MicroRNA-mRNA Regulatory Network Mediates Activation of mTOR and VEGF Signaling in African American Prostate Cancer
Abstract
:1. Introduction
2. Results
2.1. MirPath v.3 Analysis Reveals 68 Signaling Pathways Differentially Regulated by miRNA-mRNA Networks in AA PCa and EA PCa
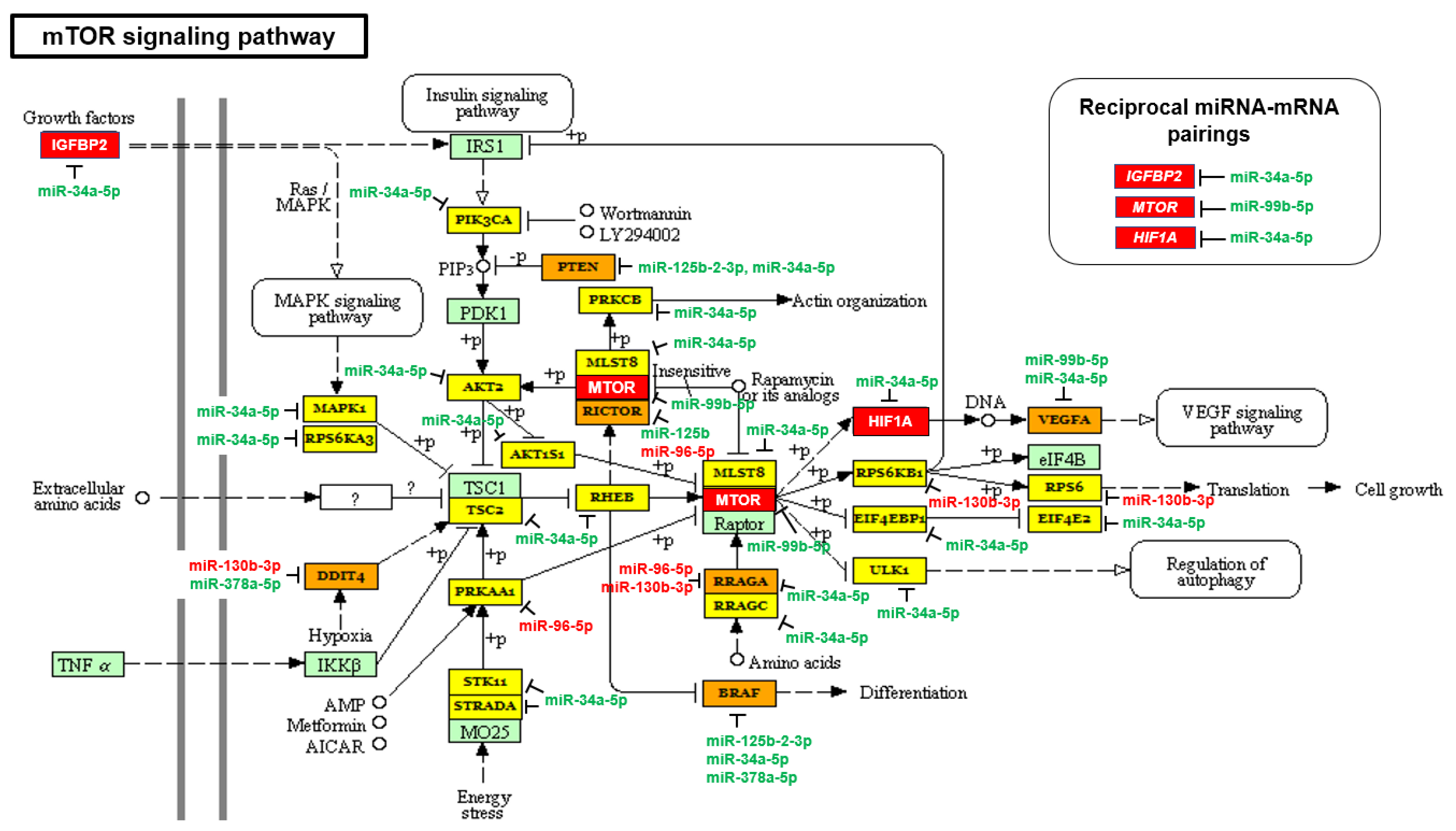
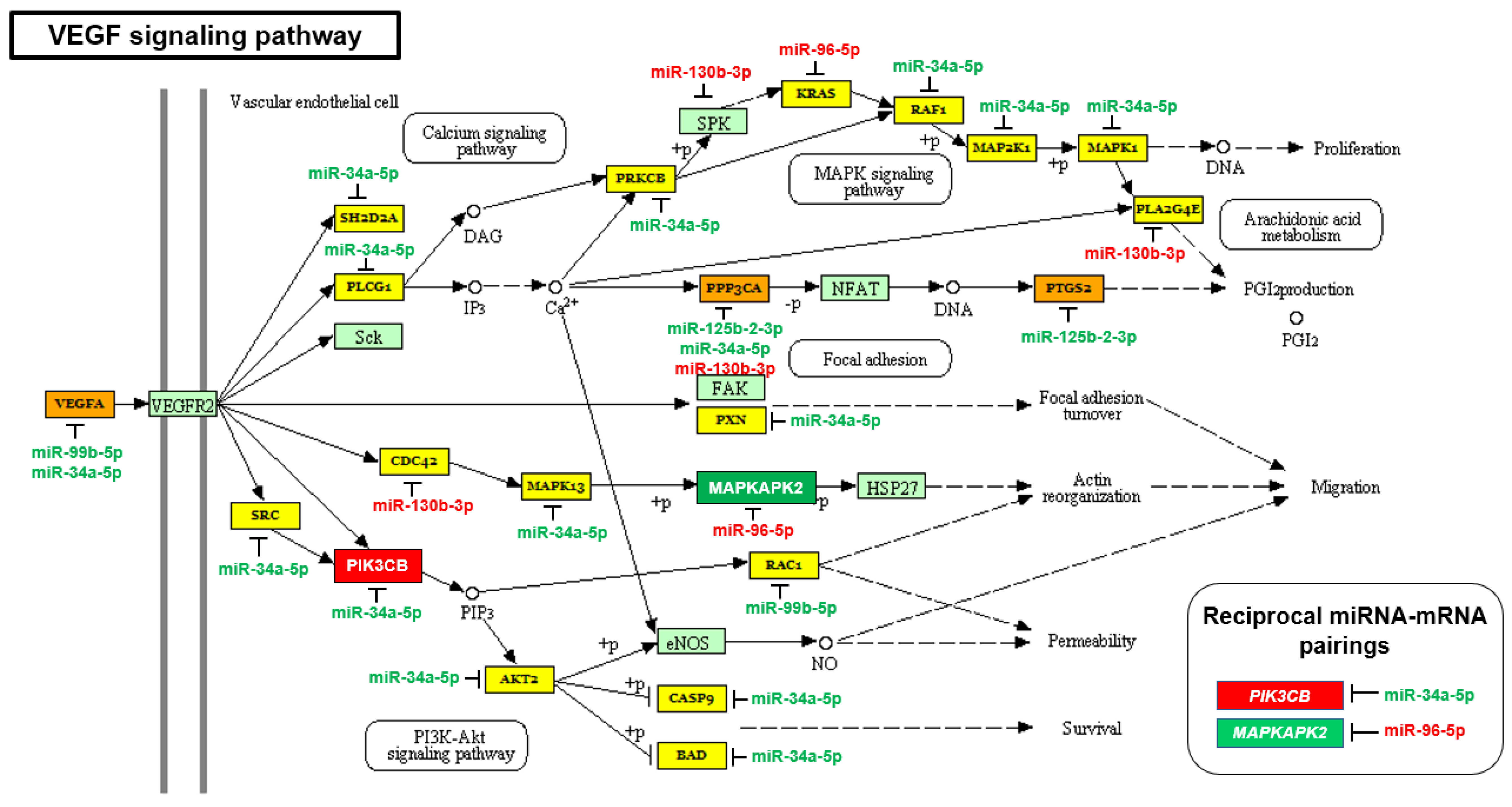
2.2. The mTOR and VEGF Signaling Pathways Are Upregulated in AA PCa Compared to EA PCa
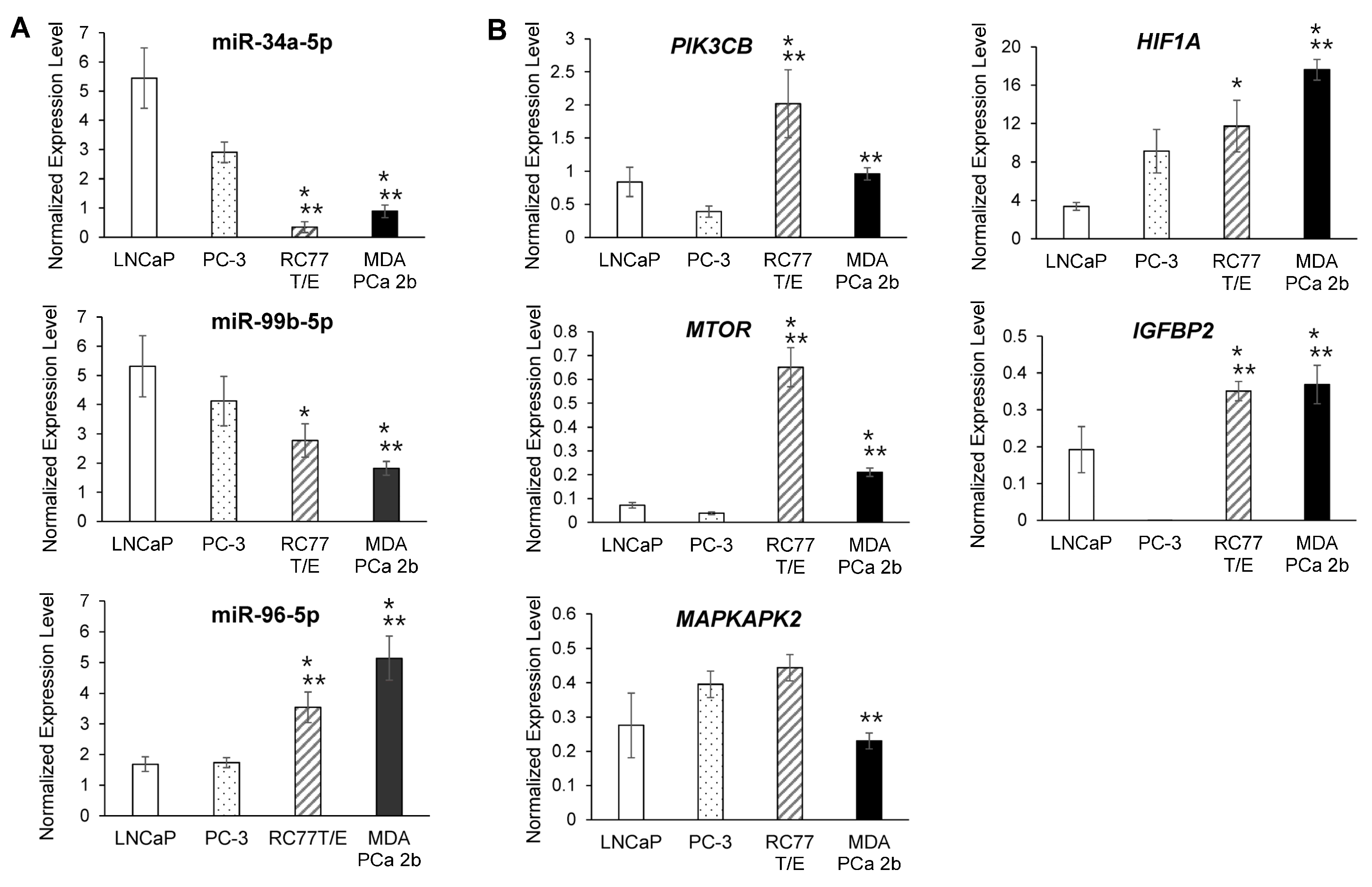
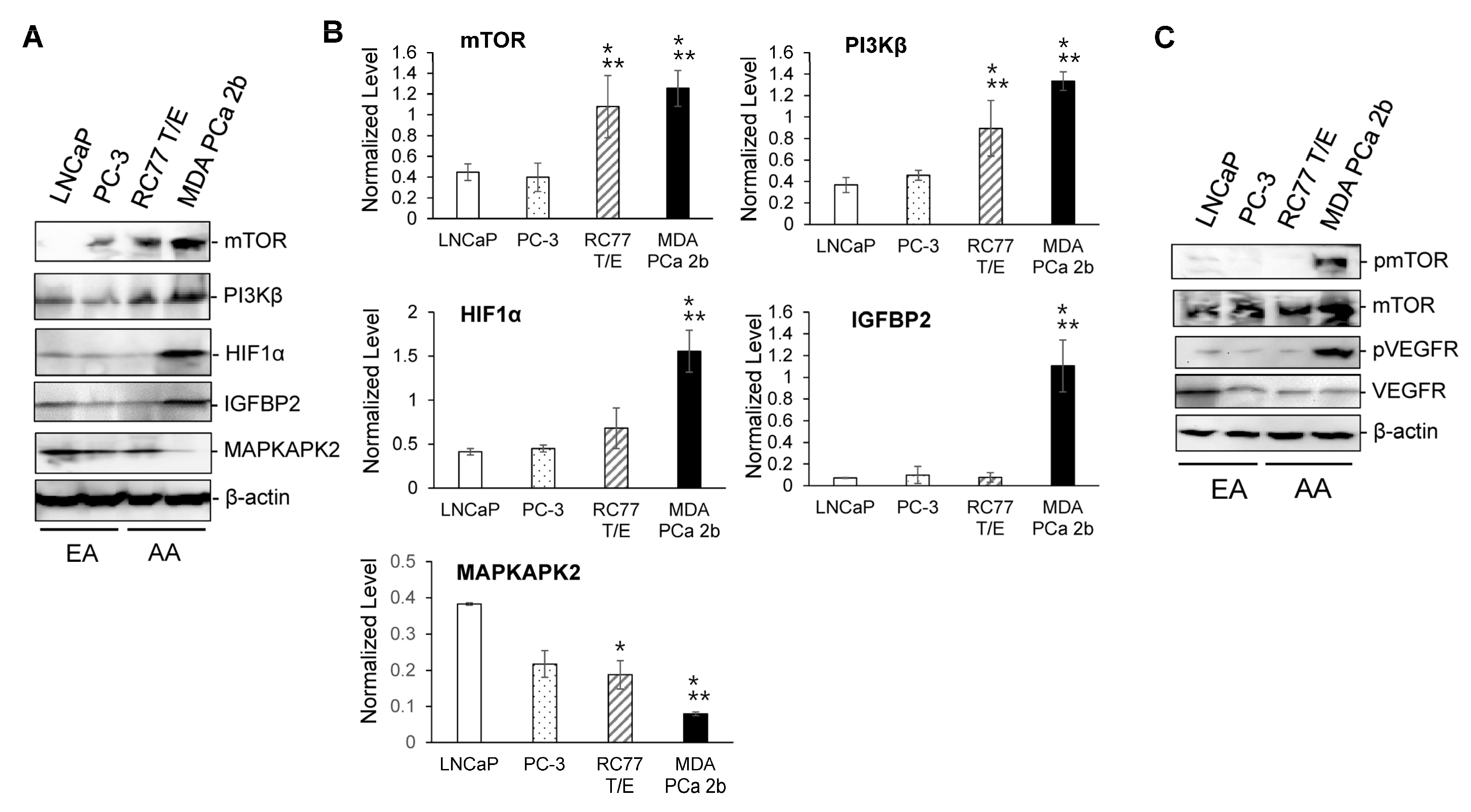
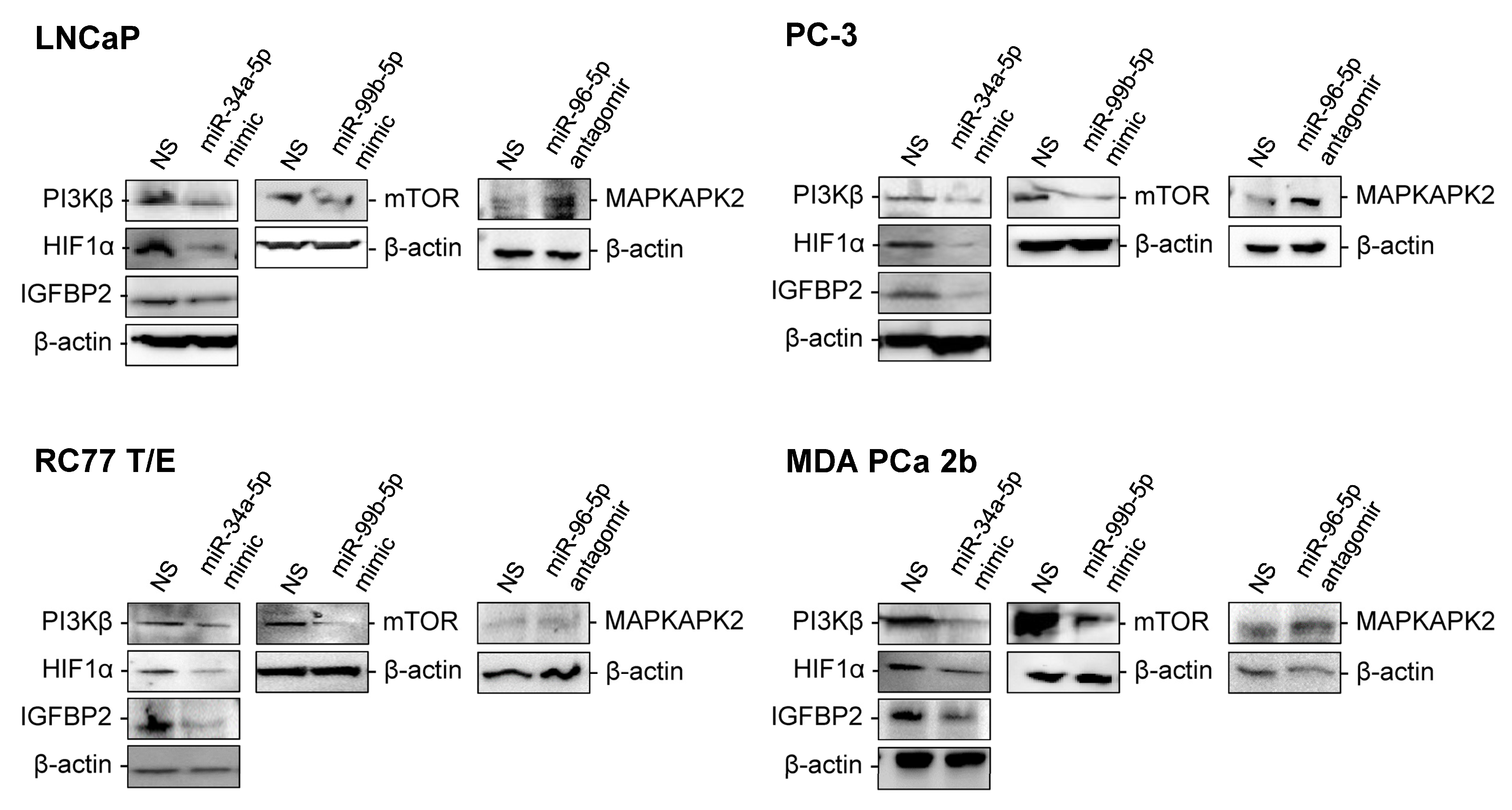
2.3. RT-qPCR Validations and Western Blot Analysis for the Candidate Reciprocal miRNA-mRNA Pairings in EA and AA PCa Tissues and Cell Models
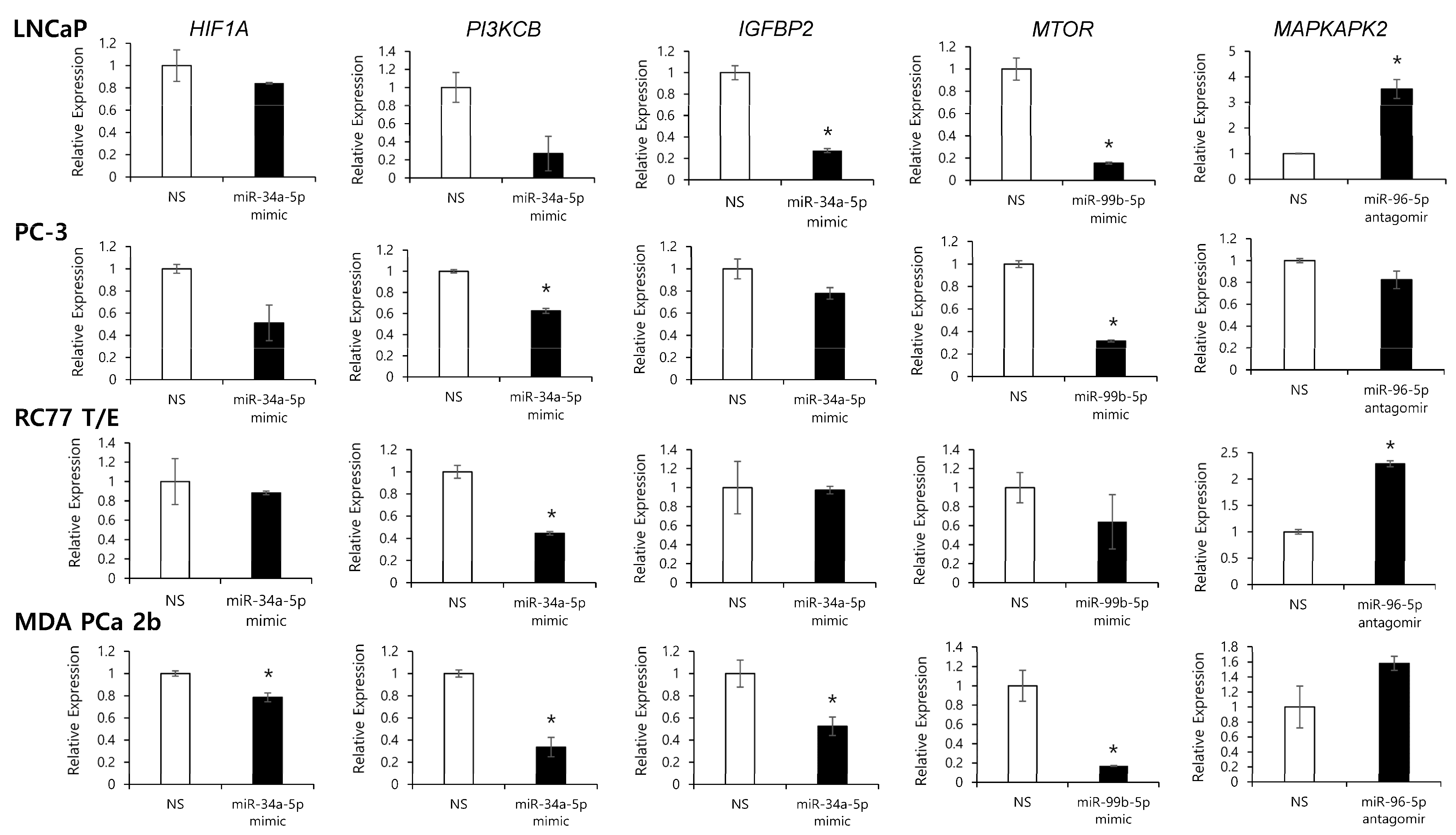
2.4. Assessment of Regulatory Relationship in Reciprocal miRNA-mRNA Pairings by Modulating AA-Depleted or AA-Enriched miRNA Expression Level
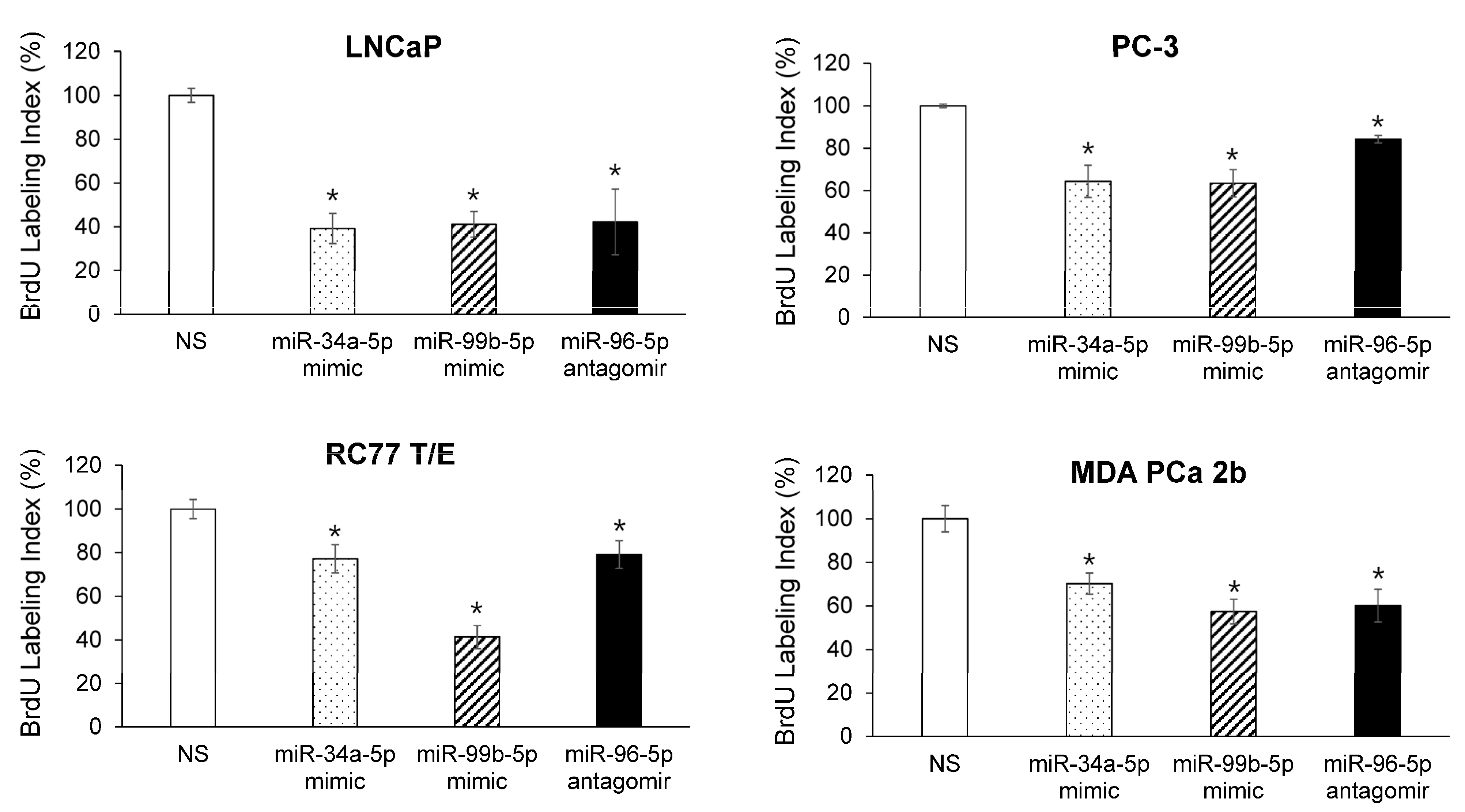
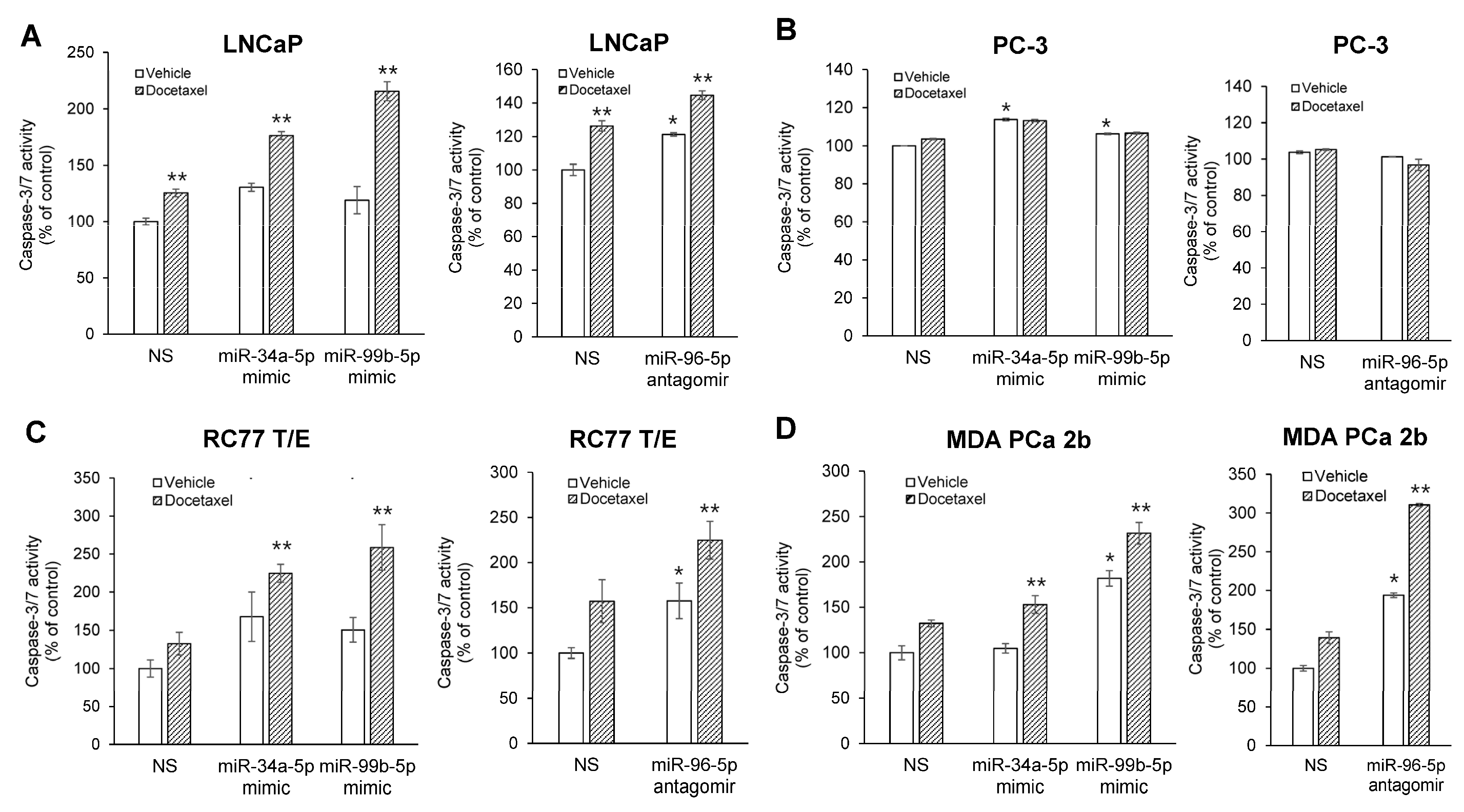
2.5. Modulating Expression Profile of Candidate Reciprocal miRNA-mRNA Pairings Inhibits Cell Proliferation and Sensitizes Docetaxel-Induced Cytotoxicity in PCa Cells
3. Discussion
4. Materials and Methods
4.1. Pathway Analysis and Identification of miRNA-mRNA Pairings
4.2. Cell Culture and TRANSFECTION
4.3. RT-qPCR Validation of mRNA and miRNA Expression
4.4. Western Blot Analysis
4.5. BrdU-Labeling Cell Proliferation Assay
4.6. Caspase 3/7 Activity-Based Apoptosis Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2008, 19, 92–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates that Thousands of Human Genes are MicroRNA Targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Li, H.; Zeng, X.; Yang, P.; Liu, X.; Zhao, X.; Liang, S. Roles of microRNA on cancer cell metabolism. J. Transl. Med. 2012, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Pedroza-Torres, A.; Romero-Córdoba, S.L.; Justo-Garrido, M.; Salido-Guadarrama, I.; Rodríguez-Bautista, R.; Montaño, S.; Muñiz-Mendoza, R.; Arriaga-Canon, C.; Fragoso-Ontiveros, V.; Álvarez-Gómez, R.M.; et al. MicroRNAs in Tumor Cell Metabolism: Roles and Therapeutic Opportunities. Front. Oncol. 2019, 9, 1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, R.J.; Standart, N. How do microRNAs regulate gene expression? Sci. STKE 2007, 2007, re1. [Google Scholar] [CrossRef] [Green Version]
- Tomari, Y.; Zamore, P.D. MicroRNA Biogenesis: Drosha Can’t Cut It without a Partner. Curr. Biol. 2005, 15, R61–R64. [Google Scholar] [CrossRef] [Green Version]
- Michlewski, G.; Cáceres, J.F. Post-transcriptional control of miRNA biogenesis. RNA 2018, 25, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Powell, I.J. Epidemiology and Pathophysiology of Prostate Cancer in African-American Men. J. Urol. 2007, 177, 444–449. [Google Scholar] [CrossRef]
- Reddy, S.; Shapiro, M.; Morton, J.R.; Brawley, O.W. Prostate cancer in black and white Americans. Cancer Metastasis Rev. 2003, 22, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Robbins, A.S.; Whittemore, A.S.; Thom, D.H. Differences in socioeconomic status and survival among white and black men with prostate cancer. Am. J. Epidemiol. 2000, 151, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Metcalfe, C.; Ibrahim, F.; Persad, R.; Ben-Shlomo, Y. Investigating Black-White differences in prostate cancer prognosis: A systematic review and meta-analysis. Int. J. Cancer 2008, 123, 430–435. [Google Scholar] [CrossRef]
- Freedland, S.J.; Isaacs, W.B. Explaining racial differences in prostate cancer in the United States: Sociology or biology? Prostate 2004, 62, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Maruyama, R.; Yamamoto, E.; Kai, M. Epigenetic alteration and microRNA dysregulation in cancer. Front. Genet. 2013, 4, 258. [Google Scholar] [CrossRef] [Green Version]
- Ramalho-Carvalho, J.; Fromm, B.; Henrique, R.; Jerónimo, C. Deciphering the function of non-coding RNAs in prostate cancer. Cancer Metastasis Rev. 2016, 35, 235–262. [Google Scholar] [CrossRef]
- Ozen, M.; Creighton, C.J.; Ozdemir, M.; Ittmann, M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene 2007, 27, 1788–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef]
- Abramovic, I.; Ulamec, M.; Bojanac, A.K.; Bulic-Jakus, F.; Jezek, D.; Sincic, N. miRNA in prostate cancer: Challenges toward translation. Epigenomics 2020, 12, 543–558. [Google Scholar] [CrossRef]
- Pełka, K.; Klicka, K.; Grzywa, T.M.; Gondek, A.; Marczewska, J.M.; Garbicz, F.; Szczepaniak, K.; Paskal, W.; Włodarski, P.K. miR-96-5p, miR-134-5p, miR-181b-5p and miR-200b-3p heterogenous expression in sites of prostate cancer versus benign prostate hyperplasia-archival samples study. Histochem. Cell Biol. 2021, 155, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Shiina, M.; Dasgupta, P.; Kulkarni, P.; Kato, T.; Wong, R.K.; Tanaka, Y.; Shahryari, V.; Maekawa, S.; Yamamura, S.; et al. Upregulation of miR-130b Contributes to Risk of Poor Prognosis and Racial Disparity in African-American Prostate Cancer. Cancer Prev. Res. 2019, 12, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Shiina, M.; Hashimoto, Y.; Kulkarni, P.; Dasgupta, P.; Shahryari, V.; Yamamura, S.; Tanaka, Y.; Dahiya, R. Role of miR-182/PDCD4 axis in aggressive behavior of prostate cancer in the African Americans. BMC Cancer 2021, 21, 1028. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M.; Banerjee, N.N.; Banerjee, H.N.; Rawat, K.; Chen, L.; Worthington, M.; Hodge, S.; Walker, R.; Verma, M.; Sarkar, F.; et al. A health disparities study of MicroRNA-146a expression in prostate cancer samples derived from African American and European American patients. J. Solid Tumors 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Shiina, M.; Hashimoto, Y.; Kato, T.; Yamamura, S.; Tanaka, Y.; Majid, S.; Saini, S.; Varahram, S.; Kulkarni, P.; Dasgupta, P.; et al. Differential expression of miR-34b and androgen receptor pathway regulate prostate cancer aggressiveness between African-Americans and Caucasians. Oncotarget 2016, 8, 8356–8368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.-D.; Ceniccola, K.; Yang, Q.; Andrawis, R.; Patel, V.; Ji, Y.; Rhim, J.; Olender, J.; Popratiloff, A.; Latham, P.; et al. Identification and Functional Validation of Reciprocal microRNA-mRNA Pairings in African American Prostate Cancer Disparities. Clin. Cancer Res. 2015, 21, 4970–4984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.-D.; Yang, Q.; Ceniccola, K.; Bianco, F.; Andrawis, R.; Jarrett, T.; Frazier, H.; Patierno, S.R.; Lee, N.H. Androgen Receptor-Target Genes in African American Prostate Cancer Disparities. Prostate Cancer 2013, 2013, 763569. [Google Scholar] [CrossRef] [Green Version]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Beltran, H.; Morris, M.J. Docetaxel for Early Prostate Cancer: What Have We Learned? Eur. Urol. 2020, 77, 573–575. [Google Scholar] [CrossRef]
- Nalls, D.; Tang, S.-N.; Rodova, M.; Srivastava, R.K.; Shankar, S. Targeting Epigenetic Regulation of miR-34a for Treatment of Pancreatic Cancer by Inhibition of Pancreatic Cancer Stem Cells. PLOS ONE 2011, 6, e24099. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wu, Q.M.; Chang, L.Y.; Liu, J.C. miR-34a and miR-125a-5p inhibit proliferation and metastasis but induce apoptosis in hepatocellular carcinoma cells via repressing the MACC1-mediated PI3K/AKT/mTOR pathway. Neoplasma 2020, 67, 1042–1053. [Google Scholar] [CrossRef]
- Paccez, J.D.; Duncan, K.; Sekar, D.; Correa, R.G.; Wang, Y.; Gu, X.; Bashin, M.; Chibale, K.; Libermann, T.A.; Zerbini, L.F. Dihydroartemisinin inhibits prostate cancer via JARID2/miR-7/miR-34a-dependent downregulation of Axl. Oncogenesis 2019, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, B.; Xu, G.-C.; Liu, S.-T.; Liu, T.; Geng, B. MiR-34a affects G2 arrest in prostate cancer PC3 cells via Wnt pathway and inhibits cell growth and migration. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8349–8358. [Google Scholar] [PubMed]
- Wang, Z.; Zhao, Z.; Yang, Y.; Luo, M.; Zhang, M.; Wang, X.; Liu, L.; Hou, N.; Guo, Q.; Song, T.; et al. MiR-99b-5p and miR-203a-3p Function as Tumor Suppressors by Targeting IGF-1R in Gastric Cancer. Sci. Rep. 2018, 8, 10119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Guan, X. MicroRNA-99b predicts clinical outcome of osteosarcoma and suppresses tumor cell proliferation, migration and invasion. Diagn. Pathol. 2019, 14, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Zhang, J.; Yang, D. miR-96-5p promotes the proliferation and migration of ovarian cancer cells by suppressing Caveolae1. J. Ovarian Res. 2019, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shi, W.; Peng, L.; Qin, X.; Hui, Y. MiR-96 enhances cellular proliferation and tumorigenicity of human cervical carcinoma cells through PTPN9. Saudi J. Biol. Sci. 2018, 25, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Haflidadóttir, B.S.; Larne, O.; Martin, M.; Persson, M.; Edsjö, A.; Bjartell, A.; Ceder, Y. Upregulation of miR-96 Enhances Cellular Proliferation of Prostate Cancer Cells through FOXO1. PLOS ONE 2013, 8, e72400. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Tang, B.; Sun, X. Development of Inhibitors Targeting Hypoxia-Inducible Factor 1 and 2 for Cancer Therapy. Yonsei Med. J. 2017, 58, 489–496. [Google Scholar] [CrossRef]
- Tran, M.G.B.; Bibby, B.A.S.; Yang, L.; Lo, F.; Warren, A.Y.; Shukla, D.; Osborne, M.; Hadfield, J.; Carroll, T.; Stark, R.; et al. Independence of HIF1a and androgen signaling pathways in prostate cancer. BMC Cancer 2020, 20, 469. [Google Scholar] [CrossRef]
- Mansoori, B.; Silvestris, N.; Mohammadi, A.; Khaze, V.; Baghbani, E.; Mokhtarzadeh, A.; Shanehbandi, D.; Derakhshani, A.; Duijf, P.H.G.; Baradaran, B. miR-34a and miR-200c Have an Additive Tumor-Suppressive Effect on Breast Cancer Cells and Patient Prognosis. Genes 2021, 12, 267. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rokavec, M.; Jiang, L.; Horst, D.; Hermeking, H. Antagonistic Effects of p53 and HIF1A on microRNA-34a Regulation of PPP1R11 and STAT3 and Hypoxia-induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells. Gastroenterology 2017, 153, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.Y.; Liu, Y.; Granberg, K.J.; Hu, L.; Haapasalo, H.; Annala, M.J.; Cogdell, D.E.; Verploegen, M.; Moore, L.M.; Fuller, G.; et al. IGFBP2 potentiates nuclear EGFR–STAT3 signaling. Oncogene 2015, 35, 738–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardsen, E.; Ukkonen, T.; Bjørnsen, T.; Mortensen, E.; Egevad, L.; Busch, C. Overexpression of IGBFB2 is a marker for malignant transformation in prostate epithelium. Virchows Arch. 2003, 442, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Azar, W.; Yau, S.; Sabin, M.; Werther, G. IGFBP-2: The dark horse in metabolism and cancer. Cytokine Growth Factor Rev. 2014, 26, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Li, Z.; Abdalla, B.A.; Chen, Y.; Nie, Q. MiR-34b-5p Mediates the Proliferation and Differentiation of Myoblasts by Targeting IGFBP2. Cells 2019, 8, 360. [Google Scholar] [CrossRef] [Green Version]
- Dbouk, H.A.; Pang, H.; Fiser, A.; Backer, J.M. A biochemical mechanism for the oncogenic potential of the p110beta catalytic subunit of phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. USA 2010, 107, 19897–19902. [Google Scholar] [CrossRef] [Green Version]
- Hart, M.; Walch-Rückheim, B.; Friedmann, K.S.; Rheinheimer, S.; Tänzer, T.; Glombitza, B.; Sester, M.; Lenhof, H.-P.; Hoth, M.; Schwarz, E.C.; et al. miR-34a: A new player in the regulation of T cell function by modulation of NF-kappaB signaling. Cell Death Dis. 2019, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Houede, N.; Pourquier, P. Targeting the genetic alterations of the PI3K-AKT-mTOR pathway: Its potential use in the treatment of bladder cancers. Pharmacol. Ther. 2015, 145, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.-H.; Liu, L.-Z. Role of mTOR in anticancer drug resistance: Perspectives for improved drug treatment. Drug Resist. Updates 2008, 11, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.-F.; Lin, J.-F.; Chou, K.-Y.; Lin, Y.-C.; Chen, H.-E.; Hwang, T.I.-S. miR-99a-5p acts as tumor suppressor via targeting to mTOR and enhances RAD001-induced apoptosis in human urinary bladder urothelial carcinoma cells. OncoTargets Ther. 2018, 11, 239–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, A.; Torres, K.; Pesci, A.; Ceccaroni, M.; Paszkowski, T.; Cassandrini, P.; Zamboni, G.; Maciejewski, R. Deregulation of miR-100, miR-99a and miR-199b in tissues and plasma coexists with increased expression of mTOR kinase in endometrioid endometrial carcinoma. BMC Cancer 2012, 12, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.J.; Wang, Y.; Wang, Y.Y. MicroRNA-99b suppresses human cervical cancer cell activity by inhibiting the PI3K/AKT/mTOR signaling pathway. J. Cell Physiol. 2019, 234, 9577–9591. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chang, J.; Wang, S.; Liu, X.; Peng, J.; Huang, D.; Sun, M.; Chen, Z.; Zhang, W.; Guo, W.; et al. miRNA-99b-5p suppresses liver metastasis of colorectal cancer by down-regulating mTOR. Oncotarget 2015, 6, 24448–24462. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Liu, Y.; Guo, Y.; Xiang, A.; Wang, G.; Xue, X.; Lu, Z. miR-99b-targeted mTOR induction contributes to irradiation resistance in pancreatic cancer. Mol. Cancer 2013, 12, 81. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Xue, J.; Yang, S.; Chen, Y.; Wang, Y.; Zhu, Y.; Wang, X.; Kuang, D.; Ruan, Q.; Duan, Y.; et al. Co-targeting of IGF1R/mTOR pathway by miR-497 and miR-99a impairs hepatocellular carcinoma development. Oncotarget 2017, 8, 47984–47997. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [Green Version]
- Canovas, B.; Nebreda, A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 346–366. [Google Scholar] [CrossRef]
- Martínez-Limón, A.; Joaquin, M.; Caballero, M.; Posas, F.; De Nadal, E. The p38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Yang, L.; Huang, B.; Cheng, M.; Wang, H.; Li, Y.; Huang, D.; Zheng, J.; Li, Q.; Zhang, X.; et al. A Functional Copy-Number Variation in MAPKAPK2 Predicts Risk and Prognosis of Lung Cancer. Am. J. Hum. Genet. 2012, 91, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Soni, S.; Anand, P.; Padwad, Y.S. MAPKAPK2: The master regulator of RNA-binding proteins modulates transcript stability and tumor progression. J. Exp. Clin. Cancer Res. 2019, 38, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| KEGG Pathway | p-Value | #Genes | #miRNAs |
|---|---|---|---|
| MicroRNAs in cancer | 3.88 × 10−54 | 100 | 8 |
| Proteoglycans in cancer | 8.70 × 10−12 | 99 | 8 |
| Adherens junction | 4.02 × 10−10 | 45 | 7 |
| Viral carcinogenesis | 1.29 × 10−7 | 105 | 8 |
| Glioma | 3.22 × 10−7 | 39 | 8 |
| Transcriptional misregulation in cancer | 3.22 × 10−7 | 88 | 8 |
| Cell cycle | 4.05 × 10−7 | 71 | 7 |
| Prostate cancer | 6.70 × 10−7 | 55 | 8 |
| Pancreatic cancer | 1.27 × 10−6 | 44 | 7 |
| Bacterial invasion of epithelial cells | 2.81 × 10−6 | 44 | 8 |
| Colorectal cancer | 4.14 × 10−6 | 40 | 7 |
| Thyroid cancer | 9.18 × 10−6 | 21 | 6 |
| Fatty acid biosynthesis | 1.35 × 10−5 | 5 | 3 |
| ErbB signaling pathway | 1.35 × 10−5 | 48 | 8 |
| Pathways in cancer | 1.89 × 10−5 | 166 | 8 |
| Chronic myeloid leukemia | 2.45 × 10−5 | 43 | 6 |
| Central carbon metabolism in cancer | 3.86 × 10−5 | 39 | 7 |
| Non-small cell lung cancer | 5.33 × 10−5 | 34 | 6 |
| Shigellosis | 8.00 × 10−5 | 37 | 7 |
| Regulation of actin cytoskeleton | 9.30 × 10−5 | 100 | 7 |
| Renal cell carcinoma | 1.14 × 10−4 | 39 | 6 |
| p53 signaling pathway | 1.14 × 10−4 | 42 | 7 |
| Glycosphingolipid biosynthesis—lacto and neolacto series | 1.16 × 10−4 | 13 | 5 |
| Endometrial cancer | 1.85 × 10−4 | 31 | 6 |
| Bladder cancer | 2.18 × 10−4 | 26 | 8 |
| Hepatitis B | 2.30 × 10−4 | 68 | 7 |
| Other types of O-glycan biosynthesis | 3.88 × 10−4 | 15 | 5 |
| Alcoholism | 4.33 × 10−4 | 88 | 8 |
| Endocytosis | 5.08 × 10−4 | 93 | 7 |
| Acute myeloid leukemia | 6.12 × 10−4 | 33 | 6 |
| Melanoma | 8.26 × 10−4 | 38 | 8 |
| Neurotrophin signaling pathway | 1.746 × 10−3 | 58 | 8 |
| Focal adhesion | 2.185 × 10−3 | 96 | 8 |
| mTOR signaling pathway | 2.477 × 10−3 | 33 | 6 |
| HIF-1 signaling pathway | 2.827 × 10−3 | 53 | 8 |
| Estrogen signaling pathway | 2.827 × 10−3 | 44 | 8 |
| Protein processing in endoplasmic reticulum | 2.962 × 10−3 | 75 | 7 |
| Oocyte meiosis | 2.974 × 10−3 | 55 | 7 |
| N-Glycan biosynthesis | 3.345 × 10−3 | 26 | 7 |
| Fc gamma R-mediated phagocytosis | 5.007 × 10−3 | 46 | 6 |
| Hippo signaling pathway | 5.356 × 10−3 | 64 | 7 |
| RNA transport | 5.788 × 10−3 | 76 | 6 |
| Thyroid hormone signaling pathway | 6.076 × 10−3 | 58 | 7 |
| Progesterone-mediated oocyte maturation | 1.0333 × 10−2 | 44 | 6 |
| Salmonella infection | 1.1305 × 10−2 | 42 | 6 |
| Axon guidance | 1.2697 × 10−2 | 54 | 6 |
| Ubiquitin mediated proteolysis | 1.2697 × 10−2 | 62 | 7 |
| Oxytocin signaling pathway | 1.2697 × 10−2 | 72 | 8 |
| Insulin signaling pathway | 1.5334 × 10−2 | 65 | 8 |
| VEGF signaling pathway | 1.5546 × 10−2 | 32 | 5 |
| Glycosaminoglycan biosynthesis—keratan sulfate | 1.9381 × 10−2 | 32 | 5 |
| Prolactin signaling pathway | 2.3342 × 10−2 | 35 | 6 |
| Small cell lung cancer | 2.7242 × 10−2 | 41 | 7 |
| FoxO signaling pathway | 3.0606 × 10−2 | 62 | 8 |
| NF-kappa B signaling pathway | 3.4130 × 10−2 | 38 | 7 |
| TNF signaling pathway | 3.6058 × 10−2 | 47 | 6 |
| HTLV-I infection | 4.0449 × 10−2 | 107 | 8 |
| Choline metabolism in cancer | 4.1519 × 10−2 | 46 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gujrati, H.; Ha, S.; Mohamed, A.; Wang, B.-D. MicroRNA-mRNA Regulatory Network Mediates Activation of mTOR and VEGF Signaling in African American Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 2926. https://doi.org/10.3390/ijms23062926
Gujrati H, Ha S, Mohamed A, Wang B-D. MicroRNA-mRNA Regulatory Network Mediates Activation of mTOR and VEGF Signaling in African American Prostate Cancer. International Journal of Molecular Sciences. 2022; 23(6):2926. https://doi.org/10.3390/ijms23062926
Chicago/Turabian StyleGujrati, Himali, Siyoung Ha, Azah Mohamed, and Bi-Dar Wang. 2022. "MicroRNA-mRNA Regulatory Network Mediates Activation of mTOR and VEGF Signaling in African American Prostate Cancer" International Journal of Molecular Sciences 23, no. 6: 2926. https://doi.org/10.3390/ijms23062926
APA StyleGujrati, H., Ha, S., Mohamed, A., & Wang, B.-D. (2022). MicroRNA-mRNA Regulatory Network Mediates Activation of mTOR and VEGF Signaling in African American Prostate Cancer. International Journal of Molecular Sciences, 23(6), 2926. https://doi.org/10.3390/ijms23062926






