Hypothyroidism and Diabetes-Related Dementia: Focused on Neuronal Dysfunction, Insulin Resistance, and Dyslipidemia
Abstract
1. Introduction
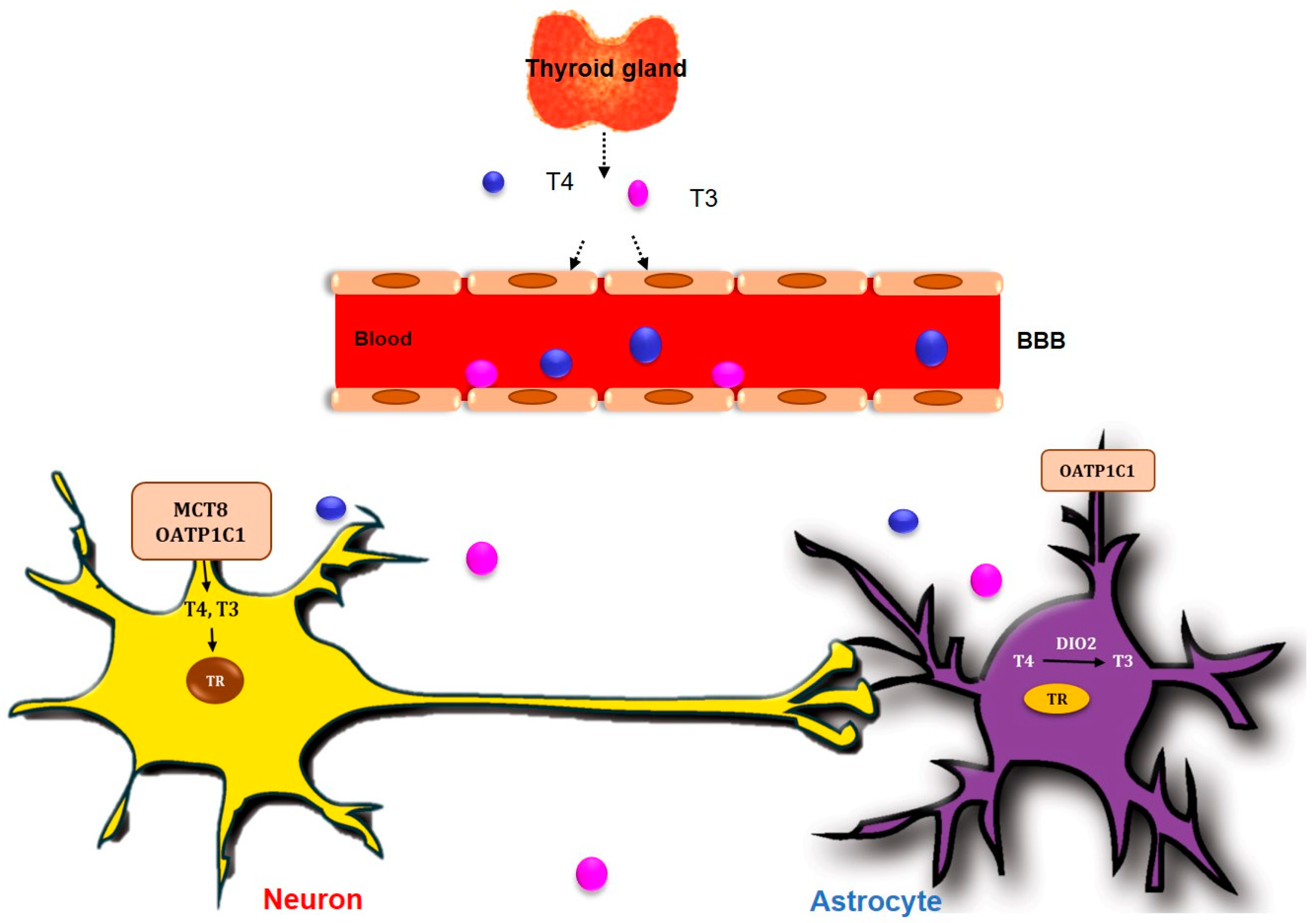
2. TH Dysfunction in the CNS
3. Diabetes-Related Dementia and THs
4. Neuronal Cell Damage and Imbalanced Neurotransmitters in Hypothyroidism
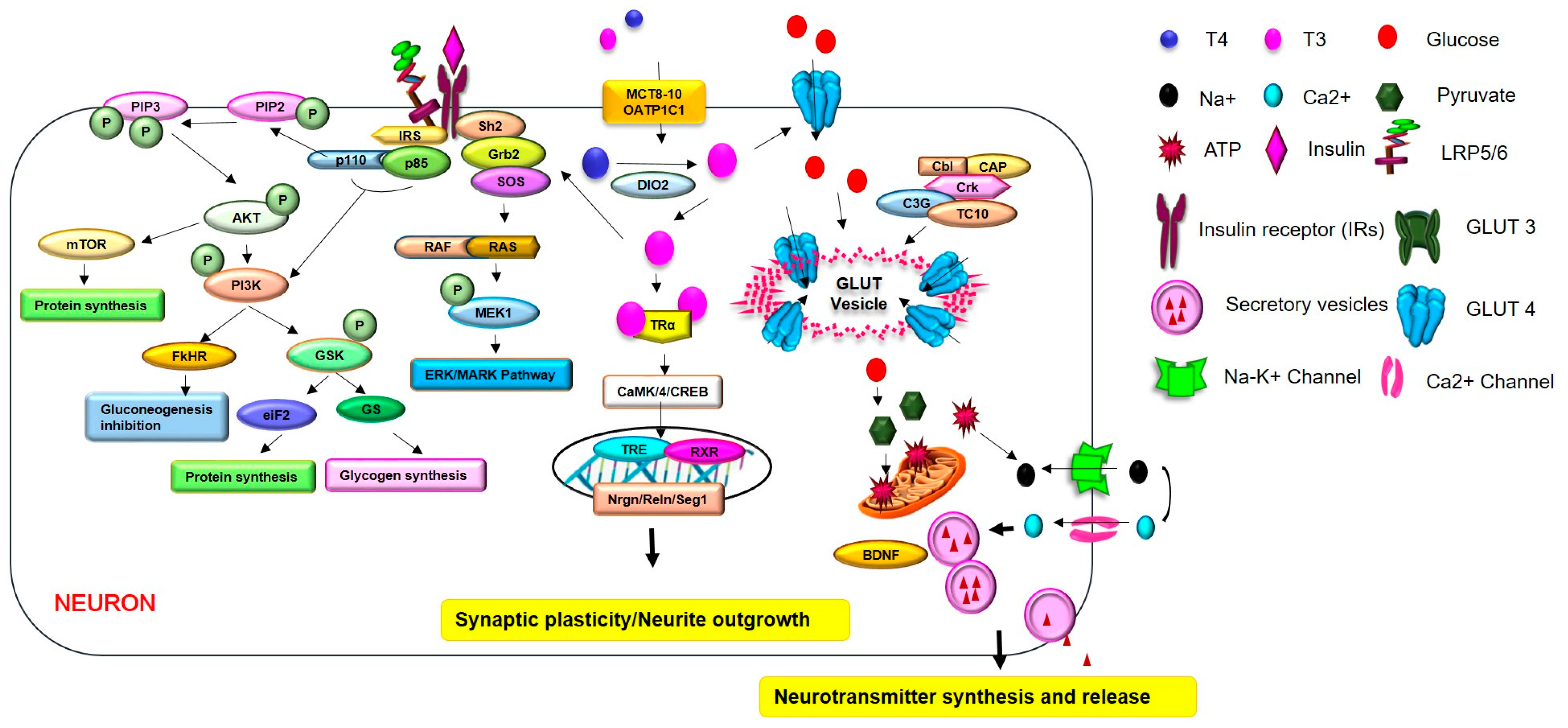
5. Brain Insulin Resistance in Hypothyroidism
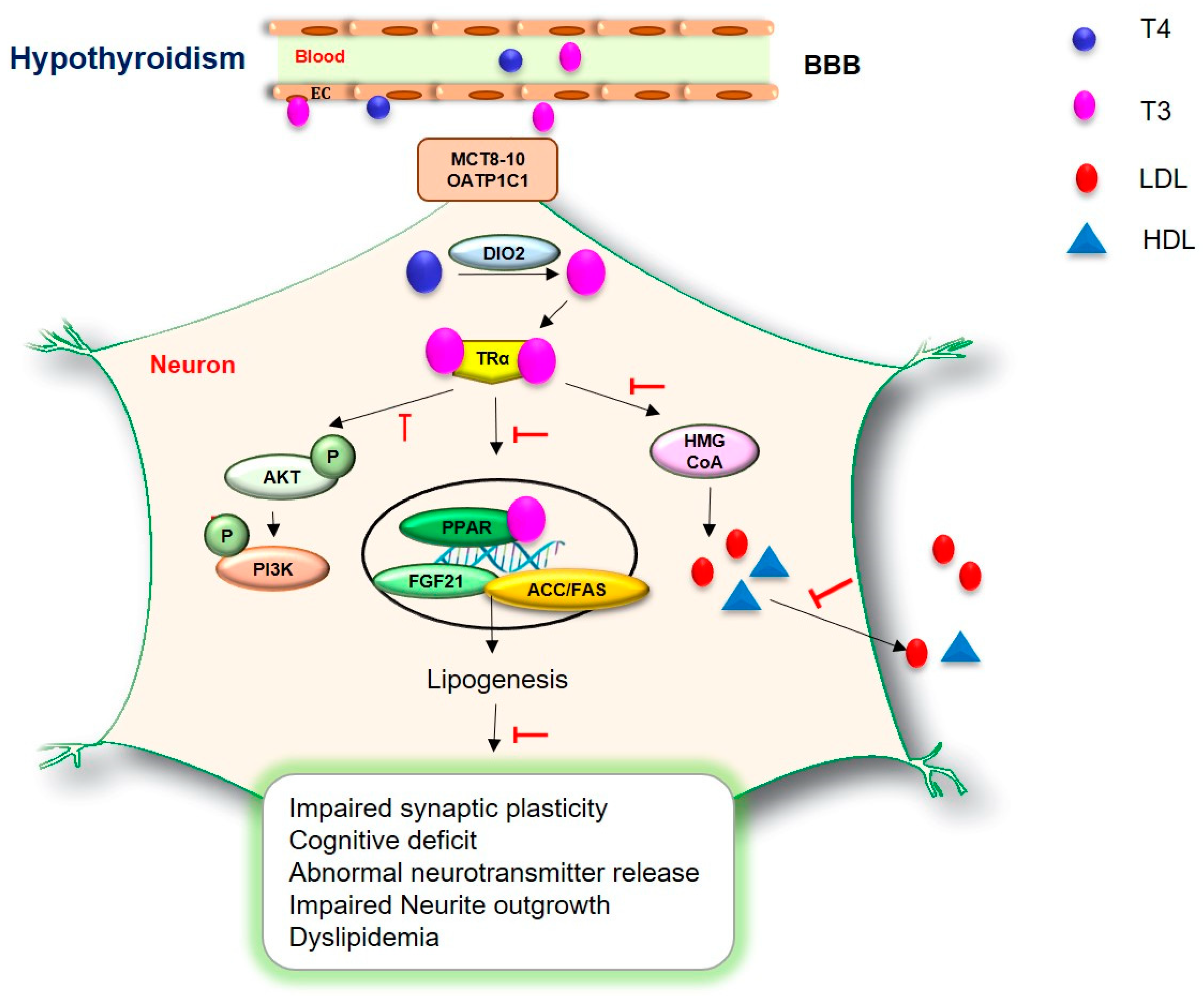
6. Dyslipidemia in Hypothyroidism
7. Neurological Problems in Hypothyroidism
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Eerdekens, A.; Verhaeghe, J.; Darras, V.; Naulaers, G.; Van den Berghe, G.; Langouche, L.; Vanhole, C. The placenta in fetal thyroid hormone delivery: From normal physiology to adaptive mechanisms in complicated pregnancies. J. Matern. Fetal Neonatal Med. 2020, 33, 3857–3866. [Google Scholar] [CrossRef] [PubMed]
- Raymaekers, S.R.; Darras, V.M. Thyroid hormones and learning-associated neuroplasticity. Gen. Comp. Endocrinol. 2017, 247, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Barez-Lopez, S.; Guadano-Ferraz, A. Thyroid Hormone Availability and Action during Brain Development in Rodents. Front. Cell. Neurosci. 2017, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, Y.; Li, W.C.; Chen, C.Y.; Chiu, Y.H.; Chien, H.Y.; Wang, Y. Association of thyroid-stimulating hormone and cardiovascular risk factors. Intern. Med. 2015, 54, 2537–2544. [Google Scholar] [CrossRef]
- Remaud, S.; Gothie, J.D.; Morvan-Dubois, G.; Demeneix, B.A. Thyroid hormone signaling and adult neurogenesis in mammals. Front. Endocrinol. 2014, 5, 62. [Google Scholar] [CrossRef]
- Apostol, M.; Keeran, M.; Klempf, N.; McCoskey, V.; Ernst, A.A.; Weiss, S.J.; Sarangarm, D. Thyroid stimulating hormone testing in ED evaluation of patients with atrial fibrillation and various psychiatric diagnoses. Am. J. Emerg. Med. 2019, 37, 1114–1117. [Google Scholar] [CrossRef]
- Biondi, B.; Kahaly, G.J.; Robertson, R.P. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr. Rev. 2019, 40, 789–824. [Google Scholar] [CrossRef]
- Iwen, K.A.; Schroder, E.; Brabant, G. Thyroid hormones and the metabolic syndrome. Eur. Thyroid J. 2013, 2, 83–92. [Google Scholar] [CrossRef]
- Taylor, P.N.; Razvi, S.; Pearce, S.H.; Dayan, C.M. Clinical review: A review of the clinical consequences of variation in thyroid function within the reference range. J. Clin. Endocrinol. Metab. 2013, 98, 3562–3571. [Google Scholar] [CrossRef]
- Nakajima, Y.; Yamada, M.; Akuzawa, M.; Ishii, S.; Masamura, Y.; Satoh, T.; Hashimoto, K.; Negishi, M.; Shimomura, Y.; Kobayashi, I.; et al. Subclinical hypothyroidism and indices for metabolic syndrome in Japanese women: One-year follow-up study. J. Clin. Endocrinol. Metab. 2013, 98, 3280–3287. [Google Scholar] [CrossRef]
- Oh, J.Y.; Sung, Y.A.; Lee, H.J. Elevated thyroid stimulating hormone levels are associated with metabolic syndrome in euthyroid young women. Korean J. Intern. Med. 2013, 28, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Ligthart, S.; Korevaar, T.I.; Hofman, A.; Franco, O.H.; Peeters, R.P.; Dehghan, A. Thyroid function and risk of type 2 diabetes: A population-based prospective cohort study. BMC Med. 2016, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Aggarwal, S.; Khandelwal, D. Thyroid Dysfunction and Type 2 Diabetes Mellitus: Screening Strategies and Implications for Management. Diabetes Ther. 2019, 10, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Gronich, N.; Deftereos, S.N.; Lavi, I.; Persidis, A.S.; Abernethy, D.R.; Rennert, G. Hypothyroidism is a Risk Factor for New-Onset Diabetes: A Cohort Study. Diabetes Care 2015, 38, 1657–1664. [Google Scholar] [CrossRef]
- De Jong, F.J.; Masaki, K.; Chen, H.; Remaley, A.T.; Breteler, M.M.; Petrovitch, H.; White, L.R.; Launer, L.J. Thyroid function, the risk of dementia and neuropathologic changes: The Honolulu-Asia aging study. Neurobiol. Aging 2009, 30, 600–606. [Google Scholar] [CrossRef]
- Kalmijn, S.; Mehta, K.M.; Pols, H.A.; Hofman, A.; Drexhage, H.A.; Breteler, M.M. Subclinical hyperthyroidism and the risk of dementia. The Rotterdam study. Clin. Endocrinol. 2000, 53, 733–737. [Google Scholar] [CrossRef]
- Rieben, C.; Segna, D.; da Costa, B.R.; Collet, T.H.; Chaker, L.; Aubert, C.E.; Baumgartner, C.; Almeida, O.P.; Hogervorst, E.; Trompet, S.; et al. Subclinical Thyroid Dysfunction and the Risk of Cognitive Decline: A Meta-Analysis of Prospective Cohort Studies. J. Clin. Endocrinol. Metab. 2016, 101, 4945–4954. [Google Scholar] [CrossRef]
- Pasqualetti, G.; Pagano, G.; Rengo, G.; Ferrara, N.; Monzani, F. Subclinical Hypothyroidism and Cognitive Impairment: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2015, 100, 4240–4248. [Google Scholar] [CrossRef]
- George, K.M.; Lutsey, P.L.; Selvin, E.; Palta, P.; Windham, B.G.; Folsom, A.R. Association Between Thyroid Dysfunction and Incident Dementia in the Atherosclerosis Risk in Communities Neurocognitive Study. J. Endocrinol. Metab. 2019, 9, 82–89. [Google Scholar] [CrossRef]
- Akintola, A.A.; Jansen, S.W.; van Bodegom, D.; van der Grond, J.; Westendorp, R.G.; de Craen, A.J.; van Heemst, D. Subclinical hypothyroidism and cognitive function in people over 60 years: A systematic review and meta-analysis. Front. Aging Neurosci. 2015, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Goetz, T.; Glenn, T.; Whybrow, P.C. The thyroid-brain interaction in thyroid disorders and mood disorders. J. Neuroendocrinol. 2008, 20, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Correia, N.; Mullally, S.; Cooke, G.; Tun, T.K.; Phelan, N.; Feeney, J.; Fitzgibbon, M.; Boran, G.; O’Mara, S.; Gibney, J. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2009, 94, 3789–3797. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.J.; Parsons, T.D.; Whybrow, P.C.; Van Herle, K.; Rasgon, N.; Van Herle, A.; Martinez, D.; Silverman, D.H.; Bauer, M. Verbal memory retrieval deficits associated with untreated hypothyroidism. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 132–136. [Google Scholar] [CrossRef]
- Gibney, S.M.; Drexhage, H.A. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J. Neuroimmune Pharmacol. 2013, 8, 900–920. [Google Scholar] [CrossRef]
- Davis, J.D.; Tremont, G. Impact of frontal systems behavioral functioning in dementia on caregiver burden. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 43–49. [Google Scholar] [CrossRef]
- Bavarsad, K.; Hosseini, M.; Hadjzadeh, M.A.; Sahebkar, A. The effects of thyroid hormones on memory impairment and Alzheimer’s disease. J. Cell. Physiol. 2019, 234, 14633–14640. [Google Scholar] [CrossRef]
- Thvilum, M.; Brandt, F.; Almind, D.; Christensen, K.; Brix, T.H.; Hegedus, L. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: A nationwide register study. Thyroid 2014, 24, 802–808. [Google Scholar] [CrossRef]
- He, X.S.; Ma, N.; Pan, Z.L.; Wang, Z.X.; Li, N.; Zhang, X.C.; Zhou, J.N.; Zhu, D.F.; Zhang, D.R. Functional magnetic resource imaging assessment of altered brain function in hypothyroidism during working memory processing. Eur. J. Endocrinol. 2011, 164, 951–959. [Google Scholar] [CrossRef]
- Miller, K.J.; Parsons, T.D.; Whybrow, P.C.; van Herle, K.; Rasgon, N.; van Herle, A.; Martinez, D.; Silverman, D.H.; Bauer, M. Memory improvement with treatment of hypothyroidism. Int. J. Neurosci. 2006, 116, 895–906. [Google Scholar] [CrossRef]
- Zhu, D.F.; Wang, Z.X.; Zhang, D.R.; Pan, Z.L.; He, S.; Hu, X.P.; Chen, X.C.; Zhou, J.N. fMRI revealed neural substrate for reversible working memory dysfunction in subclinical hypothyroidism. Brain 2006, 129, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.M.; McLelland, V.C.; Sheard, E.; McAndrews, M.P.; Rovet, J.F. Hippocampal Functioning and Verbal Associative Memory in Adolescents with Congenital Hypothyroidism. Front. Endocrinol. 2015, 6, 163. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Ali, G.C.; Guerchet, M.; Prina, A.M.; Albanese, E.; Wu, Y.T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s Res. Ther. 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- McCrimmon, R.J.; Ryan, C.M.; Frier, B.M. Diabetes and cognitive dysfunction. Lancet 2012, 379, 2291–2299. [Google Scholar] [CrossRef]
- Exalto, L.G.; Whitmer, R.A.; Kappele, L.J.; Biessels, G.J. An update on type 2 diabetes, vascular dementia and Alzheimer’s disease. Exp. Gerontol. 2012, 47, 858–864. [Google Scholar] [CrossRef]
- Hanyu, H. Diabetes-Related Dementia. Adv. Exp. Med. Biol. 2019, 1128, 147–160. [Google Scholar] [CrossRef]
- Bhatia, P.; Singh, N. Thromboxane A2 synthase inhibition ameliorates endothelial dysfunction, memory deficits, oxidative stress and neuroinflammation in rat model of streptozotocin diabetes induced dementia. Physiol. Behav. 2021, 241, 113592. [Google Scholar] [CrossRef]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef]
- Koekkoek, P.S.; Kappelle, L.J.; van den Berg, E.; Rutten, G.E.; Biessels, G.J. Cognitive function in patients with diabetes mellitus: Guidance for daily care. Lancet Neurol. 2015, 14, 329–340. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Hua, S.; Liao, H.; Wang, M.; Xiong, Y.; Cao, F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017, 124, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Moran, C.; Beare, R.; Phan, T.; Starkstein, S.; Bruce, D.; Romina, M.; Srikanth, V. Neuroimaging and its Relevance to Understanding Pathways Linking Diabetes and Cognitive Dysfunction. J. Alzheimer’s Dis. 2017, 59, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Reijmer, Y.D. Brain changes underlying cognitive dysfunction in diabetes: What can we learn from MRI? Diabetes 2014, 63, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Ly, H.; Verma, N.; Wu, F.; Liu, M.; Saatman, K.E.; Nelson, P.T.; Slevin, J.T.; Goldstein, L.B.; Biessels, G.J.; Despa, F. Brain microvascular injury and white matter disease provoked by diabetes-associated hyperamylinemia. Ann. Neurol. 2017, 82, 208–222. [Google Scholar] [CrossRef]
- Gudala, K.; Bansal, D.; Schifano, F.; Bhansali, A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J. Diabetes Investig. 2013, 4, 640–650. [Google Scholar] [CrossRef]
- Feinkohl, I.; Price, J.F.; Strachan, M.W.; Frier, B.M. The impact of diabetes on cognitive decline: Potential vascular, metabolic, and psychosocial risk factors. Alzheimer’s Res. Ther. 2015, 7, 46. [Google Scholar] [CrossRef]
- Haroon, N.N.; Austin, P.C.; Shah, B.R.; Wu, J.; Gill, S.S.; Booth, G.L. Risk of dementia in seniors with newly diagnosed diabetes: A population-based study. Diabetes Care 2015, 38, 1868–1875. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Biessels, G.J.; Reagan, L.P. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 2015, 16, 660–671. [Google Scholar] [CrossRef]
- Van Vliet, N.A.; van Heemst, D.; Almeida, O.P.; Asvold, B.O.; Aubert, C.E.; Bae, J.B.; Barnes, L.E.; Bauer, D.C.; Blauw, G.J.; Brayne, C.; et al. Association of Thyroid Dysfunction with Cognitive Function: An Individual Participant Data Analysis. JAMA Intern. Med. 2021, 181, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Ahn, J.; Yoon, U.; Im, K.; Lee, J.M.; Tae Kim, S.; Ahn, H.J.; Chin, J.; Jeong, Y.; Na, D.L. Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2010, 20, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Z.C.; Guo, Q.H.; Cheng, W.; Chen, Y.W. Is thyroid status associated with cognitive impairment in elderly patients in China? BMC Endocr. Disord. 2016, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.P.; Plenge, P.; Sachs, B.D.; Pehrson, A.L.; Cajina, M.; Du, Y.; Roberts, W.; Rudder, M.L.; Dalvi, P.; Robinson, T.J.; et al. The interaction of escitalopram and R-citalopram at the human serotonin transporter investigated in the mouse. Psychopharmacology 2014, 231, 4527–4540. [Google Scholar] [CrossRef]
- Samuels, M.H. Thyroid disease and cognition. Endocrinol. Metab. Clin. N. Am. 2014, 43, 529–543. [Google Scholar] [CrossRef]
- Taylor, E.; Heyland, A. Evolution of thyroid hormone signaling in animals: Non-genomic and genomic modes of action. Mol. Cell. Endocrinol. 2017, 459, 14–20. [Google Scholar] [CrossRef]
- Koromilas, C.; Liapi, C.; Schulpis, K.H.; Kalafatakis, K.; Zarros, A.; Tsakiris, S. Structural and functional alterations in the hippocampus due to hypothyroidism. Metab. Brain Dis. 2010, 25, 339–354. [Google Scholar] [CrossRef]
- Fernandez-Lamo, I.; Montero-Pedrazuela, A.; Delgado-Garcia, J.M.; Guadano-Ferraz, A.; Gruart, A. Effects of thyroid hormone replacement on associative learning and hippocampal synaptic plasticity in adult hypothyroid rats. Eur. J. Neurosci. 2009, 30, 679–692. [Google Scholar] [CrossRef]
- Lauffer, P.; Zwaveling-Soonawala, N.; Naafs, J.C.; Boelen, A.; van Trotsenburg, A.S.P. Diagnosis and Management of Central Congenital Hypothyroidism. Front. Endocrinol. 2021, 12, 686317. [Google Scholar] [CrossRef]
- Dratman, M.B.; Crutchfield, F.L.; Schoenhoff, M.B. Transport of iodothyronines from bloodstream to brain: Contributions by blood:brain and choroid plexus:cerebrospinal fluid barriers. Brain Res. 1991, 554, 229–236. [Google Scholar] [CrossRef]
- Ceballos, A.; Belinchon, M.M.; Sanchez-Mendoza, E.; Grijota-Martinez, C.; Dumitrescu, A.M.; Refetoff, S.; Morte, B.; Bernal, J. Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3’-triiodo-L-thyronine. Endocrinology 2009, 150, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Heuer, H. The importance of thyroid hormone transporters for brain development and function. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.H.; Zhu, C.Z.; Yang, Y.; Zuo, X.N.; Long, X.Y.; Cao, Q.J.; Wang, Y.F.; Zang, Y.F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef]
- Sandler, B.; Webb, P.; Apriletti, J.W.; Huber, B.R.; Togashi, M.; Cunha Lima, S.T.; Juric, S.; Nilsson, S.; Wagner, R.; Fletterick, R.J.; et al. Thyroxine-thyroid hormone receptor interactions. J. Biol. Chem. 2004, 279, 55801–55808. [Google Scholar] [CrossRef]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Gusdon, A.M.; Qu, S. Cross-talk between the thyroid and liver: A new target for nonalcoholic fatty liver disease treatment. World J. Gastroenterol. 2013, 19, 8238–8246. [Google Scholar] [CrossRef]
- Schwartz, H.L.; Strait, K.A.; Ling, N.C.; Oppenheimer, J.H. Quantitation of rat tissue thyroid hormone binding receptor isoforms by immunoprecipitation of nuclear triiodothyronine binding capacity. J. Biol. Chem. 1992, 267, 11794–11799. [Google Scholar] [CrossRef]
- Heuer, H.; Mason, C.A. Thyroid hormone induces cerebellar Purkinje cell dendritic development via the thyroid hormone receptor α1. J. Neurosci. 2003, 23, 10604–10612. [Google Scholar] [CrossRef]
- Lopez-Juarez, A.; Remaud, S.; Hassani, Z.; Jolivet, P.; Pierre Simons, J.; Sontag, T.; Yoshikawa, K.; Price, J.; Morvan-Dubois, G.; Demeneix, B.A. Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell 2012, 10, 531–543. [Google Scholar] [CrossRef]
- Kapoor, R.; Desouza, L.A.; Nanavaty, I.N.; Kernie, S.G.; Vaidya, V.A. Thyroid hormone accelerates the differentiation of adult hippocampal progenitors. J. Neuroendocrinol. 2012, 24, 1259–1271. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Tousson, E.; Ibrahim, W.; Arafa, N.; Akela, M.A. Monoamine concentrations changes in the PTU-induced hypothyroid rat brain and the ameliorating role of folic acid. Hum. Exp. Toxicol. 2012, 31, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Miura, I.; Kunii, Y.; Asano, S.; Kanno-Nozaki, K.; Mashiko, H.; Yabe, H. Hashimoto encephalopathy with high plasma monoamine metabolite levels: A case report. Neuropsychiatr. Dis. Treat. 2017, 13, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Carageorgiou, H.; Pantos, C.; Zarros, A.; Stolakis, V.; Mourouzis, I.; Cokkinos, D.; Tsakiris, S. Changes in acetylcholinesterase, Na+,K+-ATPase, and Mg2+-ATPase activities in the frontal cortex and the hippocampus of hyper- and hypothyroid adult rats. Metabolism 2007, 56, 1104–1110. [Google Scholar] [CrossRef]
- Bell, R.J.; Rivera-Woll, L.; Davison, S.L.; Topliss, D.J.; Donath, S.; Davis, S.R. Well-being, health-related quality of life and cardiovascular disease risk profile in women with subclinical thyroid disease—A community-based study. Clin. Endocrinol. 2007, 66, 548–556. [Google Scholar] [CrossRef]
- DeLong, G.R.; Stanbury, J.B.; Fierro-Benitez, R. Neurological signs in congenital iodine-deficiency disorder (endemic cretinism). Dev. Med. Child Neurol. 1985, 27, 317–324. [Google Scholar] [CrossRef]
- Gulseren, S.; Gulseren, L.; Hekimsoy, Z.; Cetinay, P.; Ozen, C.; Tokatlioglu, B. Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch. Med. Res. 2006, 37, 133–139. [Google Scholar] [CrossRef]
- Demartini, B.; Ranieri, R.; Masu, A.; Selle, V.; Scarone, S.; Gambini, O. Depressive symptoms and major depressive disorder in patients affected by subclinical hypothyroidism: A cross-sectional study. J. Nerv. Ment. Dis. 2014, 202, 603–607. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Rostant, O.S.; Dore, G.A.; Fanelli-Kuczmarski, M.T.; Evans, M.K.; Zonderman, A.B. Thyroid hormones are associated with longitudinal cognitive change in an urban adult population. Neurobiol. Aging 2015, 36, 3056–3066. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Beydoun, H.A.; Kitner-Triolo, M.H.; Kaufman, J.S.; Evans, M.K.; Zonderman, A.B. Thyroid hormones are associated with cognitive function: Moderation by sex, race, and depressive symptoms. J. Clin. Endocrinol. Metab. 2013, 98, 3470–3481. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; von Muhlen, D.; Kritz-Silverstein, D.; Barrett-Connor, E. Treated hypothyroidism, cognitive function, and depressed mood in old age: The Rancho Bernardo Study. Eur. J. Endocrinol. 2009, 161, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Grondin, R.; LeMaster, W.; Martin, B.; Gold, B.T.; Ain, K.B. Reversible cognitive, motor, and driving impairments in severe hypothyroidism. Thyroid 2015, 25, 28–36. [Google Scholar] [CrossRef] [PubMed]
- SO, E.S.; Chan, I.T.; Lobo Santos, M.A.; Cohen, M.; de La Roque, P.A.M.; da Silva Almeida, J.; Simoes, A.; Givigi, H.R.; Vaisman, M.; Paixao, C.M., Jr.; et al. Impact of thyroid status and age on comprehensive geriatric assessment. Endocrine 2014, 47, 255–265. [Google Scholar] [CrossRef]
- Brownlie, B.E.; Rae, A.M.; Walshe, J.W.; Wells, J.E. Psychoses associated with thyrotoxicosis—‘thyrotoxic psychosis.’ A report of 18 cases, with statistical analysis of incidence. Eur. J. Endocrinol. 2000, 142, 438–444. [Google Scholar] [CrossRef]
- Ceresini, G.; Lauretani, F.; Maggio, M.; Ceda, G.P.; Morganti, S.; Usberti, E.; Chezzi, C.; Valcavi, R.; Bandinelli, S.; Guralnik, J.M.; et al. Thyroid function abnormalities and cognitive impairment in elderly people: Results of the Invecchiare in Chianti study. J. Am. Geriatr. Soc. 2009, 57, 89–93. [Google Scholar] [CrossRef]
- Nelson, P.T.; Katsumata, Y.; Nho, K.; Artiushin, S.C.; Jicha, G.A.; Wang, W.X.; Abner, E.L.; Saykin, A.J.; Kukull, W.A.; Fardo, D.W. Genomics and CSF analyses implicate thyroid hormone in hippocampal sclerosis of aging. Acta Neuropathol. 2016, 132, 841–858. [Google Scholar] [CrossRef]
- Accorroni, A.; Giorgi, F.S.; Donzelli, R.; Lorenzini, L.; Prontera, C.; Saba, A.; Vergallo, A.; Tognoni, G.; Siciliano, G.; Baldacci, F.; et al. Thyroid hormone levels in the cerebrospinal fluid correlate with disease severity in euthyroid patients with Alzheimer’s disease. Endocrine 2017, 55, 981–984. [Google Scholar] [CrossRef]
- Rutigliano, G.; Accorroni, A.; Zucchi, R. The Case for TAAR1 as a Modulator of Central Nervous System Function. Front. Pharmacol. 2017, 8, 987. [Google Scholar] [CrossRef]
- Wortmann, M. Dementia: A global health priority-highlights from an ADI and World Health Organization report. Alzheimer’s Res. Ther. 2012, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Han, K.; Park, S.; Cho, H.; Lee, D.Y.; Kim, J.W.; Seo, J.A.; Kim, S.G.; Baik, S.H.; Park, Y.G.; et al. Incidence and Risk Factors for Dementia in Type 2 Diabetes Mellitus: A Nationwide Population-Based Study in Korea. Diabetes Metab. J. 2020, 44, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Mirza, Z.; Kamal, M.A.; Buzenadah, A.M.; Al-Qahtani, M.H.; Karim, S. Establishing genomic/transcriptomic links between Alzheimer’s disease and type 2 diabetes mellitus by meta-analysis approach. Former. Curr. Drug Targets-CNS Neurol. Disord. 2014, 13, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.C.; Lai, S.W.; Hung, H.C.; Muo, C.H.; Hung, S.C.; Liu, L.L.; Chang, C.W.; Hwu, Y.J.; Chen, S.L.; Sung, F.C. Association between comorbidities and dementia in diabetes mellitus patients: Population-based retrospective cohort study. J. Diabetes Its Complicat. 2015, 29, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Kopf, D.; Frolich, L. Risk of incident Alzheimer’s disease in diabetic patients: A systematic review of prospective trials. J. Alzheimer’s Dis. 2009, 16, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Macauley, S.L.; Stanley, M.; Caesar, E.E.; Yamada, S.A.; Raichle, M.E.; Perez, R.; Mahan, T.E.; Sutphen, C.L.; Holtzman, D.M. Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J. Clin. Investig. 2015, 125, 2463–2467. [Google Scholar] [CrossRef]
- Boles, A.; Kandimalla, R.; Reddy, P.H. Dynamics of diabetes and obesity: Epidemiological perspective. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1026–1036. [Google Scholar] [CrossRef]
- Bomfim, T.R.; Forny-Germano, L.; Sathler, L.B.; Brito-Moreira, J.; Houzel, J.C.; Decker, H.; Silverman, M.A.; Kazi, H.; Melo, H.M.; McClean, P.L.; et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. J. Clin. Investig. 2012, 122, 1339–1353. [Google Scholar] [CrossRef]
- Verdile, G.; Fuller, S.J.; Martins, R.N. The role of type 2 diabetes in neurodegeneration. Neurobiol. Dis. 2015, 84, 22–38. [Google Scholar] [CrossRef]
- Rudolph, J.D.; de Graauw, M.; van de Water, B.; Geiger, T.; Sharan, R. Elucidation of Signaling Pathways from Large-Scale Phosphoproteomic Data Using Protein Interaction Networks. Cell Syst. 2016, 3, 585–593 e583. [Google Scholar] [CrossRef]
- Kimura, N. Diabetes Mellitus Induces Alzheimer’s Disease Pathology: Histopathological Evidence from Animal Models. Int. J. Mol. Sci. 2016, 17, 503. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Sato, N.; Uchio-Yamada, K.; Sawada, K.; Kunieda, T.; Takeuchi, D.; Kurinami, H.; Shinohara, M.; Rakugi, H.; Morishita, R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. Proc. Natl. Acad. Sci. USA 2010, 107, 7036–7041. [Google Scholar] [CrossRef] [PubMed]
- Breteler, M.M. Vascular risk factors for Alzheimer’s disease: An epidemiologic perspective. Neurobiol. Aging 2000, 21, 153–160. [Google Scholar] [CrossRef]
- Marseglia, A.; Fratiglioni, L.; Laukka, E.J.; Santoni, G.; Pedersen, N.L.; Backman, L.; Xu, W. Early Cognitive Deficits in Type 2 Diabetes: A Population-Based Study. J. Alzheimer’s Dis. 2016, 53, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.O.; Beauquis, J.; Revsin, Y.; Banzan, A.M.; Roig, P.; De Nicola, A.F.; Saravia, F. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav. Brain Res. 2009, 198, 224–230. [Google Scholar] [CrossRef]
- Taurino, F.; Stanca, E.; Siculella, L.; Trentadue, R.; Papa, S.; Zanotti, F.; Gnoni, A. Mitochondrial proteome analysis reveals depression of the Ndufs3 subunit and activity of complex I in diabetic rat brain. J. Proteom. 2012, 75, 2331–2341. [Google Scholar] [CrossRef]
- Duarte, J.M.; Oses, J.P.; Rodrigues, R.J.; Cunha, R.A. Modification of purinergic signaling in the hippocampus of streptozotocin-induced diabetic rats. Neuroscience 2007, 149, 382–391. [Google Scholar] [CrossRef]
- Neumann, K.F.; Rojo, L.; Navarrete, L.P.; Farias, G.; Reyes, P.; Maccioni, R.B. Insulin resistance and Alzheimer’s disease: Molecular links & clinical implications. Curr. Alzheimer Res. 2008, 5, 438–447. [Google Scholar] [CrossRef]
- Perez-Taboada, I.; Alberquilla, S.; Martin, E.D.; Anand, R.; Vietti-Michelina, S.; Tebeka, N.N.; Cantley, J.; Cragg, S.J.; Moratalla, R.; Vallejo, M. Diabetes Causes Dysfunctional Dopamine Neurotransmission Favoring Nigrostriatal Degeneration in Mice. Mov. Disord. 2020, 35, 1636–1648. [Google Scholar] [CrossRef]
- Grunberger, G. Novel therapies for the management of type 2 diabetes mellitus: Part 1. pramlintide and bromocriptine-QR. J. Diabetes 2013, 5, 110–117. [Google Scholar] [CrossRef]
- Andersen, J.V.; Nissen, J.D.; Christensen, S.K.; Markussen, K.H.; Waagepetersen, H.S. Impaired Hippocampal Glutamate and Glutamine Metabolism in the db/db Mouse Model of Type 2 Diabetes Mellitus. Neural Plast. 2017, 2017, 2107084. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, J.A.; Hen, R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology 2001, 155, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Marquez, R.; Hernandez-Rodriguez, J.; Medina-Serrano, J.; Boyzo-Montes de Oca, A.; Manjarrez-Gutierrez, G. Association of metabolic syndrome with reduced central serotonergic activity. Metab. Brain Dis. 2011, 26, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Maratou, E.; Hadjidakis, D.J.; Peppa, M.; Alevizaki, M.; Tsegka, K.; Lambadiari, V.; Mitrou, P.; Boutati, E.; Kollias, A.; Economopoulos, T.; et al. Studies of insulin resistance in patients with clinical and subclinical hyperthyroidism. Eur. J. Endocrinol. 2010, 163, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Dessein, P.H.; Joffe, B.I.; Stanwix, A.E. Subclinical hypothyroidism is associated with insulin resistance in rheumatoid arthritis. Thyroid 2004, 14, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Khassawneh, A.H.; Al-Mistarehi, A.H.; Zein Alaabdin, A.M.; Khasawneh, L.; AlQuran, T.M.; Kheirallah, K.A.; Saadeh, N.A.; Beni Yonis, O.; Shawkat, M.; Obeidat, N. Prevalence and Predictors of Thyroid Dysfunction Among Type 2 Diabetic Patients: A Case-Control Study. Int. J. Gen. Med. 2020, 13, 803–816. [Google Scholar] [CrossRef]
- Jun, J.E.; Jee, J.H.; Bae, J.C.; Jin, S.M.; Hur, K.Y.; Lee, M.K.; Kim, T.H.; Kim, S.W.; Kim, J.H. Association Between Changes in Thyroid Hormones and Incident Type 2 Diabetes: A Seven-Year Longitudinal Study. Thyroid 2017, 27, 29–38. [Google Scholar] [CrossRef]
- Zhao, W.; Zeng, H.; Zhang, X.; Liu, F.; Pan, J.; Zhao, J.; Zhao, J.; Li, L.; Bao, Y.; Liu, F.; et al. A high thyroid stimulating hormone level is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2016, 115, 122–129. [Google Scholar] [CrossRef]
- Fan, J.; Pan, Q.; Gao, Q.; Li, W.; Xiao, F.; Guo, L. TSH Combined with TSHR Aggravates Diabetic Peripheral Neuropathy by Promoting Oxidative Stress and Apoptosis in Schwann Cells. Oxidative Med. Cell. Longev. 2021, 2021, 2482453. [Google Scholar] [CrossRef]
- Volke, L.; Krause, K. Effect of Thyroid Hormones on Adipose Tissue Flexibility. Eur. Thyroid J. 2021, 10, 1–9. [Google Scholar] [CrossRef]
- Sinha, R.A.; You, S.H.; Zhou, J.; Siddique, M.M.; Bay, B.H.; Zhu, X.; Privalsky, M.L.; Cheng, S.Y.; Stevens, R.D.; Summers, S.A.; et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Investig. 2012, 122, 2428–2438. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, Y.; Hillgartner, F.B. Sterol regulatory element-binding protein-1 interacts with the nuclear thyroid hormone receptor to enhance acetyl-CoA carboxylase-α transcription in hepatocytes. J. Biol. Chem. 2002, 277, 19554–19565. [Google Scholar] [CrossRef] [PubMed]
- Perra, A.; Simbula, G.; Simbula, M.; Pibiri, M.; Kowalik, M.A.; Sulas, P.; Cocco, M.T.; Ledda-Columbano, G.M.; Columbano, A. Thyroid hormone (T3) and TRβ agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats. FASEB J. 2008, 22, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Cable, E.E.; Finn, P.D.; Stebbins, J.W.; Hou, J.; Ito, B.R.; van Poelje, P.D.; Linemeyer, D.L.; Erion, M.D. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology 2009, 49, 407–417. [Google Scholar] [CrossRef]
- Dentin, R.; Girard, J.; Postic, C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): Two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie 2005, 87, 81–86. [Google Scholar] [CrossRef]
- Ritter, M.J.; Amano, I.; Hollenberg, A.N. Thyroid Hormone Signaling and the Liver. Hepatology 2020, 72, 742–752. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhou, L.; Song, Y.; Ma, S.; Yu, C.; Zhao, J.; Xu, C.; Gao, L. Thyroid stimulating hormone increases hepatic gluconeogenesis via CRTC2. Mol. Cell. Endocrinol. 2017, 446, 70–80. [Google Scholar] [CrossRef]
- Teng, X.; Liu, Y.Y.; Teng, W.; Brent, G.A. COUP-TF1 Modulates Thyroid Hormone Action in an Embryonic Stem-Cell Model of Cortical Pyramidal Neuronal Differentiation. Thyroid 2018, 28, 667–678. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Brent, G.A. Thyroid hormone-dependent gene expression in differentiated embryonic stem cells and embryonal carcinoma cells: Identification of novel thyroid hormone target genes by deoxyribonucleic acid microarray analysis. Endocrinology 2005, 146, 776–783. [Google Scholar] [CrossRef]
- Morte, B.; Diez, D.; Auso, E.; Belinchon, M.M.; Gil-Ibanez, P.; Grijota-Martinez, C.; Navarro, D.; de Escobar, G.M.; Berbel, P.; Bernal, J. Thyroid hormone regulation of gene expression in the developing rat fetal cerebral cortex: Prominent role of the Ca2+/calmodulin-dependent protein kinase IV pathway. Endocrinology 2010, 151, 810–820. [Google Scholar] [CrossRef]
- Navarro, D.; Alvarado, M.; Morte, B.; Berbel, D.; Sesma, J.; Pacheco, P.; Morreale de Escobar, G.; Bernal, J.; Berbel, P. Late maternal hypothyroidism alters the expression of Camk4 in neocortical subplate neurons: A comparison with Nurr1 labeling. Cereb. Cortex 2014, 24, 2694–2706. [Google Scholar] [CrossRef] [PubMed]
- Redmond, L.; Kashani, A.H.; Ghosh, A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron 2002, 34, 999–1010. [Google Scholar] [CrossRef]
- Li, J.; Abe, K.; Milanesi, A.; Liu, Y.Y.; Brent, G.A. Thyroid Hormone Protects Primary Cortical Neurons Exposed to Hypoxia by Reducing DNA Methylation and Apoptosis. Endocrinology 2019, 160, 2243–2256. [Google Scholar] [CrossRef] [PubMed]
- Iniguez, M.A.; De Lecea, L.; Guadano-Ferraz, A.; Morte, B.; Gerendasy, D.; Sutcliffe, J.G.; Bernal, J. Cell-specific effects of thyroid hormone on RC3/neurogranin expression in rat brain. Endocrinology 1996, 137, 1032–1041. [Google Scholar] [CrossRef]
- Zhong, L.; Gerges, N.Z. Neurogranin targets calmodulin and lowers the threshold for the induction of long-term potentiation. PLoS ONE 2012, 7, e41275. [Google Scholar] [CrossRef]
- Miyakawa, T.; Yared, E.; Pak, J.H.; Huang, F.L.; Huang, K.P.; Crawley, J.N. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus 2001, 11, 763–775. [Google Scholar] [CrossRef]
- Alvarez-Dolado, M.; Ruiz, M.; Del Rio, J.A.; Alcantara, S.; Burgaya, F.; Sheldon, M.; Nakajima, K.; Bernal, J.; Howell, B.W.; Curran, T.; et al. Thyroid hormone regulates reelin and dab1 expression during brain development. J. Neurosci. 1999, 19, 6979–6993. [Google Scholar] [CrossRef]
- Sui, L.; Ren, W.W.; Li, B.M. Administration of thyroid hormone increases reelin and brain-derived neurotrophic factor expression in rat hippocampus in vivo. Brain Res. 2010, 1313, 9–24. [Google Scholar] [CrossRef]
- Jossin, Y.; Cooper, J.A. Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat. Neurosci. 2011, 14, 697–703. [Google Scholar] [CrossRef]
- Herz, J.; Chen, Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat. Rev. Neurosci. 2006, 7, 850–859. [Google Scholar] [CrossRef]
- Thompson, C.C.; Potter, G.B. Thyroid hormone action in neural development. Cereb. Cortex 2000, 10, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Weeks, P.R.; Fournier, A.E. Neuronal cytoskeleton in synaptic plasticity and regeneration. J. Neurochem. 2014, 129, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.E.; Rudas, P. Effects of congenital hypothyroidism on microtubule-associated protein-2 expression in the cerebellum of the rat. Endocrinology 1990, 126, 1276–1282. [Google Scholar] [CrossRef]
- Aniello, F.; Couchie, D.; Bridoux, A.M.; Gripois, D.; Nunez, J. Splicing of juvenile and adult tau mRNA variants is regulated by thyroid hormone. Proc. Natl. Acad. Sci. USA 1991, 88, 4035–4039. [Google Scholar] [CrossRef] [PubMed]
- Koibuchi, N.; Chin, W.W. Thyroid hormone action and brain development. Trends Endocrinol. Metab. 2000, 11, 123–128. [Google Scholar] [CrossRef]
- Kapoor, R.; Fanibunda, S.E.; Desouza, L.A.; Guha, S.K.; Vaidya, V.A. Perspectives on thyroid hormone action in adult neurogenesis. J. Neurochem. 2015, 133, 599–616. [Google Scholar] [CrossRef]
- Morte, B.; Bernal, J. Thyroid hormone action: Astrocyte-neuron communication. Front. Endocrinol. 2014, 5, 82. [Google Scholar] [CrossRef]
- Kincaid, A.E. Spontaneous circling behavior and dopamine neuron loss in a genetically hypothyroid mouse. Neuroscience 2001, 105, 891–898. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, S.M.; Kim, C.H.; Pagire, S.H.; Pagire, H.S.; Chung, H.Y.; Ahn, J.H.; Park, C.H. Dopamine neuron induction and the neuroprotective effects of thyroid hormone derivatives. Sci. Rep. 2019, 9, 13659. [Google Scholar] [CrossRef]
- Mendes-de-Aguiar, C.B.; Alchini, R.; Decker, H.; Alvarez-Silva, M.; Tasca, C.I.; Trentin, A.G. Thyroid hormone increases astrocytic glutamate uptake and protects astrocytes and neurons against glutamate toxicity. J. Neurosci. Res. 2008, 86, 3117–3125. [Google Scholar] [CrossRef]
- Losi, G.; Garzon, G.; Puia, G. Nongenomic regulation of glutamatergic neurotransmission in hippocampus by thyroid hormones. Neuroscience 2008, 151, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Guyot, R.; Rey-Millet, M.; Prieux, M.; Markossian, S.; Aubert, D.; Flamant, F. A Pivotal Genetic Program Controlled by Thyroid Hormone during the Maturation of GABAergic Neurons. iScience 2020, 23, 100899. [Google Scholar] [CrossRef]
- Hashimoto, H.; Walker, C.H.; Prange, A.J., Jr.; Mason, G.A. The effects of thyroid hormones on potassium-stimulated release of 3H-GABA by synaptosomes of rat cerebral cortex. Neuropsychopharmacology 1991, 5, 49–54. [Google Scholar] [PubMed]
- Da Settimo, A.; Primofiore, G.; Da Settimo, F.; Novellino, E.; Greco, G.; Martini, C.; Senatore, G.; Lucacehini, A. Indole derivatives as probes to study the benzodiazepine binding site in GABA receptor complex. Adv. Exp. Med. Biol. 1996, 398, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Manzano, J.; Cuadrado, M.; Morte, B.; Bernal, J. Influence of thyroid hormone and thyroid hormone receptors in the generation of cerebellar gamma-aminobutyric acid-ergic interneurons from precursor cells. Endocrinology 2007, 148, 5746–5751. [Google Scholar] [CrossRef][Green Version]
- Mason, G.A.; Walker, C.H.; Prange, A.J., Jr.; Bondy, S.C. GABA uptake is inhibited by thyroid hormones: Implications for depression. Psychoneuroendocrinology 1987, 12, 53–59. [Google Scholar] [CrossRef]
- Cortes, C.; Eugenin, E.; Aliaga, E.; Carreno, L.J.; Bueno, S.M.; Gonzalez, P.A.; Gayol, S.; Naranjo, D.; Noches, V.; Marassi, M.P.; et al. Hypothyroidism in the adult rat causes incremental changes in brain-derived neurotrophic factor, neuronal and astrocyte apoptosis, gliosis, and deterioration of postsynaptic density. Thyroid 2012, 22, 951–963. [Google Scholar] [CrossRef]
- McNay, E.C.; Recknagel, A.K. Brain insulin signaling: A key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol. Learn. Mem. 2011, 96, 432–442. [Google Scholar] [CrossRef]
- McNay, E.C.; Ong, C.T.; McCrimmon, R.J.; Cresswell, J.; Bogan, J.S.; Sherwin, R.S. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem. 2010, 93, 546–553. [Google Scholar] [CrossRef]
- Heni, M.; Kullmann, S.; Preissl, H.; Fritsche, A.; Haring, H.U. Impaired insulin action in the human brain: Causes and metabolic consequences. Nat. Rev. Endocrinol. 2015, 11, 701–711. [Google Scholar] [CrossRef]
- Babri, S.; Badie, H.G.; Khamenei, S.; Seyedlar, M.O. Intrahippocampal insulin improves memory in a passive-avoidance task in male wistar rats. Brain Cogn. 2007, 64, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.P.; Bartness, T.J.; Mielke, J.G.; Parent, M.B. A high fructose diet impairs spatial memory in male rats. Neurobiol. Learn Mem. 2009, 92, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Jolivalt, C.G.; Hurford, R.; Lee, C.A.; Dumaop, W.; Rockenstein, E.; Masliah, E. Type 1 diabetes exaggerates features of Alzheimer’s disease in APP transgenic mice. Exp. Neurol. 2010, 223, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, L.; Gouras, G.K.; Wang, R.; Gross, R.S.; Beal, M.F.; Greengard, P.; Xu, H. Stimulation of β-amyloid precursor protein trafficking by insulin reduces intraneuronal β-amyloid and requires mitogen-activated protein kinase signaling. J. Neurosci. 2001, 21, 2561–2570. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.V.; Shanley, J.; Correll, M.P.; Fieles, W.E.; Keith, R.A.; Scott, C.W.; Lee, C.M. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3β in cellular and animal models of neuronal degeneration. Proc. Natl. Acad. Sci. USA 2000, 97, 11074–11079. [Google Scholar] [CrossRef]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef]
- Tsygankova, O.M.; Feshchenko, E.; Klein, P.S.; Meinkoth, J.L. Thyroid-stimulating hormone/cAMP and glycogen synthase kinase 3β elicit opposing effects on Rap1GAP stability. J. Biol. Chem. 2004, 279, 5501–5507. [Google Scholar] [CrossRef]
- Joffe, B.I.; Distiller, L.A. Diabetes mellitus and hypothyroidism: Strange bedfellows or mutual companions? World J. Diabetes 2014, 5, 901–904. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Passaro, A.; Dalla Nora, E.; Morieri, M.L.; Soavi, C.; Sanz, J.M.; Zurlo, A.; Fellin, R.; Zuliani, G. Brain-derived neurotrophic factor plasma levels: Relationship with dementia and diabetes in the elderly population. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 294–302. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Lasley, S.M. Developmental thyroid hormone insufficiency and brain development: A role for brain-derived neurotrophic factor (BDNF)? Neuroscience 2013, 239, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Ohguni, S.; Notsu, K.; Kato, Y. Correlation of plasma free thyroxine levels with insulin sensitivity and metabolic clearance rate of insulin in patients with hyperthyroid Graves’ disease. Intern. Med. 1995, 34, 339–341. [Google Scholar] [CrossRef]
- Ruhla, S.; Arafat, A.M.; Weickert, M.O.; Osterhoff, M.; Isken, F.; Spranger, J.; Schofl, C.; Pfeiffer, A.F.; Mohlig, M. T3/rT3-ratio is associated with insulin resistance independent of TSH. Horm. Metab. Res. 2011, 43, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Mills, I.; Barge, R.M.; Silva, J.E.; Larsen, P.R. Insulin stimulation of iodothyronine 5’-deiodinase in rat brown adipocytes. Biochem. Biophys. Res. Commun. 1987, 143, 81–86. [Google Scholar] [CrossRef]
- Lisboa, P.C.; Cabanelas, A.P.; Curty, F.H.; Oliveira, K.J.; Ortiga-Carvalho, T.M.; Moura, E.G.; Nascimento-Saba, C.C.; Rosenthal, D.; Pazos-Moura, C.C. Modulation of type 2 iodothyronine deiodinase activity in rat thyroid gland. Horm. Metab. Res. 2007, 39, 538–541. [Google Scholar] [CrossRef]
- Ruhla, S.; Weickert, M.O.; Arafat, A.M.; Osterhoff, M.; Isken, F.; Spranger, J.; Schofl, C.; Pfeiffer, A.F.; Mohlig, M. A high normal TSH is associated with the metabolic syndrome. Clin. Endocrinol. 2010, 72, 696–701. [Google Scholar] [CrossRef]
- Rong, F.; Dai, H.; Wu, Y.; Li, J.; Liu, G.; Chen, H.; Zhang, X. Association between thyroid dysfunction and type 2 diabetes: A meta-analysis of prospective observational studies. BMC Med. 2021, 19, 257. [Google Scholar] [CrossRef]
- Buchberger, B.; Huppertz, H.; Krabbe, L.; Lux, B.; Mattivi, J.T.; Siafarikas, A. Symptoms of depression and anxiety in youth with type 1 diabetes: A systematic review and meta-analysis. Psychoneuroendocrinology 2016, 70, 70–84. [Google Scholar] [CrossRef]
- Wessels, A.M.; Scheltens, P.; Barkhof, F.; Heine, R.J. Hyperglycaemia as a determinant of cognitive decline in patients with type 1 diabetes. Eur. J. Pharmacol. 2008, 585, 88–96. [Google Scholar] [CrossRef]
- Salkovic-Petrisic, M.; Tribl, F.; Schmidt, M.; Hoyer, S.; Riederer, P. Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J. Neurochem. 2006, 96, 1005–1015. [Google Scholar] [CrossRef]
- Choi, Y.M.; Kim, M.K.; Kwak, M.K.; Kim, D.; Hong, E.G. Association between thyroid hormones and insulin resistance indices based on the Korean National Health and Nutrition Examination Survey. Sci. Rep. 2021, 11, 21738. [Google Scholar] [CrossRef] [PubMed]
- Heemstra, K.A.; Smit, J.W.; Eustatia-Rutten, C.F.; Heijboer, A.C.; Frolich, M.; Romijn, J.A.; Corssmit, E.P. Glucose tolerance and lipid profile in longterm exogenous subclinical hyperthyroidism and the effects of restoration of euthyroidism, a randomised controlled trial. Clin. Endocrinol. 2006, 65, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Jahagirdar, V.; McNay, E.C. Thyroid hormone’s role in regulating brain glucose metabolism and potentially modulating hippocampal cognitive processes. Metab. Brain Dis. 2012, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Zhang, S.; Guan, Y.H.; Ye, H.Y.; Zhang, Z.Y.; Zhang, Q.Y.; Xue, R.D.; Zeng, M.F.; Zuo, C.T.; Li, Y.M. Reversible changes in brain glucose metabolism following thyroid function normalization in hyperthyroidism. Am. J. Neuroradiol. 2011, 32, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, E.L.; Teixeira Sda, S.; Giannocco, G.; Machado, U.F.; Nunes, M.T. T3 rapidly increases SLC2A4 gene expression and GLUT4 trafficking to the plasma membrane in skeletal muscle of rat and improves glucose homeostasis. Thyroid 2012, 22, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Panveloski-Costa, A.C.; Silva Teixeira, S.; Ribeiro, I.M.; Serrano-Nascimento, C.; das Neves, R.X.; Favaro, R.R.; Seelaender, M.; Antunes, V.R.; Nunes, M.T. Thyroid hormone reduces inflammatory cytokines improving glycaemia control in alloxan-induced diabetic wistar rats. Acta Physiol. 2016, 217, 130–140. [Google Scholar] [CrossRef]
- Bos, M.M.; Smit, R.A.J.; Trompet, S.; van Heemst, D.; Noordam, R. Thyroid Signaling, Insulin Resistance, and 2 Diabetes Mellitus: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2017, 102, 1960–1970. [Google Scholar] [CrossRef]
- Hage, M.; Zantout, M.S.; Azar, S.T. Thyroid disorders and diabetes mellitus. J. Thyroid Res. 2011, 2011, 439463. [Google Scholar] [CrossRef]
- Caria, M.A.; Dratman, M.B.; Kow, L.M.; Mameli, O.; Pavlides, C. Thyroid hormone action: Nongenomic modulation of neuronal excitability in the hippocampus. J. Neuroendocrinol. 2009, 21, 98–107. [Google Scholar] [CrossRef]
- Cao, X.; Kambe, F.; Yamauchi, M.; Seo, H. Thyroid-hormone-dependent activation of the phosphoinositide 3-kinase/Akt cascade requires Src and enhances neuronal survival. Biochem. J. 2009, 424, 201–209. [Google Scholar] [CrossRef]
- Prieto-Almeida, F.; Panveloski-Costa, A.C.; Crunfli, F.; da Silva Teixeira, S.; Nunes, M.T.; Torrao, A.D.S. Thyroid hormone improves insulin signaling and reduces the activation of neurodegenerative pathway in the hippocampus of diabetic adult male rats. Life Sci. 2018, 192, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.S.; Cho, H.; Mu, J.; Birnbaum, M.J. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J. Biol. Chem. 2003, 278, 49530–49536. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.J.; Moloney, A.; Kelliher, M.; Johnston, J.A.; Ravid, R.; Dockery, P.; O’Connor, R.; O’Neill, C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J. Neurochem. 2005, 93, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D.; Girgis, W.; Shah, G.N. Thyroid hormone-induced GLUT-1 expression in rat cerebral tissue: Effect of age. Brain Res. 1997, 747, 144–146. [Google Scholar] [CrossRef]
- Kuruvilla, A.K.; Perez, C.; Ismail-Beigi, F.; Loeb, J.N. Regulation of glucose transport in Clone 9 cells by thyroid hormone. Biochim. Biophys. Acta 1991, 1094, 300–308. [Google Scholar] [CrossRef]
- Grozovsky, R.; Ribich, S.; Rosene, M.L.; Mulcahey, M.A.; Huang, S.A.; Patti, M.E.; Bianco, A.C.; Kim, B.W. Type 2 deiodinase expression is induced by peroxisomal proliferator-activated receptor-gamma agonists in skeletal myocytes. Endocrinology 2009, 150, 1976–1983. [Google Scholar] [CrossRef][Green Version]
- Estivalet, A.A.; Leiria, L.B.; Dora, J.M.; Rheinheimer, J.; Boucas, A.P.; Maia, A.L.; Crispim, D. D2 Thr92Ala and PPARgamma2 Pro12Ala polymorphisms interact in the modulation of insulin resistance in type 2 diabetic patients. Obesity 2011, 19, 825–832. [Google Scholar] [CrossRef]
- Sutherland, M.K.; Wong, L.; Somerville, M.J.; Handley, P.; Yoong, L.; Bergeron, C.; McLachlan, D.R. Reduction of thyroid hormone receptor c-ERB A α mRNA levels in the hippocampus of Alzheimer as compared to Huntington brain. Neurobiol. Aging 1992, 13, 301–312. [Google Scholar] [CrossRef]
- Carlsson, C.M. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, 711–722. [Google Scholar] [CrossRef]
- Bowman, G.L.; Kaye, J.A.; Moore, M.; Waichunas, D.; Carlson, N.E.; Quinn, J.F. Blood-brain barrier impairment in Alzheimer disease: Stability and functional significance. Neurology 2007, 68, 1809–1814. [Google Scholar] [CrossRef]
- Bowman, G.L.; Kaye, J.A.; Quinn, J.F. Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr. Gerontol. Geriatr. Res. 2012, 2012, 184042. [Google Scholar] [CrossRef] [PubMed]
- Heverin, M.; Bogdanovic, N.; Lutjohann, D.; Bayer, T.; Pikuleva, I.; Bretillon, L.; Diczfalusy, U.; Winblad, B.; Bjorkhem, I. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. J. Lipid Res. 2004, 45, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Papassotiropoulos, A.; Lutjohann, D.; Bagli, M.; Locatelli, S.; Jessen, F.; Buschfort, R.; Ptok, U.; Bjorkhem, I.; von Bergmann, K.; Heun, R. 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J. Psychiatr. Res. 2002, 36, 27–32. [Google Scholar] [CrossRef]
- Refolo, L.M.; Malester, B.; LaFrancois, J.; Bryant-Thomas, T.; Wang, R.; Tint, G.S.; Sambamurti, K.; Duff, K.; Pappolla, M.A. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 2000, 7, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Raffaitin, C.; Gin, H.; Empana, J.P.; Helmer, C.; Berr, C.; Tzourio, C.; Portet, F.; Dartigues, J.F.; Alperovitch, A.; Barberger-Gateau, P. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: The Three-City Study. Diabetes Care 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Bogdanovic, N.; Bretillon, L.; Lund, E.G.; Diczfalusy, U.; Lannfelt, L.; Winblad, B.; Russell, D.W.; Bjorkhem, I. On the turnover of brain cholesterol in patients with Alzheimer’s disease. Abnormal induction of the cholesterol-catabolic enzyme CYP46 in glial cells. Neurosci. Lett. 2001, 314, 45–48. [Google Scholar] [CrossRef]
- Kojro, E.; Gimpl, G.; Lammich, S.; Marz, W.; Fahrenholz, F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α-secretase ADAM 10. Proc. Natl. Acad. Sci. USA 2001, 98, 5815–5820. [Google Scholar] [CrossRef]
- Refolo, L.M.; Pappolla, M.A.; LaFrancois, J.; Malester, B.; Schmidt, S.D.; Thomas-Bryant, T.; Tint, G.S.; Wang, R.; Mercken, M.; Petanceska, S.S.; et al. A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2001, 8, 890–899. [Google Scholar] [CrossRef]
- McLaurin, J.; Darabie, A.A.; Morrison, M.R. Cholesterol, a modulator of membrane-associated Aβ-fibrillogenesis. Pharmacopsychiatry 2003, 36 (Suppl. 2), S130–S135. [Google Scholar] [CrossRef]
- Murakami, K.; Shimizu, M.; Yamada, N.; Ishibashi, S.; Shimano, H.; Yazaki, Y.; Akanuma, Y. Apolipoprotein E polymorphism is associated with plasma cholesterol response in a 7-day hospitalization study for metabolic and dietary control in NIDDM. Diabetes Care 1993, 16, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Graff-Radford, N.R.; Green, R.C.; Go, R.C.; Hutton, M.L.; Edeki, T.; Bachman, D.; Adamson, J.L.; Griffith, P.; Willis, F.B.; Williams, M.; et al. Association between apolipoprotein E genotype and Alzheimer disease in African American subjects. Arch. Neurol. 2002, 59, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.B.; Lin, V.W.; Boudreau, D.; Devine, E.B. Statins in the prevention of dementia and Alzheimer’s disease: A meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol. Drug Saf. 2013, 22, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Hyman, B.T.; Strickland, D.; Rebeck, G.W. Role of the low-density lipoprotein receptor-related protein in β-amyloid metabolism and Alzheimer disease. Arch. Neurol. 2000, 57, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.C.; Hsu, J.L.; Tung, H.Y.; Chou, C.C.; Bai, C.H. Increased dementia risk predominantly in diabetes mellitus rather than in hypertension or hyperlipidemia: A population-based cohort study. Alzheimer’s Res. Ther. 2017, 9, 7. [Google Scholar] [CrossRef]
- Helzner, E.P.; Luchsinger, J.A.; Scarmeas, N.; Cosentino, S.; Brickman, A.M.; Glymour, M.M.; Stern, Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch. Neurol. 2009, 66, 343–348. [Google Scholar] [CrossRef]
- Watanabe, T.; Koba, S.; Kawamura, M.; Itokawa, M.; Idei, T.; Nakagawa, Y.; Iguchi, T.; Katagiri, T. Small dense low-density lipoprotein and carotid atherosclerosis in relation to vascular dementia. Metabolism 2004, 53, 476–482. [Google Scholar] [CrossRef]
- Evans, R.M.; Emsley, C.L.; Gao, S.; Sahota, A.; Hall, K.S.; Farlow, M.R.; Hendrie, H. Serum cholesterol, APOE genotype, and the risk of Alzheimer’s disease: A population-based study of African Americans. Neurology 2000, 54, 240–242. [Google Scholar] [CrossRef]
- Solomon, A.; Kareholt, I.; Ngandu, T.; Wolozin, B.; Macdonald, S.W.; Winblad, B.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Serum total cholesterol, statins and cognition in non-demented elderly. Neurobiol. Aging 2009, 30, 1006–1009. [Google Scholar] [CrossRef]
- Burgess, B.L.; McIsaac, S.A.; Naus, K.E.; Chan, J.Y.; Tansley, G.H.; Yang, J.; Miao, F.; Ross, C.J.; van Eck, M.; Hayden, M.R.; et al. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer’s disease mouse models with abundant Aβ in plasma. Neurobiol. Dis. 2006, 24, 114–127. [Google Scholar] [CrossRef]
- Reynolds, C.A.; Gatz, M.; Prince, J.A.; Berg, S.; Pedersen, N.L. Serum lipid levels and cognitive change in late life. J. Am. Geriatr. Soc. 2010, 58, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Trkanjec, Z.; Bene, R.; Martinic-Popovic, I.; Jurasic, J.M.; Lisak, M.; Seric, V.; Demarin, V. Serum HDL, LDL and total cholesterol in patients with late-life onset of Alzheimer’s disease versus vascular dementia. Acta Clin. Croat 2009, 48, 259–263. [Google Scholar] [PubMed]
- Reitz, C.; Tang, M.X.; Schupf, N.; Manly, J.J.; Mayeux, R.; Luchsinger, J.A. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch. Neurol. 2010, 67, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, Y.; Feng, M.; Zhou, X.; Lu, Y.; Gao, L.; Yu, C.; Jiang, X.; Zhao, J. Thyroid-stimulating hormone decreases HMG-CoA reductase phosphorylation via AMP-activated protein kinase in the liver. J. Lipid Res. 2015, 56, 963–971. [Google Scholar] [CrossRef]
- Kuusi, T.; Saarinen, P.; Nikkila, E.A. Evidence for the role of hepatic endothelial lipase in the metabolism of plasma high density lipoprotein2 in man. Atherosclerosis 1980, 36, 589–593. [Google Scholar] [CrossRef]
- Gambo, Y.; Matsumura, M.; Fujimori, K. Triiodothyronine enhances accumulation of intracellular lipids in adipocytes through thyroid hormone receptor α via direct and indirect mechanisms. Mol. Cell. Endocrinol. 2016, 431, 1–11. [Google Scholar] [CrossRef]
- Liu, S.; Jing, F.; Yu, C.; Gao, L.; Qin, Y.; Zhao, J. AICAR-Induced Activation of AMPK Inhibits TSH/SREBP-2/HMGCR Pathway in Liver. PLoS ONE 2015, 10, e0124951. [Google Scholar] [CrossRef]
- Gao, C.X.; Yang, B.; Guo, Q.; Wei, L.H.; Tian, L.M. High thyroid-stimulating hormone level is associated with the risk of developing atherosclerosis in subclinical hypothyroidism. Horm. Metab. Res. 2015, 47, 220–224. [Google Scholar] [CrossRef]
- Klieverik, L.P.; Coomans, C.P.; Endert, E.; Sauerwein, H.P.; Havekes, L.M.; Voshol, P.J.; Rensen, P.C.; Romijn, J.A.; Kalsbeek, A.; Fliers, E. Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology 2009, 150, 5639–5648. [Google Scholar] [CrossRef]
- Nedvidkova, J.; Haluzik, M.; Bartak, V.; Dostalova, I.; Vlcek, P.; Racek, P.; Taus, M.; Behanova, M.; Svacina, S.; Alesci, S.; et al. Changes of noradrenergic activity and lipolysis in the subcutaneous abdominal adipose tissue of hypo- and hyperthyroid patients: An in vivo microdialysis study. Ann. N. Y. Acad. Sci. 2004, 1018, 541–549. [Google Scholar] [CrossRef]
- Lam, K.S.; Chan, M.K.; Yeung, R.T. High-density lipoprotein cholesterol, hepatic lipase and lipoprotein lipase activities in thyroid dysfunction--effects of treatment. QJM Int. J. Med. 1986, 59, 513–521. [Google Scholar]
- D’Ambrosio, R.; Campi, I.; Maggioni, M.; Perbellini, R.; Giammona, E.; Stucchi, R.; Borghi, M.; Degasperi, E.; De Silvestri, A.; Persani, L.; et al. The relationship between liver histology and thyroid function tests in patients with non-alcoholic fatty liver disease (NAFLD). PLoS ONE 2021, 16, e0249614. [Google Scholar] [CrossRef]
- Hazlehurst, J.M.; Tomlinson, J.W. Non-alcoholic fatty liver disease in common endocrine disorders. Eur. J. Endocrinol. 2013, 169, R27–R37. [Google Scholar] [CrossRef]
- Tan, K.C.; Shiu, S.W.; Kung, A.W. Plasma cholesteryl ester transfer protein activity in hyper- and hypothyroidism. J. Clin. Endocrinol. Metab. 1998, 83, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, L.; Wang, F.; Yuan, Z.; Zhang, X.; Xu, C.; Song, Y.; Guan, Q.; Gao, L.; Shan, Z.; et al. A Worthy Finding: Decrease in Total Cholesterol and Low-Density Lipoprotein Cholesterol in Treated Mild Subclinical Hypothyroidism. Thyroid 2016, 26, 1019–1029. [Google Scholar] [CrossRef]
- Pearce, E.N. Update in lipid alterations in subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2012, 97, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Willard, D.L.; Leung, A.M.; Pearce, E.N. Thyroid function testing in patients with newly diagnosed hyperlipidemia. JAMA Intern. Med. 2014, 174, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Arikan, S.; Bahceci, M.; Tuzcu, A.; Celik, F.; Gokalp, D. Postprandial hyperlipidemia in overt and subclinical hypothyroidism. Eur. J. Intern. Med. 2012, 23, e141–e145. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Daya, N.; Lutsey, P.L.; Matsushita, K.; Fretz, A.; McEvoy, J.W.; Blumenthal, R.S.; Coresh, J.; Greenland, P.; Kottgen, A.; et al. Thyroid Function, Cardiovascular Risk Factors, and Incident Atherosclerotic Cardiovascular Disease: The Atherosclerosis Risk in Communities (ARIC) Study. J. Clin. Endocrinol. Metab. 2017, 102, 3306–3315. [Google Scholar] [CrossRef]
- Dong, X.; Yao, Z.; Hu, Y.; Yang, N.; Gao, X.; Xu, Y.; Wang, G. Potential harmful correlation between homocysteine and low-density lipoprotein cholesterol in patients with hypothyroidism. Medicine 2016, 95, e4291. [Google Scholar] [CrossRef]
- Stein, S.; Stepan, H.; Kratzsch, J.; Verlohren, M.; Verlohren, H.J.; Drynda, K.; Lossner, U.; Bluher, M.; Stumvoll, M.; Fasshauer, M. Serum fibroblast growth factor 21 levels in gestational diabetes mellitus in relation to insulin resistance and dyslipidemia. Metabolism 2010, 59, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zeng, J.; Huang, P.; Yan, B.; Zeng, X.; Liu, C.; Shi, X.; Wang, L.; Song, H.; Lin, M.; et al. Independent Association of Serum Fibroblast Growth Factor 21 Levels With Impaired Liver Enzymes in Hyperthyroid Patients. Front. Endocrinol. 2018, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.C.; Astapova, I.; Fisher, F.M.; Badman, M.K.; Kurgansky, K.E.; Flier, J.S.; Hollenberg, A.N.; Maratos-Flier, E. Thyroid hormone regulates hepatic expression of fibroblast growth factor 21 in a PPARα-dependent manner. J. Biol. Chem. 2010, 285, 14078–14082. [Google Scholar] [CrossRef] [PubMed]
- van der Boom, T.; Jia, C.; Lefrandt, J.D.; Connelly, M.A.; Links, T.P.; Tietge, U.J.F.; Dullaart, R.P.F. HDL Cholesterol Efflux Capacity is Impaired in Severe Short-Term Hypothyroidism Despite Increased HDL Cholesterol. J. Clin. Endocrinol. Metab. 2020, 105, E3355–E3362. [Google Scholar] [CrossRef]
- Gharib, H.; Tuttle, R.M.; Baskin, H.J.; Fish, L.H.; Singer, P.A.; McDermott, M.T.; American Association of Clinical, E.; American Thyroid, A.; Endocrine, S. Consensus Statement #1: Subclinical thyroid dysfunction: A joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and The Endocrine Society. Thyroid 2005, 15, 24–28, response 32–23. [Google Scholar] [CrossRef]
- Hugo, J.; Ganguli, M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef]
- Vanderpump, M.P. The epidemiology of thyroid disease. Br. Med. Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef]
- Modesto, T.; Tiemeier, H.; Peeters, R.P.; Jaddoe, V.W.; Hofman, A.; Verhulst, F.C.; Ghassabian, A. Maternal Mild Thyroid Hormone Insufficiency in Early Pregnancy and Attention-Deficit/Hyperactivity Disorder Symptoms in Children. JAMA Pediatr. 2015, 169, 838–845. [Google Scholar] [CrossRef]
- Sweatt, J.D. Hippocampal function in cognition. Psychopharmacology 2004, 174, 99–110. [Google Scholar] [CrossRef]
- Salazar, P.; Cisternas, P.; Codocedo, J.F.; Inestrosa, N.C. Induction of hypothyroidism during early postnatal stages triggers a decrease in cognitive performance by decreasing hippocampal synaptic plasticity. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 870–883. [Google Scholar] [CrossRef]
- Chaalal, A.; Poirier, R.; Blum, D.; Laroche, S.; Enderlin, V. Thyroid Hormone Supplementation Restores Spatial Memory, Hippocampal Markers of Neuroinflammation, Plasticity-Related Signaling Molecules, and β-Amyloid Peptide Load in Hypothyroid Rats. Mol. Neurobiol. 2019, 56, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Kim, J.W.; Yoo, D.Y.; Jung, H.Y.; Chung, J.Y.; Kim, D.W.; Hwang, I.K.; Yoon, Y.S. Hypothyroidism increases cyclooxygenase-2 levels and pro-inflammatory response and decreases cell proliferation and neuroblast differentiation in the hippocampus. Mol. Med. Rep. 2018, 17, 5782–5788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lin, X.; Liu, A.; Chen, J.; Shan, Z.; Teng, W.; Yu, X. Maternal Subclinical Hypothyroidism in Rats Impairs Spatial Learning and Memory in Offspring by Disrupting Balance of the TrkA/p75(NTR) Signal Pathway. Mol. Neurobiol. 2021, 58, 4237–4250. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Anderson, W.L.; Gilbert, M.E. Impairment in short-term but enhanced long-term synaptic potentiation and ERK activation in adult hippocampal area CA1 following developmental thyroid hormone insufficiency. Toxicol. Sci. 2005, 85, 647–656. [Google Scholar] [CrossRef]
- Parsaik, A.K.; Singh, B.; Roberts, R.O.; Pankratz, S.; Edwards, K.K.; Geda, Y.E.; Gharib, H.; Boeve, B.F.; Knopman, D.S.; Petersen, R.C. Hypothyroidism and risk of mild cognitive impairment in elderly persons: A population-based study. JAMA Neurol. 2014, 71, 201–207. [Google Scholar] [CrossRef]
- Dong, J.; Yin, H.; Liu, W.; Wang, P.; Jiang, Y.; Chen, J. Congenital iodine deficiency and hypothyroidism impair LTP and decrease C-fos and C-jun expression in rat hippocampus. Neurotoxicology 2005, 26, 417–426. [Google Scholar] [CrossRef]
- Sui, L.; Gilbert, M.E. Pre- and postnatal propylthiouracil-induced hypothyroidism impairs synaptic transmission and plasticity in area CA1 of the neonatal rat hippocampus. Endocrinology 2003, 144, 4195–4203. [Google Scholar] [CrossRef]
- Gerges, N.Z.; Alkadhi, K.A. Hypothyroidism impairs late LTP in CA1 region but not in dentate gyrus of the intact rat hippocampus: MAPK involvement. Hippocampus 2004, 14, 40–45. [Google Scholar] [CrossRef]
- Gilbert, M.E. Alterations in synaptic transmission and plasticity in area CA1 of adult hippocampus following developmental hypothyroidism. Brain Res. Dev. Brain Res. 2004, 148, 11–18. [Google Scholar] [CrossRef]
- Harper, P.C.; Roe, C.M. Thyroid medication use and subsequent development of dementia of the Alzheimer type. J. Geriatr. Psychiatry Neurol. 2010, 23, 63–69. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.K.; Song, J. Hypothyroidism and Diabetes-Related Dementia: Focused on Neuronal Dysfunction, Insulin Resistance, and Dyslipidemia. Int. J. Mol. Sci. 2022, 23, 2982. https://doi.org/10.3390/ijms23062982
Kim HK, Song J. Hypothyroidism and Diabetes-Related Dementia: Focused on Neuronal Dysfunction, Insulin Resistance, and Dyslipidemia. International Journal of Molecular Sciences. 2022; 23(6):2982. https://doi.org/10.3390/ijms23062982
Chicago/Turabian StyleKim, Hee Kyung, and Juhyun Song. 2022. "Hypothyroidism and Diabetes-Related Dementia: Focused on Neuronal Dysfunction, Insulin Resistance, and Dyslipidemia" International Journal of Molecular Sciences 23, no. 6: 2982. https://doi.org/10.3390/ijms23062982
APA StyleKim, H. K., & Song, J. (2022). Hypothyroidism and Diabetes-Related Dementia: Focused on Neuronal Dysfunction, Insulin Resistance, and Dyslipidemia. International Journal of Molecular Sciences, 23(6), 2982. https://doi.org/10.3390/ijms23062982






