Role of Nuclear-Receptor-Related 1 in the Synergistic Neuroprotective Effect of Umbilical Cord Blood and Erythropoietin Combination Therapy in Hypoxic Ischemic Encephalopathy
Abstract
1. Introduction
2. Results
2.1. Comprehensive Analysis of Gene Expression Profiles in Each Treatment Sample after HIE
2.2. Identified Pathways of Genes Upregulated in UCB+EPO Compared with HIE
2.3. Temporal Gene Expression Profiling the Effect of EPO or UCB or UCB+EPO Combination Treatment on the HIE Model
2.4. Gene Enrichment Analyses of the Effect of EPO or UCB or UCB+EPO Combination Treatments on the HIE Model
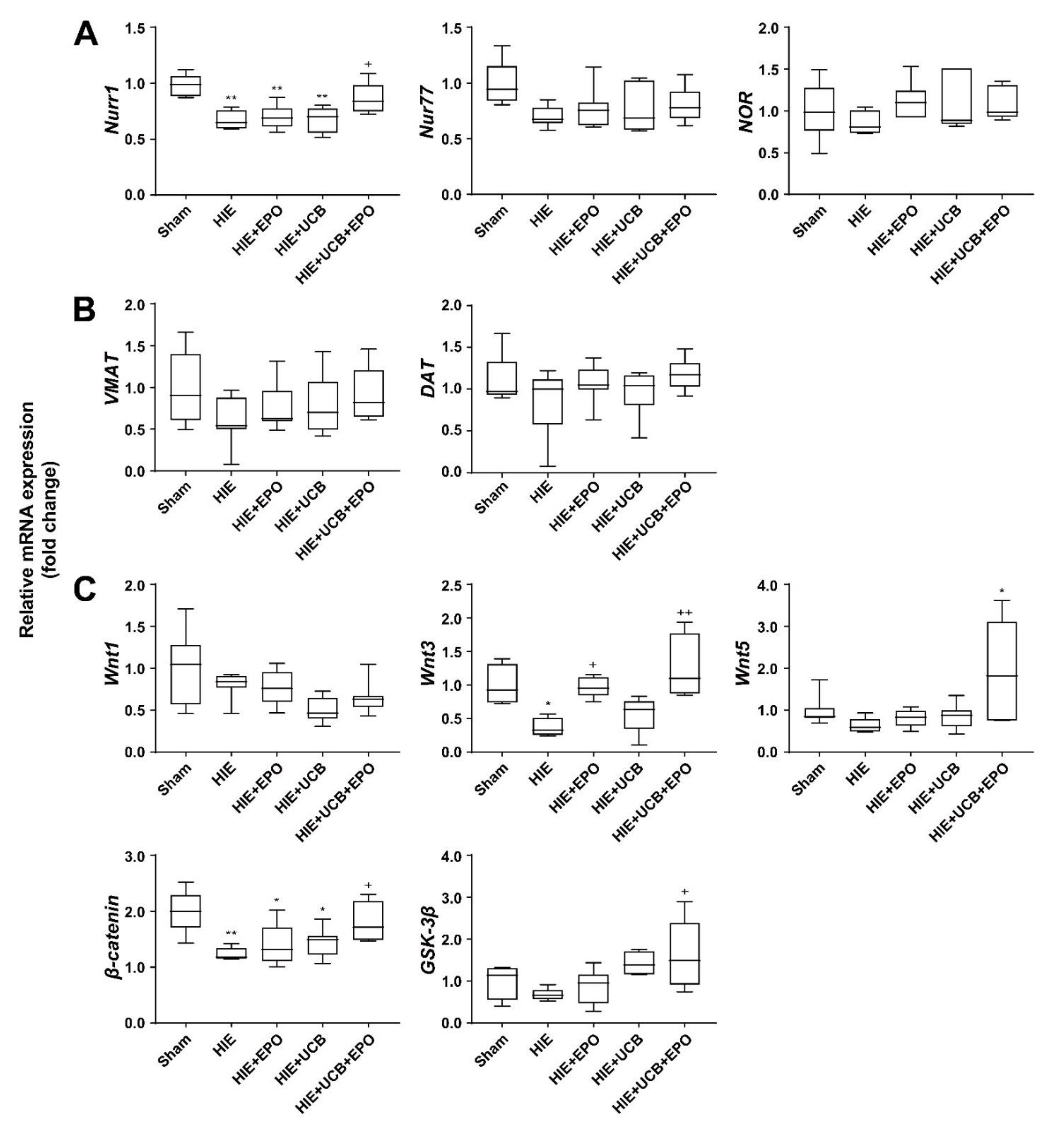
2.5. Neuroprotective Effect of UCB+EPO Treatment via the Activation of Nurr1 Which Is Related to the Wnt/β-Catenin Pathway
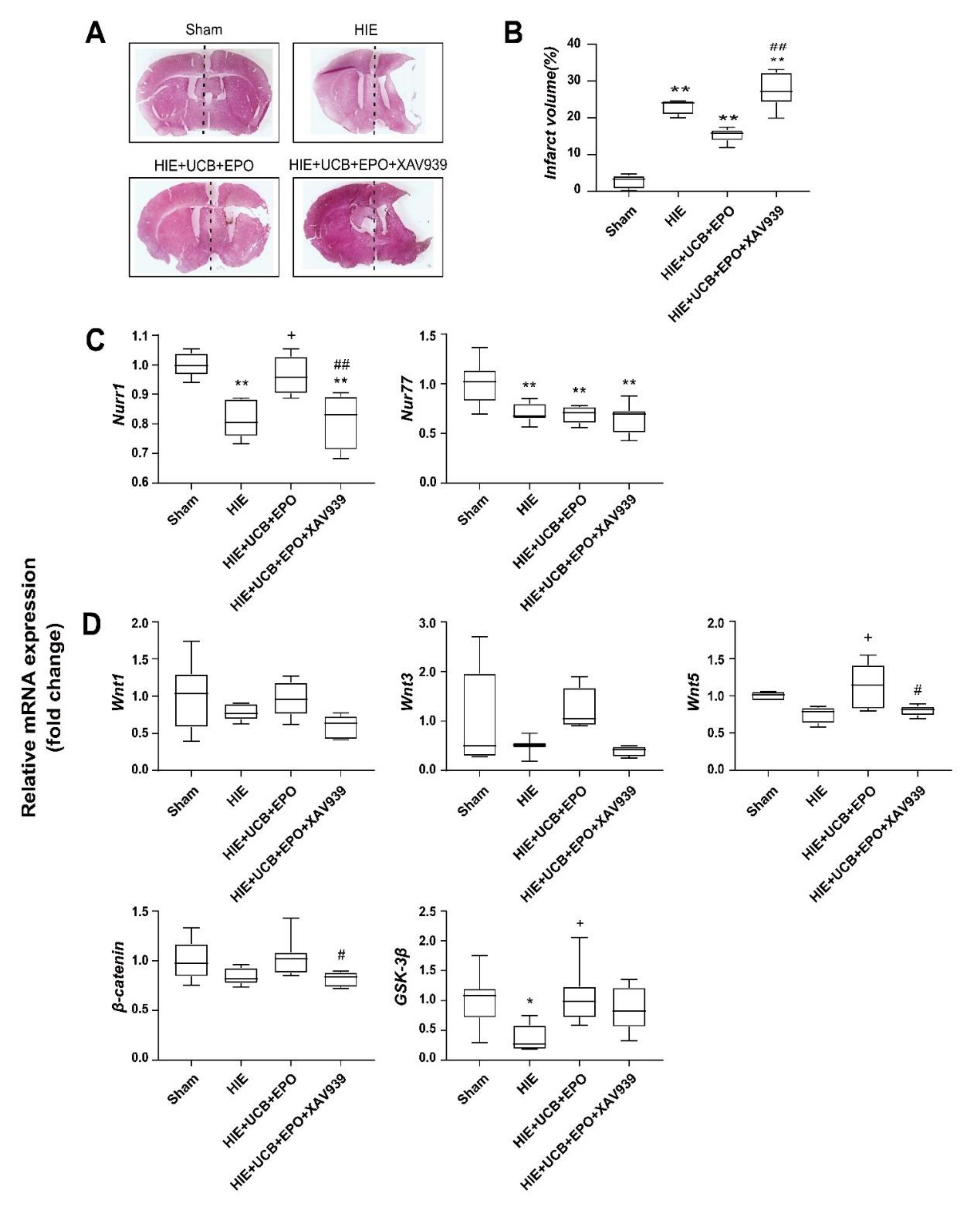
2.6. Countervailed Neuroprotective Effect of UCB+EPO Combination Treatment by Inhibition of the Wnt/β-Catenin Pathway
2.7. Analysis for Evaluating Effect Caused by Gender Difference
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Hypoxic Ischemic Encephalopathy (HIE) Model
4.3. Human Umbilical Cord Blood (UCB) Isolation
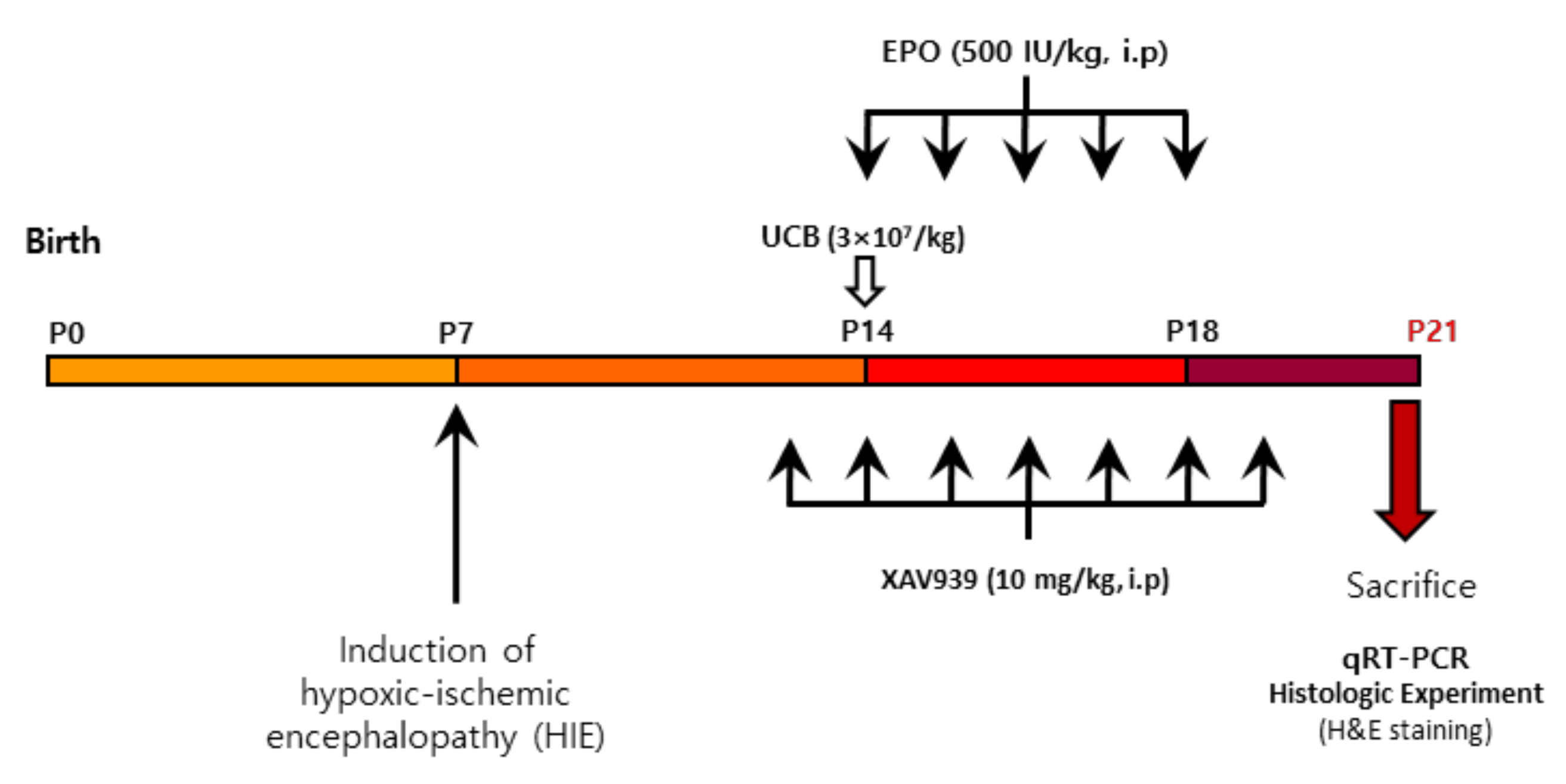
4.4. Experimental Design
4.5. Infarct Volume Measurement
4.6. Microarray Analysis
4.7. Principal Component Analysis
4.8. Analysis of Differentially Expressed Genes and Hierarchical Clustering
4.9. Gene Enrichment Analysis
4.10. Total RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, M.-C.; Yang, S.-N. Perinatal hypoxic-ischemic encephalopathy. J. Biomed. Biotechnol. 2010, 2011, 609813. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.A.; Brandon, D.H. Hypoxic ischemic encephalopathy: Pathophysiology and experimental treatments. Newborn Infant Nurs. Rev. 2011, 11, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, S.J.; Hagberg, H. Hypoxia–ischemia in the immature brain. J. Exp. Biol. 2004, 207, 3149–3154. [Google Scholar] [CrossRef] [PubMed]
- Tetorou, K.; Sisa, C.; Iqbal, A.; Dhillon, K.; Hristova, M. Current Therapies for Neonatal Hypoxic–Ischaemic and Infection-Sensitised Hypoxic–Ischaemic Brain Damage. Front. Synaptic Neurosci. 2021, 13, 709301. [Google Scholar] [CrossRef]
- Fatemi, A.; Wilson, M.A.; Johnston, M.V. Hypoxic-ischemic encephalopathy in the term infant. Clin. Perinatol. 2009, 36, 835–858. [Google Scholar] [CrossRef]
- Michael-Asalu, A.; Taylor, G.; Campbell, H.; Lelea, L.-L.; Kirby, R.S. Cerebral palsy: Diagnosis, epidemiology, genetics, and clinical update. Adv. Pediatrics 2019, 66, 189–208. [Google Scholar] [CrossRef]
- Gonzales-Portillo, G.S.; Reyes, S.; Aguirre, D.; Pabon, M.M.; Borlongan, C.V. Stem cell therapy for neonatal hypoxic-ischemic encephalopathy. Front. Neurol. 2014, 5, 147. [Google Scholar] [CrossRef]
- Sato, Y.; Ueda, K.; Kondo, T.; Hattori, T.; Mikrogeorgiou, A.; Sugiyama, Y.; Suzuki, T.; Yamamoto, M.; Hirata, H.; Hirakawa, A. Administration of bone marrow-derived mononuclear cells contributed to the reduction of hypoxic-ischemic brain injury in neonatal rats. Front. Neurol. 2018, 9, 987. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, L.; Qu, Y.; Xiao, G.; Li, S.; Bao, S.; Lu, Q.R.; Mu, D. Mesenchymal stem cells protect against hypoxia-ischemia brain damage by enhancing autophagy through brain derived neurotrophic factor/mammalin target of rapamycin signaling pathway. Stem Cells 2018, 36, 1109–1121. [Google Scholar] [CrossRef]
- Eggenberger, S.; Boucard, C.; Schoeberlein, A.; Guzman, R.; Limacher, A.; Surbek, D.; Mueller, M. Stem cell treatment and cerebral palsy: Systemic review and meta-analysis. World J. Stem Cells 2019, 11, 891. [Google Scholar] [CrossRef]
- Yang, L.; Qian, J.; Yang, B.; He, Q.; Wang, J.; Weng, Q. Challenges and Improvements of Novel Therapies for Ischemic Stroke. Front. Pharmacol. 2021, 12, 2209. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Kim, E.H.; Cha, J.M.; Moon, G.J. Adult stem cell therapy for stroke: Challenges and progress. J. Stroke 2016, 18, 256. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-K.; Cho, S.-R. Exploring erythropoietin and G-CSF combination therapy in chronic stroke patients. Int. J. Mol. Sci. 2016, 17, 463. [Google Scholar] [CrossRef] [PubMed]
- Larpthaveesarp, A.; Pathipati, P.; Ostrin, S.; Rajah, A.; Ferriero, D.; Gonzalez, F.F. Enhanced Mesenchymal Stromal Cells or Erythropoietin Provide Long-Term Functional Benefit After Neonatal Stroke. Stroke 2021, 52, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Esneault, E.; Pacary, E.; Eddi, D.; Freret, T.; Tixier, E.; Toutain, J.; Touzani, O.; Schumann-Bard, P.; Petit, E.; Roussel, S. Combined therapeutic strategy using erythropoietin and mesenchymal stem cells potentiates neurogenesis after transient focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2008, 28, 1552–1563. [Google Scholar] [CrossRef]
- Kang, M.; Min, K.; Jang, J.; Kim, S.C.; Kang, M.S.; Jang, S.J.; Lee, J.Y.; Kim, S.H.; Kim, M.K.; An, S.A. Involvement of immune responses in the efficacy of cord blood cell therapy for cerebral palsy. Stem Cells Dev. 2015, 24, 2259–2268. [Google Scholar] [CrossRef]
- Hwang, S.; Choi, J.; Kim, M. Combining human umbilical cord blood cells with erythropoietin enhances angiogenesis/neurogenesis and behavioral recovery after stroke. Front. Neurol. 2019, 10, 357. [Google Scholar] [CrossRef]
- Cao, Q.-Q.; Li, S.; Lu, Y.; Wu, D.; Feng, W.; Shi, Y.; Zhang, L.-P. Transcriptome analysis of molecular mechanisms underlying facial nerve injury repair in rats. Neural Regen. Res. 2021, 16, 2316. [Google Scholar]
- Cai, Y.; Zhang, Y.; Ke, X.; Guo, Y.; Yao, C.; Tang, N.; Pang, P.; Xie, G.; Fang, L.; Zhang, Z. Transcriptome sequencing unravels potential biomarkers at different stages of cerebral ischemic stroke. Front. Genet. 2019, 10, 814. [Google Scholar] [CrossRef]
- Jiang, X.; Qin, W.; Wu, J.; Xiao, J.; Zhong, Y.; Yuan, C.; Yuan, Q. Transcriptome analysis and differentially expressed gene screening for hypoxic-ischemic brain damage in rats treated with acupuncture. Acupuncture 2021. [Google Scholar] [CrossRef]
- Juul, S.E.; Beyer, R.P.; Bammler, T.K.; McPherson, R.J.; Wilkerson, J.; Farin, F.M. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatric Res. 2009, 65, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Mary-Huard, T.; Daudin, J.-J.; Baccini, M.; Biggeri, A.; Bar-Hen, A. Biases induced by pooling samples in microarray experiments. Bioinformatics 2007, 23, i313–i318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schurch, N.J.; Schofield, P.; Gierliński, M.; Cole, C.; Sherstnev, A.; Singh, V.; Wrobel, N.; Gharbi, K.; Simpson, G.G.; Owen-Hughes, T. How many biological replicates are needed in an RNA-seq experiment and which differential expression tool should you use? RNA 2016, 22, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C. Pathway enrichment analysis and visualization of omics data using g: Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.M. Principal Components Analysis (PCA); Department of Geology, University of Georgia: Athens, GA, USA, 2008; pp. 30602–32501. [Google Scholar]
- Cai, H.; Chen, H.; Yi, T.; Daimon, C.M.; Boyle, J.P.; Peers, C.; Maudsley, S.; Martin, B. VennPlex–a novel Venn diagram program for comparing and visualizing datasets with differentially regulated datapoints. PLoS ONE 2013, 8, e53388. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.Z.; Comin, C.H.; Casanova, D.; Bruno, O.M.; Amancio, D.R.; Costa, L.d.F.; Rodrigues, F.A. Clustering algorithms: A comparative approach. PLoS ONE 2019, 14, e0210236. [Google Scholar] [CrossRef]
- Tilford, C.A.; Siemers, N.O. Gene set enrichment analysis. In Protein Networks and Pathway Analysis; Springer: Berlin/Heidelberg, Germany, 2009; pp. 99–121. [Google Scholar]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Perlmann, T.; Wallén-Mackenzie, Å. Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells. Cell Tissue Res. 2004, 318, 45–52. [Google Scholar] [CrossRef]
- Frasch, M.G.; Schulkin, J.; Metz, G.A.; Antonelli, M. Animal models of fetal programming: Focus on chronic maternal stress during pregnancy and neurodevelopment. In Animal Models for the Study of Human Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 839–849. [Google Scholar]
- Luo, S.X.; Huang, E.J. Dopaminergic neurons and brain reward pathways: From neurogenesis to circuit assembly. Am. J. Pathol. 2016, 186, 478–488. [Google Scholar] [CrossRef]
- Fei, Y.-x.; Zhu, J.-p.; Zhao, B.; Yin, Q.-y.; Fang, W.-r.; Li, Y.-m. XQ-1H regulates Wnt/GSK3β/β-catenin pathway and ameliorates the integrity of blood brain barrier in mice with acute ischemic stroke. Brain Res. Bull. 2020, 164, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Y.; Liu, H. The role of Wnt/β-catenin pathway in the protection process by dexmedetomidine against cerebral ischemia/reperfusion injury in rats. Life Sci. 2019, 236, 116921. [Google Scholar] [CrossRef] [PubMed]
- Armoskus, C.; Moreira, D.; Bollinger, K.; Jimenez, O.; Taniguchi, S.; Tsai, H.-W. Identification of sexually dimorphic genes in the neonatal mouse cortex and hippocampus. Brain Res. 2014, 1562, 23–38. [Google Scholar] [CrossRef]
- Wang, L.; Shilatifard, A. UTX mutations in human cancer. Cancer Cell 2019, 35, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.I.; Choi, J.-W.; Shim, K.-H.; Choung, J.S.; Kim, H.-J.; Sim, H.R.; Suh, M.R.; Jung, J.E.; Kim, M. Synergistic Effect in Neurological Recovery via Anti-Apoptotic Akt Signaling in Umbilical Cord Blood and Erythropoietin Combination Therapy for Neonatal Hypoxic-Ischemic Brain Injury. Int. J. Mol. Sci. 2021, 22, 11995. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Vaishnav, P.K.; Dinda, A.K.; Mohanty, S. Evaluation of priming efficiency of forskolin in tissue-specific human mesenchymal stem cells into dopaminergic neurons: An in vitro comparative study. Cells 2020, 9, 2058. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, D.; Qi, M.; Kim, D.-h.; Kitamura, Y.; Inden, M.; Tsuchiya, D.; Takata, K.; Taniguchi, T.; Yoshimoto, K.; Shimohama, S. Improvement of focal ischemia-induced rat dopaminergic dysfunction by striatal transplantation of mouse embryonic stem cells. Neurosci. Lett. 2006, 407, 74–79. [Google Scholar] [CrossRef]
- Chen, X.X.; Qian, Y.; Wang, X.P.; Tang, Z.W.; Xu, J.T.; Lin, H.; Yang, Z.Y.; Song, X.B.; Lu, D.; Guo, J.Z. Nurr1 promotes neurogenesis of dopaminergic neuron and represses inflammatory factors in the transwell coculture system of neural stem cells and microglia. CNS Neurosci. Ther. 2018, 24, 790–800. [Google Scholar] [CrossRef]
- Gao, H.; Wang, D.; Jiang, S.; Mao, J.; Yang, X. NF-κB is negatively associated with Nurr1 to reduce the inflammatory response in Parkinson’s disease. Mol. Med. Rep. 2021, 23, 1–9. [Google Scholar] [CrossRef]
- Yu, S.; Doycheva, D.M.; Gamdzyk, M.; Yang, Y.; Lenahan, C.; Li, G.; Li, D.; Lian, L.; Tang, J.; Lu, J. Activation of MC1R with BMS-470539 attenuates neuroinflammation via cAMP/PKA/Nurr1 pathway after neonatal hypoxic-ischemic brain injury in rats. J. Neuroinflammation 2021, 18, 1–20. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Bi, J.; Chen, Y.; Jain, S.; Zhao, Y. Human cord blood-derived multipotent stem cells (CB-SCs) treated with all-trans-retinoic acid (ATRA) give rise to dopamine neurons. Biochem. Biophys. Res. Commun. 2012, 419, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Frazzini, V.; Rockabrand, E.; Mocchegiani, E.; Sensi, S. Oxidative stress and brain aging: Is zinc the link? Biogerontology 2006, 7, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Peng, L.; Zhu, J.; Zhou, Y.; Li, L.; Chen, Y.; Yu, S.; Zhao, Y. miR-145-5p/Nurr1/TNF-α signaling-induced microglia activation regulates neuron injury of acute cerebral ischemic/reperfusion in rats. Front. Mol. Neurosci. 2017, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Chen, S.; Le, W. The role of Nurr1 in the development of dopaminergic neurons and Parkinson’s disease. Prog. Neurobiol. 2005, 77, 128–138. [Google Scholar] [CrossRef]
- Tito, A.J., Jr.; Zhang, S. Characterization of Vesicular Monoamine Transporter 2 and its Role in Parkinson’s Disease Pathogenesis Using Drosophila. Ph.D. Thesis, The University of Texas Md Anderson Cancer Center, Houston, TX, USA, December 2016; p. 12. [Google Scholar]
- Jia, L.; Piña-Crespo, J.; Li, Y. Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Mol. Brain 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Fairbanks, S.L.; Young, J.M.; Nelson, J.W.; Davis, C.; Koerner, I.P.; Alkayed, N.J. Mechanism of the sex difference in neuronal ischemic cell death. Neuroscience 2012, 219, 183–191. [Google Scholar] [CrossRef]
- Hu, C.; Bai, X.; Liu, C.; Hu, Z. Long noncoding RNA XIST participates hypoxia-induced angiogenesis in human brain microvascular endothelial cells through regulating miR-485/SOX7 axis. Am. J. Transl. Res. 2019, 11, 6487. [Google Scholar]
- Oorschot, D.E.; Sizemore, R.J.; Amer, A.R. Treatment of neonatal hypoxic-ischemic encephalopathy with erythropoietin alone, and erythropoietin combined with hypothermia: History, current status, and future research. Int. J. Mol. Sci. 2020, 21, 1487. [Google Scholar] [CrossRef]
- Herz, J.; Köster, C.; Reinboth, B.S.; Dzietko, M.; Hansen, W.; Sabir, H.; van Velthoven, C.; Bendix, I.; Felderhoff-Müser, U. Interaction between hypothermia and delayed mesenchymal stem cell therapy in neonatal hypoxic-ischemic brain injury. Brain Behav. Immun. 2018, 70, 118–130. [Google Scholar] [CrossRef]
- Park, W.S.; Sung, S.I.; Ahn, S.Y.; Yoo, H.S.; Sung, D.K.; Im, G.H.; Choi, S.J.; Chang, Y.S. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS ONE 2015, 10, e0120893. [Google Scholar] [CrossRef]
- Zhou, K.Q.; Davidson, J.O.; Bennet, L.; Gunn, A.J. Combination treatments with therapeutic hypothermia for hypoxic-ischemic neuroprotection. Dev. Med. Child Neurol. 2020, 62, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Song, J.; Lee, J.H.; Kang, M.S.; Jang, S.J.; Kim, S.H.; Kim, M. Allogenic umbilical cord blood therapy combined with erythropoietin for patients with severe traumatic brain injury: Three case reports. Restor. Neurol. Neurosci. 2013, 31, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Paton, M.C.; Finch-Edmondson, M. Research update on the state of the evidence for stem cell and regenerative medicine in cerebral palsy. Arch. Stem Cell Ther. 2021, 2, 15–19. [Google Scholar]
- Lan, K.-M.; Tien, L.-T.; Cai, Z.; Lin, S.; Pang, Y.; Tanaka, S.; Rhodes, P.G.; Bhatt, A.J.; Savich, R.D.; Fan, L.-W. Erythropoietin ameliorates neonatal hypoxia-ischemia-induced neurobehavioral deficits, neuroinflammation, and hippocampal injury in the juvenile rat. Int. J. Mol. Sci. 2016, 17, 289. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Y.; Sheng, Q.; Shyr, Y. Advanced heat map and clustering analysis using heatmap3. BioMed Res. Int. 2014, 2014, 986048. [Google Scholar] [CrossRef]

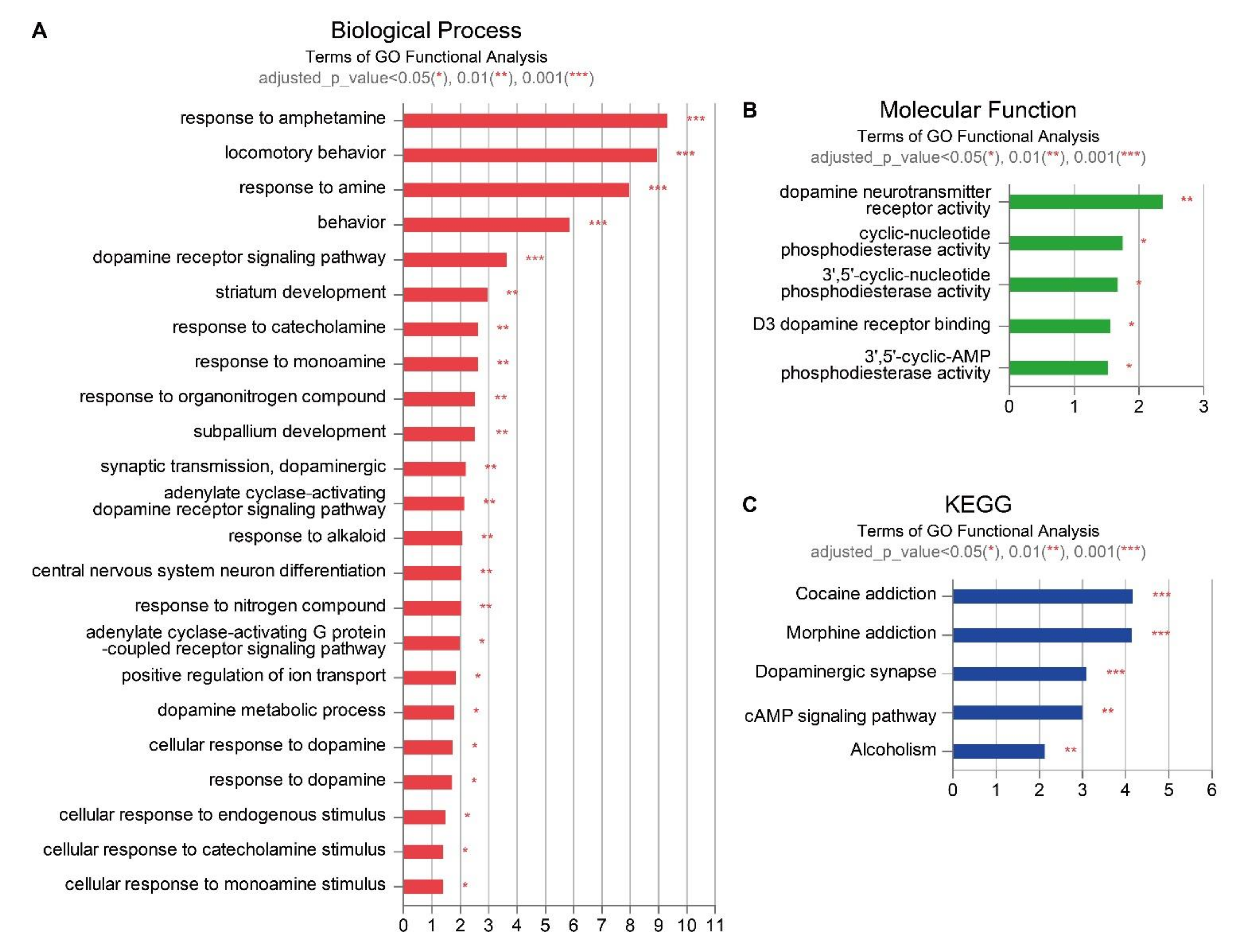

| Biological Category | Term | −log10 (adjusted p-Value) | Associated Genes |
|---|---|---|---|
| Cellular structure | positive regulation of actin cytoskeleton reorganization | 1.400660 | Baiap2l1 |
| Dopamine related pathways | response to amphetamine | 1.504507 | Nr4a2 |
| response to amine | 1.923929 | Nr4a2 | |
| response to insecticide | 1.524137 | Nr4a2 | |
| epithalamus development | 1.711028 | Nr4a2 | |
| habenula development | 1.711028 | Nr4a2 | |
| regulation of dopamine metabolic process | 1.504507 | Nr4a2 | |
| regulation of catecholamine metabolic process | 1.923929 | Nr4a2 | |
| response to corticotropin-releasing hormone | 1.524137 | Nr4a2 | |
| regulation of respiratory gaseous exchange | 1.641285 | Nr4a2 | |
| general adaptation syndrome | 1.854837 | Nr4a2 | |
| cellular response to corticotropin-releasing hormone stimulus | 1.524137 | Nr4a2 | |
| Immune response | macrophage cytokine production | 1.321089 | Sema7a |
| regulation of macrophage cytokine production | 1.321089 | Sema7a | |
| positive regulation of myeloid leukocyte cytokine production involved in immune response | 1.504507 | Sema7a | |
| zinc protein pathways | zinc ion transmembrane transporter activity | 1.641285 | Slc30a3 |
| detoxification of zinc ion | 1.711028 | Slc30a3 | |
| sequestering of zinc ion | 1.482841 | Slc30a3 | |
| sequestering of metal ion | 1.325929 | Slc30a3 | |
| regulation of sequestering of zinc ion | 1.524137 | Slc30a3 | |
| zinc ion import into organelle | 1.854837 | Slc30a3 | |
| zinc ion import into synaptic vesicle | 1.854837 | Slc30a3 | |
| stress response to zinc ion | 1.711028 | Slc30a3 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Nurr1 | GTGTTCAGGCGCAGTATGG | TGGCAGTAATTTCAGTGTTGGT |
| Nur77 | TTGAGTTCGGCAAGCCTACC | GTGTACCCGTCCATGAAGGTG |
| NOR1 | AGGATTCACTGATCTCCCCAA | GATGCAGGACAAGTCCATTGC |
| DAT | AAATGCTCCGTGGGACCAATG | GTCTCCCGCTCTTGAACCTC |
| VMAT | ATGCTGCTCACCGTCGTAG | GGACAGTCGTGTTGGTCACAG |
| Wnt1 | GGTTTCTACTACGTTGCTACTGG | GGAATCCGTCAACAGGTTCGT |
| Wnt3 | CTCGCTGGCTACCCAATTTG | CTTCACACCTTCTGCTACGCT |
| Wnt5 | CAACTGGCAGGACTTTCTCAA | CATCTCCGATGCCGGAACT |
| β-catenin | ATGCAGCCGGACAGAAAAGC | CTTGCCACTCAGGGAAGGA |
| GSK-3β | TGGCAGCAAGGTAACCACAG | CGGTTCTTAAATCGCTTGTCCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-W.; Kang, S.J.; Choi, J.I.; Kwack, K.; Kim, M. Role of Nuclear-Receptor-Related 1 in the Synergistic Neuroprotective Effect of Umbilical Cord Blood and Erythropoietin Combination Therapy in Hypoxic Ischemic Encephalopathy. Int. J. Mol. Sci. 2022, 23, 2900. https://doi.org/10.3390/ijms23052900
Choi J-W, Kang SJ, Choi JI, Kwack K, Kim M. Role of Nuclear-Receptor-Related 1 in the Synergistic Neuroprotective Effect of Umbilical Cord Blood and Erythropoietin Combination Therapy in Hypoxic Ischemic Encephalopathy. International Journal of Molecular Sciences. 2022; 23(5):2900. https://doi.org/10.3390/ijms23052900
Chicago/Turabian StyleChoi, Joo-Wan, Su Jung Kang, Jee In Choi, KyuBum Kwack, and MinYoung Kim. 2022. "Role of Nuclear-Receptor-Related 1 in the Synergistic Neuroprotective Effect of Umbilical Cord Blood and Erythropoietin Combination Therapy in Hypoxic Ischemic Encephalopathy" International Journal of Molecular Sciences 23, no. 5: 2900. https://doi.org/10.3390/ijms23052900
APA StyleChoi, J.-W., Kang, S. J., Choi, J. I., Kwack, K., & Kim, M. (2022). Role of Nuclear-Receptor-Related 1 in the Synergistic Neuroprotective Effect of Umbilical Cord Blood and Erythropoietin Combination Therapy in Hypoxic Ischemic Encephalopathy. International Journal of Molecular Sciences, 23(5), 2900. https://doi.org/10.3390/ijms23052900






