were germinated on sterile culture dish with filter paper for 7 days, and seedlings were transplanted into Hogland nutrient solution with two concentrations of Zn, supplemented in the form of ZnSO4, including no Zn (Zn deficiency) and normal Zn supply (8.6 mg/L ZnSO4.7H2O). Leaves and roots of the individuals were harvested at 1, 3, 5, 7, and 9 days after Zn-deficient treatments. All samples were stored at −80 °C for further analysis.
4.4. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
Total RNA from leaf and root samples was isolated using plant extraction kit v1.5 (Biofit Biotechnologies, Chengdu, China). First-strand cDNA synthesis was performed using the TaKaRa PrimeScriptTMRT Reagent Kit (Takara, Dalian, China) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) was performed using the Bio-Rad CFX96 Real-Time PCR System (Bio-Rad, Hercules, CA, USA) in 10 µL reaction volume containing 2 ng of cDNA, 5 µL of 1 × SYBR Premix Ex Taq (TaKaRa), and 0.5 µL (300 nM) of each primer. The GAPDH gene was used as the internal reference. The gene expression was quantified using the 2−ΔΔCT method [62]. Three biological replicates were used for each data point.
4.5. Yeast Complementation Assay
The yeast strains wild-type DY1457 and zrt1/zrt2 (DY1457 + zrt1::LEU2, zrt2::HIS3) mutant were used in this study. Yeast expression vector was pYES2, which has restriction enzyme cutting sites BamHI and EcoRI. The TdZIP6B-1 gene was connected to pYES2 using ClonExpress II One Step Cloning Kit (Vazyme Biotech Co., Ltd., Nanjing, China) and then transformed into Escherichia coli DH5α. The pYES2-TdZIP6B-1 vector was further transformed into yeast strains using EX-Yeast Transformation Kit (ZOMANBIO). Yeast cells were grown in 1% yeast extract, 2% peptone, and 2% glucose (YPD). Low-Zn media (50 µM ZnSO4) and control media (1 mM ZnSO4) were prepared as described by [25]. The pH value was adjusted to 4.2 to enhance the EDTA affinity for Zn. The SD/-Ura liquid medium with galactose was used to select pYES2-TdZIP transformants. The positive transformants were incubated in SD/-ura liquid medium at 30 °C for 4 h. Yeast liquid culture was adjusted to OD600 value of 0.5 using SD-glucose/-ura/-Zn solution. A 1:10 dilution of the culture (1, 10−1, 10−2, 10−3, and 10−4) was made and 3 µL dilution of each was dropped onto different media for 3 days incubation at 30 °C.
4.6. Rice Transformation
The cDNA of TdZIP6B-1 was cloned into the overexpression vector pCAMBIA2300-GFP (pCAMBIA2300-GFP-TdZIP6B-1). The construct had KpnI and SpeI on the 3′ side of the CaMV 35S promoter. An Agrobacterium tumefaciens strain (AGL1) carrying the above construct was used to transform rice (Oryza. Sativa L. spp. japonica) following the method of [11]. The T1 seeds obtained from the transformants were germinated on MS medium containing 50 mg L−1 hygromycin to select resistant plants. In addition, the hygromycin-resistant lines were further confirmed by PCR using gene-specific primer and qRT-PCR to detect the expression of TdZIP6B-1 in transgenic lines. The OsActin gene was used as the internal reference. Homozygous T3 transgenic lines were selected for subsequent experimental analysis.
4.7. Measurement of Metal Concentration
The roots and shoots were sampled and dried at 70 °C for 3 days. The samples were wet-ashed by HNO3 (60%) as described previously [63]. After dilution, the Zn, Fe, and Mn concentrations were determined by inductively coupled plasma atomic emission spectrometry (SPS1200VR; Seiko, Tokyo, Japan) [52]
5. Conclusions
Zn homeostasis is important for plant development and adaptation to diverse stresses. The plant ZIP family proteins play critical role in uptake and transport of Zn ion. In this study, a genome-wide analysis of ZIP family gene in wild emmer was performed. A total of 33 TdZIPs genes were identified and compared with CS TaZIP genes. We found large sequence differences for most of them, and that TdZIP5B-1 could not match TaZIP genes. We performed the phylogenetic, gene structure, chromosomal localization, expression, conserved motif, gene annotation (GO, KOG, and Swiss_Prot_annotation), cis-element, qRT-PCR, and functional analyses of TdZIP genes. TdZIP1A-2, TdZIP1A-3, TdZIP6B-1, and TdZIP7B-2 have the ability to rescue the growth defects of the zrt1/zrt2Δ mutant. The overexpression of TdZIP6B-1 in rice improved the Zn, Fe, and Mn concentrations in roots under normal Zn condition. Our results demonstrate that wild emmer can sever as a valuable reservoir of genetic variation in the study of Zn transporters and lay a significant foundation for future functional analysis and genetic improvement of Zn deficiency tolerance in wheat and TdZIP6B-1 is a candidate Zn transporter that responsible for absorption and probably root-to-shoot translocation of Zn.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23052866/s1.
Author Contributions
F.G.: formal analysis, writing—original draft preparation. T.Q.: investigation. Y.H.: investigation. Y.J.: investigation. J.L.: investigation. W.W.: investigation. J.H.: investigation. B.T.: resources. T.Z.: investigation. B.J.: investigation. Y.W.: resources. L.Z.: investigation. Y.Z.: supervision. D.L.: supervision. L.H.: writing, editing. B.W.: conceptualization, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the International Cooperation Program of Science and Technology Department of Sichuan Province (2021YFH0110) and the Key Research and Development Program of Sichuan Province, China (2021YFYZ0002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Haydon, M.J.; Cobbett, C.S. Transporters of ligands for essential metal ions in plants. New Phytol. 2007, 174, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Krithika, S.; Balachandar, D. Expression of zinc transporter genes in rice as influenced by zinc-solubilizing Enterobacter cloacae strain ZSB14. Front. Plant Sci. 2016, 7, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Ajeesh Krishna, T.P.; Maharajan, T.; Victor Roch, G.; Ignacimuthu, S.; Antony Ceasar, S. Structure, function, regulation and phylogenetic relationship of ZIP family transporters of plants. Front. Plant Sci. 2020, 11, 662. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Kumssa, D.B.; Joy, E.J.M.; Ander, E.L.; Watts, M.J.; Young, S.D.; Walker, S.; Broadley, M.R. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci. Rep. 2015, 5, 10974. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Huang, L.; Wang, C.Q.; Liu, Y.X.; Yan, Z.H.; Wang, Z.Z.; Xiang, L.; Zhong, X.Y.; Gong, F.Y.; Zheng, Y.L.; et al. Genome-wide association study reveals novel genomic regions associated with high grain protein content in wheat lines derived from wild emmer wheat. Front. Plant Sci. 2019, 10, 464. [Google Scholar] [CrossRef]
- Tiong, J.; McDonald, G.; Genc, Y.; Shirley, N.; Langridge, P.; Huang, C.Y. Increased expression of six ZIP family genes by zinc (Zn) deficiency is associated with enhanced uptake and root-to-shoot translocation of Zn in barley (Hordeum vulgare). New Phytol. 2015, 207, 1097–1109. [Google Scholar] [CrossRef]
- Eide, D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta 2006, 1763, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Olsen, L.I.; Palmgren, M.G. Many rivers to cross: The journey of zinc from soil to seed. Front. Plant Sci. 2014, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, Y.; Suzuki, M.; Kobayashi, T.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsZIP4, a novel zinc-regulated zinc transporter in rice. J. Exp. Bot. 2005, 56, 3207–3214. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jeong, H.J.; Kim, S.A.; Lee, J.; Guerinot, M.L.; An, G. OsZIP5 is a plasma membrane zinc transporter in rice. Plant Mol. Biol. 2010, 73, 507–517. [Google Scholar] [CrossRef]
- Guerinot, M.L. The ZIP family of metal transporters. Biochim. Biophys. Acta-Biomembr. 2000, 1465, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Pence, N.S.; Larsen, P.B.; Ebbs, S.D.; Letham, D.L.; Lasat, M.M.; Garvin, D.F.; Kochian, L.V. The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc. Natl. Acad. Sci. USA 2000, 97, 4956–4960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, A.; Yamaji, N.; Mitani-Ueno, N.; Kashino, M.; Ma, J.F. A node-localized transporter OsZIP3 is responsible for the preferential distribution of Zn to developing tissues in rice. Plant J. 2015, 84, 374–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.T.; Zhu, Y.X.; Fan, T.; Peng, C.; Wang, J.R.; Sun, L.; Chen, C.Y. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Biophys. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef]
- Yang, X.; Huang, J.; Jiang, Y.; Zhang, H.S. Cloning and functional identification of two members of the ZIP (Zrt, Irt-like protein) gene family in rice (Oryza sativa L.). Mol. Biol. Rep. 2009, 36, 281–287. [Google Scholar] [CrossRef]
- Grotz, N.; Fox, T.; Connolly, E.; Park, W.; Guerinot, M.L.; Eide, D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. USA 1998, 95, 7220–7224. [Google Scholar] [CrossRef] [Green Version]
- Kavitha, P.G.; Kuruvilla, S.; Mathew, M.K. Functional characterization of a transition metal ion transporter, OsZIP6 from rice (Oryza sativa L.). Plant Physiol. Biochem. 2015, 97, 165–174. [Google Scholar]
- Maurya, S.; Vishwakarma, A.K.; Dubey, M.; Shrivastava, P.; Shrivastava, R.; Chandel, G. Developing gene-tagged molecular marker for functional analysis of OsZIP10 metal transporter gene in rice. Indian J. Genet. Plant Breed. 2018, 78, 180–186. [Google Scholar] [CrossRef]
- Evens, N.P.; Buchner, P.; Williams, L.E.; Hawkesford, M.J. The role of ZIP transporters and group F bZIP transcription factors in the Zn-deficiency response of wheat (Triticum aestivum). Plant J. 2017, 92, 291–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cakmak, I.; Torun, A.; Millet, E.; Feldman, M.; Fahima, T.; Korol, A.; Nevo, E.; Braun, H.J.; Ozkan, H. Triticum dicoccoides: An important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci. Plant Nutr. 2004, 50, 1047–1054. [Google Scholar] [CrossRef] [Green Version]
- Uauy, C.; Brevis, J.C.; Dubcovsky, J. The high grain protein content gene Gpc-B1 accelerates senescence and has pleiotropic effects on protein content in wheat. J. Exp. Bot. 2006, 57, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, L.; Li, T.X.; Liu, Y.X.; Yan, Z.H.; Tang, G.; Zheng, Y.L.; Liu, D.C.; Wu, B.H. Genome-wide association study for grain micronutrient concentrations in wheat advanced lines derived from wild emmer. Front. Plant Sci. 2021, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Coruh, C.; Dinler, G.; Grusak, M.A.; Peleg, Z.; Saranga, Y.; Yazici, A.; Ozturk, L.; Cakmak, I.; Budak, H. Expression and cellular localization of ZIP1 transporter under zinc deficiency in wild emmer wheat. Plant Mol. Biol. Rep. 2011, 29, 582–596. [Google Scholar] [CrossRef]
- Avni, R.; Nave, M.; Barad, O.; Baruch, K.; Twardziok, S.O.; Gundlach, H.; Hale, I.; Mascher, M.; Spannagl, M.; Wiebe, K.; et al. Wild emmer genome architecture and diversity elucidate wheat evolution and domestication. Science 2017, 357, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Baltaci, A.K.; Yuce, K. Zinc transporter proteins. Neurochem. Res. 2017, 43, 517–530. [Google Scholar] [CrossRef]
- Hojyo, S.; Fukada, T. Zinc transporters and signaling in physiology and pathogenesis. Arch. Biochem. Biophys. 2016, 611, 43–50. [Google Scholar] [CrossRef]
- Milner, M.; Seamon, J.; Craft, E.; Kochain, L. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liu, X.; Zhou, X.; Li, Y.; Yang, W.; Chen, R. Improving zinc and iron accumulation in maize grains using the zinc and iron transporter ZmZIP5. Plant Cell Physiol. 2019, 9, 2077–2085. [Google Scholar] [CrossRef]
- Liu, X.S.; Feng, S.J.; Zhang, B.Q.; Wang, M.Q.; Yang, Z.M. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019, 19, 283. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Shin, R.; Eide, D.J.; Schachtman, D.P. Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol. 2003, 133, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, S.A.; Lee, J.; Guerinot, M.L.; An, G. Zinc deficiency inducible OsZIP8 encodes a plasma membrane-localized zinc transporter in rice. Mol. Cells 2010, 29, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, X.; Huang, Y.; Zhu, L.; Zhang, S.; Zhao, Y.; Guo, J.; Chen, J.; Chen, R. Identification and characterization of the zinc-regulated transporters, iron-regulated transporter-like protein (ZIP) gene family in maize. BMC Plant Biol. 2013, 13, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, M.W.; Lu, J.; Shah, L.; Yang, L.; Chen, C.; Mei, X.D.; Xue, L.; Manzoor, M.A.; Abdullah, M.; Rehman, S.; et al. Expansion and molecular characterization of AP2/ERF gene family in wheat (Triticum aestivum L.). Front. Genet. 2021, 12, 632155. [Google Scholar] [CrossRef] [PubMed]
- Pla, M.; Vilardell, J.; Guiltinan, M.J.; Marcotte, W.R.; Pagès, M. The cis-regulatory element CCACGTGG is involved in ABA and water-stress responses of the maize gene rab28. Plant Mol. Biol. 1993, 21, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.H.; Wang, B.A.; Jiang, Y.N.; Cheng, L.J.; Wu, T.L. GmFNSII-controlled soybean flavone metabolism responds to abiotic stresses and regulates plant salt tolerance. Plant Cell Physiol. 2014, 55, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Qiu, G. Cloning and activity analysis of the promoter of nucleotide exchange factor gene ZjFes1 from the seagrasses zostera japonica. Sci. Rep. 2020, 10, 17291. [Google Scholar] [CrossRef]
- Jenner, C.F. Starch synthesis in the kernel of wheat under high temperature conditions. Aust. J. Plant Physiol. 1994, 21, 791. [Google Scholar] [CrossRef]
- Manzoor, M.A.; Cheng, X.; Li, G.; Su, X.; Abdullah, M.; Cai, Y. Gene structure, evolution and expression analysis of the P-ATPase gene family in Chinese pear (Pyrus bretschneideri). Comput. Biol. Chem. 2020, 88, 107346. [Google Scholar] [CrossRef]
- Almasia, N.I.; Narhirñak, V.; Hopp, H.E.; Vazquez-Rovere, C. Isolation and characterization of the tissue and development-specific potato snakin-1 promoter inducible by temperature and wounding. Electron. J. Biotechnol. 2010, 13, 8–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, J.; Ji, C.; Wu, Y.; Messing, J. NAC-type transcription factors regulate accumulation of starch and protein in maize seeds. Proc. Natl. Acad. Sci. USA 2019, 116, 11223–11228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.Y.; Xuan, J.P.; Jia, X.D.; Liu, Y.Z.; Wang, X.; Guo, Z.R. Isolation and expression analysis of MdAPETALA2 gene from Fuji apple. Acta Bot. Boreali-Occident. Sin. 2012, 32, 1309–1315. [Google Scholar]
- Nakabayashi, K.; Okamoto, M.; Koshiba, T.; Kamiya, Y.; Nambara, E. Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: Epigenetic and genetic regulation of transcription in seed. Plant J. 2010, 41, 697–709. [Google Scholar] [CrossRef]
- Yoshihara, T.; Washida, H.; Takaiwa, F. Assessment of common regulatory regions required for the endosperm-specific expression of rice storage protein glutelin genes by hybrid promoters. Plant Sci. 1996, 121, 63–73. [Google Scholar] [CrossRef]
- Chen, W.R.; Feng, Y.; Chao, Y.E. Genomic analysis and expression pattern of OsZIP1, OsZIP3, and OsZIP4 in two rice (Oryza sativa L.) genotypes with different zinc efficiency. Russ. J. Plant Physiol. 2008, 55, 400–409. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. The node a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci. 2014, 19, 556–563. [Google Scholar] [CrossRef]
- Ramegowda, Y.; Venkategowda, R.; Jagadish, P.; Govind, G.; Hanumanthareddy, R.R.; Makarla, U.; Guligowda, S.A. Expression of a rice Zn transporter, OsZIP1, increases Zn concentration in tobacco and finger millet transgenic plants. Plant Biotechnol. Rep. 2013, 7, 309–319. [Google Scholar] [CrossRef]
- Zhao, H.; Eide, D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc. Natl. Acad. Sci. USA 1996, 93, 2454–2458. [Google Scholar] [CrossRef] [Green Version]
- Bird, A.J.; Blankman, E.; Stillman, J.; Eide, D.J.; Winge, D.R. The Zap1 transcriptional activator also acts as a repressor by binding downstream of the TATA box in ZRT2. EMBO J. 2004, 23, 1123–1132. [Google Scholar] [CrossRef] [Green Version]
- Pedas, P.; Schjoerring, J.K.; Husted, S. Identification and characterization of zinc-starvation-induced ZIP transporters from barley roots. Plant Physiol. Biochem. 2009, 47, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Yasuhiro, I.; Hiroshi, M.; Motofumi, S.; Khurram, B.; Michiko, T.; Hiromi, N.; Satoshi, M.; Nishizawa, N.K. Overexpression of the OsZIP4 zinc transporter confers disarrangement of zinc distribution in rice plants. J. Exp. Bot. 2007, 58, 2909. [Google Scholar]
- Li, S.; Liu, Z.H.; Guo, L.L.; Li, H.J.; Nie, X.J.; Chai, S.C.; Zheng, W.J. Genome-wide identification of ZIP gene family members in wheat. Front. Plant Sci. 2021, 12, 2481. [Google Scholar]
- Flicek, P.; Amode, M.R.; Barrell, D.; Beal, K.; Fitzgerald, S. Ensembl 2012. Nucleic Acids Res. 2012, 40, D84–D90. [Google Scholar] [CrossRef] [Green Version]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [Green Version]
- Bailey, T.L.; Mikael, B.; Buske, F.A.; Martin, F.; Grant, C.E.; Luca, C.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, 202–208. [Google Scholar] [CrossRef]
- Rombauts, S.; Dehais, P.; Van Montagu, M.; Rouze, P. Plantcare, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef] [Green Version]
- Gong, F.Y.; Qi, T.G.; Zhang, T.; Lu, Y.S.; Liu, J.; Zhong, X.Y.; He, J.S.; Li, Y.F.; Zheng, Y.L.; Liu, D.C.; et al. Comparison of the Agronomic, Cytological, Grain Protein Characteristics, as Well as Transcriptomic Profile of Two Wheat Lines Derived from Wild Emmer. Front. Genet. 2022, 12, 804481. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Yamaji, Y.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).