Effect of Bacterial Infection on Ghrelin Receptor Regulation in Periodontal Cells and Tissues
Abstract
1. Introduction
2. Results
2.1. Expression of GHS-R in Rat Periodontal Tissues In Vivo
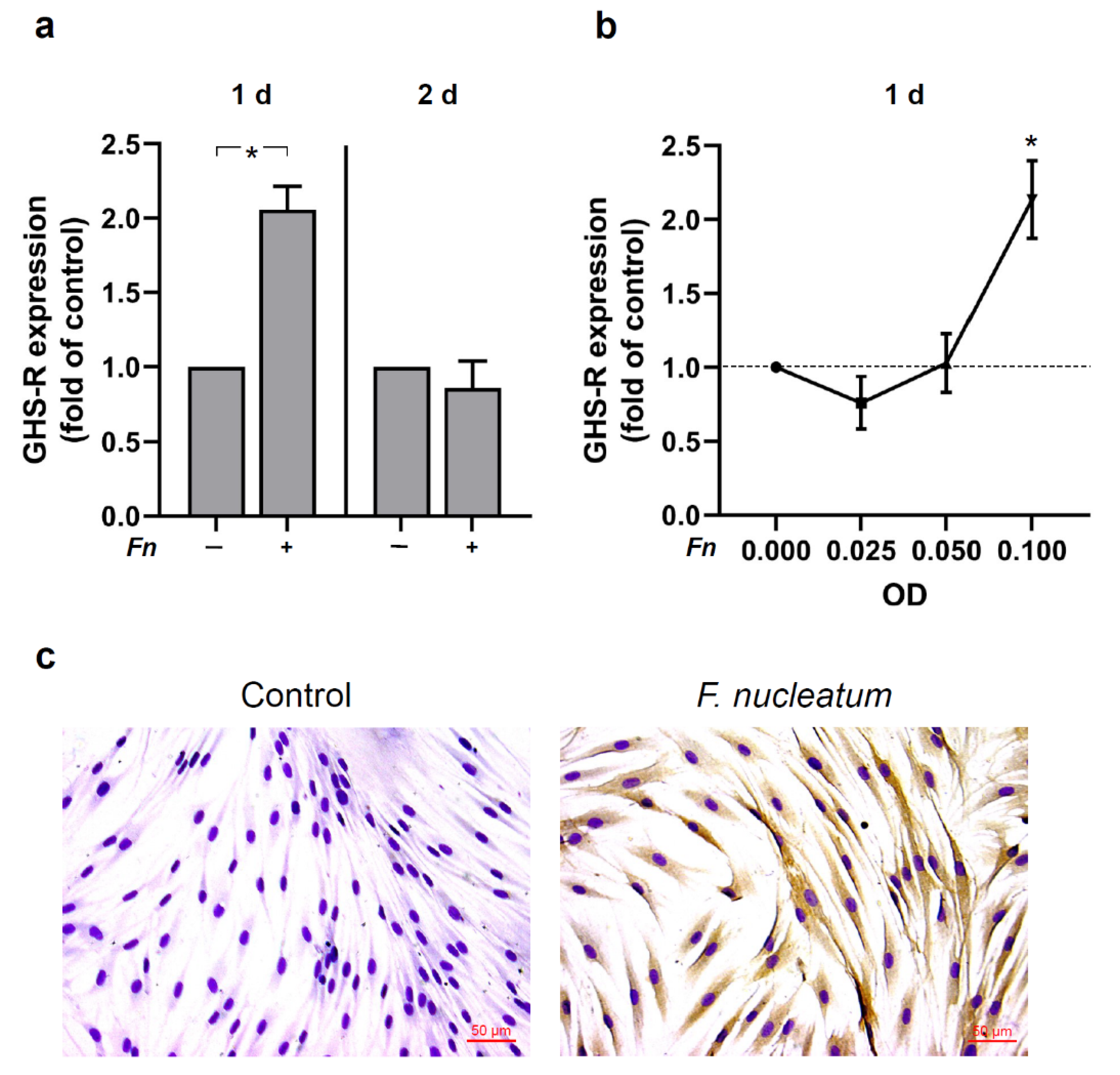
2.2. Regulation of GHS-R by F. nucleatum in HGFs In Vitro
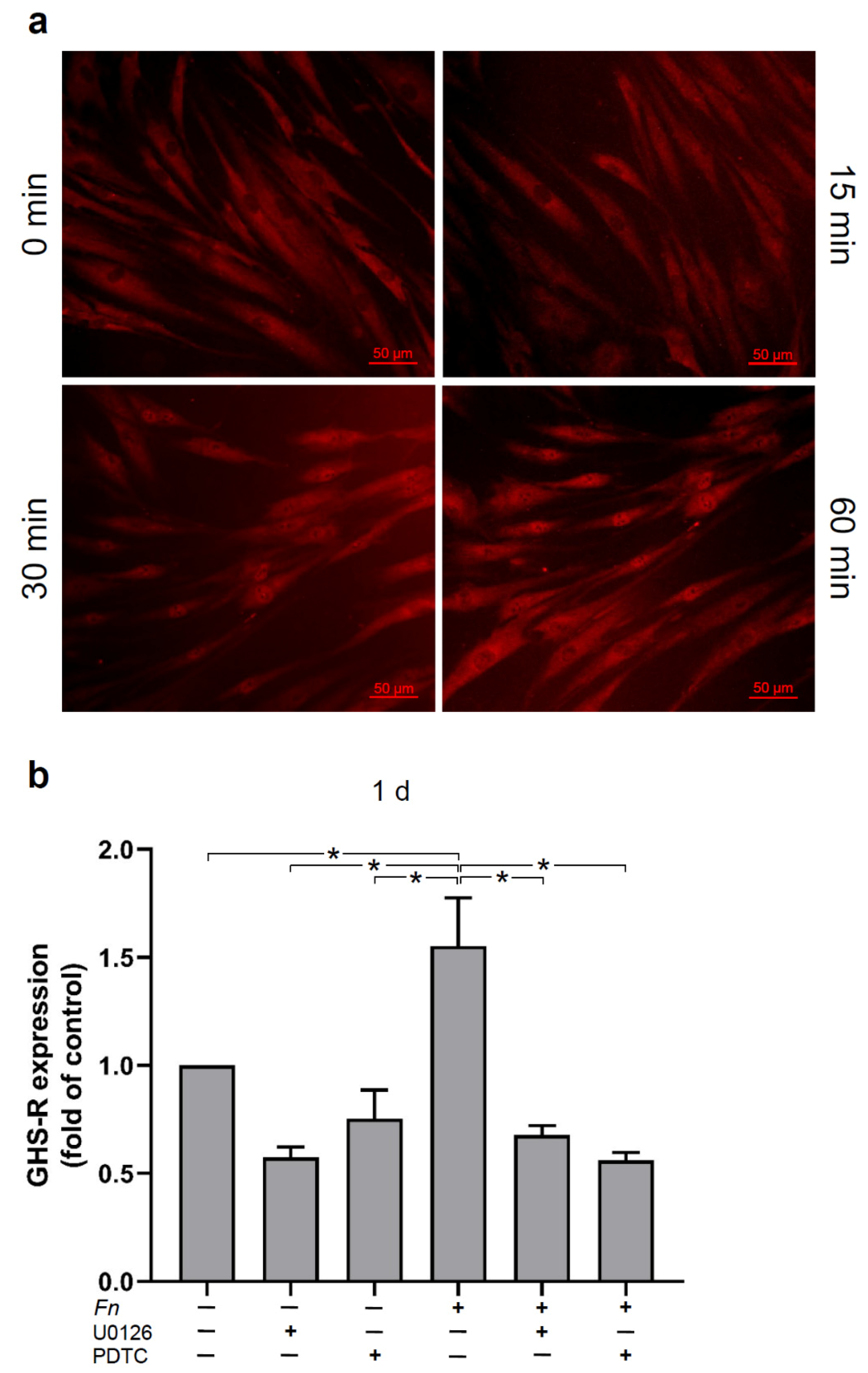
2.3. Signaling Pathways Involved in the Regulation of GHS-R by F. nucleatum in HGFs In Vitro
2.4. Effects of Ghrelin on the Expressions of Proinflammatory and Chemotactic Cytokines in HGFs In Vitro
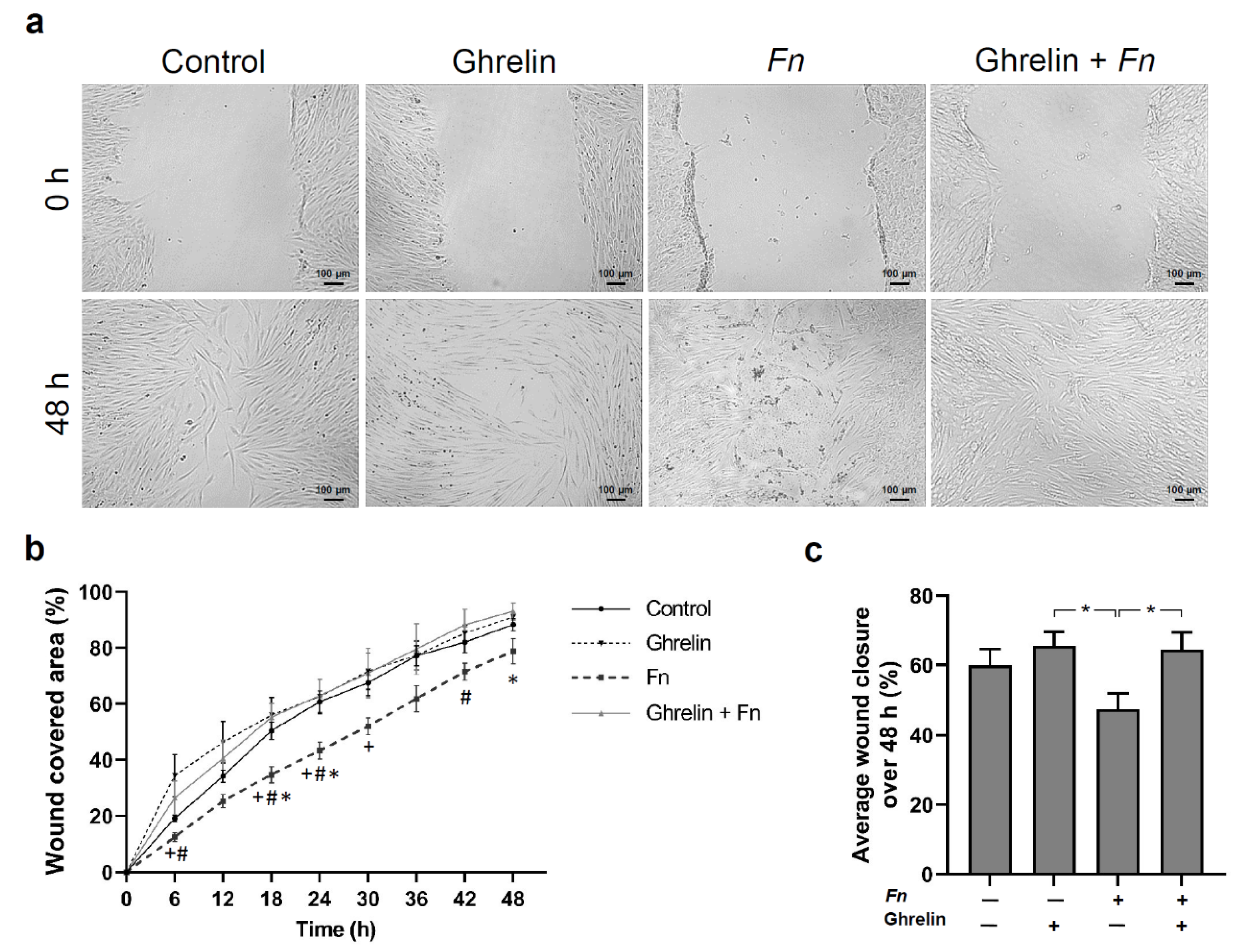
2.5. Effects of Ghrelin and F. nucleatum on Wound Healing, Proliferation, and Viability of HGFs In Vitro
3. Discussion
4. Materials and Methods
4.1. Experimental Periodontal Disease Model
4.2. Immunohistochemistry Analysis
4.3. Histomorphometric Analysis
4.4. Cell Culture and Treatment
4.5. Real-Time PCR
4.6. Immunocytochemistry
4.7. Immunofluorescence
4.8. In Vitro Wound Healing, Cell Viability, Proliferation, and Migration
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nocini, R.; Lippi, G.; Mattiuzzi, C. Periodontal disease: The portrait of an epidemic. J. Public Health Emerg. 2020, 4. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Beikler, T.; Flemmig, T.F. Oral biofilm-associated diseases: Trends and implications for quality of life, systemic health and expenditures. Periodontology 2000 2011, 55, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Machado, V.; Leira, Y.; Proenca, L.; Chambrone, L.; Mendes, J.J. Economic burden of periodontitis in the United States and Europe—An updated estimation. J. Periodontol. 2021. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Sbordone, L.; Bortolaia, C. Oral microbial biofilms and plaque-related diseases: Microbial communities and their role in the shift from oral health to disease. Clin. Oral Investig. 2003, 7, 181–188. [Google Scholar] [CrossRef]
- Yucel-Lindberg, T.; Bage, T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 2013, 15, e7. [Google Scholar] [CrossRef]
- Tatakis, D.N.; Kumar, P.S. Etiology and pathogenesis of periodontal diseases. Dent. Clin. N. Am. 2005, 49, 491–516. [Google Scholar] [CrossRef]
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-analyses. J. Dent. Res. 2021, 100, 37–49. [Google Scholar] [CrossRef]
- Stohr, J.; Barbaresko, J.; Neuenschwander, M.; Schlesinger, S. Bidirectional association between periodontal disease and diabetes mellitus: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 13686. [Google Scholar] [CrossRef]
- Gobin, R.; Tian, D.; Liu, Q.; Wang, J. Periodontal Diseases and the Risk of Metabolic Syndrome: An Updated Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Herrera, M.; Silvestre-Rangil, J.; Silvestre, F.J. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e708–e715. [Google Scholar] [CrossRef] [PubMed]
- Gaio, E.J.; Haas, A.N.; Rosing, C.K.; Oppermann, R.V.; Albandar, J.M.; Susin, C. Effect of obesity on periodontal attachment loss progression: A 5-year population-based prospective study. J. Clin. Periodontol. 2016, 43, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Moura-Grec, P.G.; Marsicano, J.A.; Carvalho, C.A.; Sales-Peres, S.H. Obesity and periodontitis: Systematic review and meta-analysis. Cien. Saude Colet. 2014, 19, 1763–1772. [Google Scholar] [CrossRef]
- Slotwinska, S.M. Ghrelin and oral diseases. Cent. Eur. J. Immunol. 2020, 45, 433–438. [Google Scholar] [CrossRef]
- Alamri, B.N.; Shin, K.; Chappe, V.; Anini, Y. The role of ghrelin in the regulation of glucose homeostasis. Horm. Mol. Biol. Clin. Investig. 2016, 26, 3–11. [Google Scholar] [CrossRef]
- Gil-Campos, M.; Aguilera, C.M.; Canete, R.; Gil, A. Ghrelin: A hormone regulating food intake and energy homeostasis. Br. J. Nutr. 2006, 96, 201–226. [Google Scholar] [CrossRef]
- Cummings, D.E.; Weigle, D.S.; Frayo, R.S.; Breen, P.A.; Ma, M.K.; Dellinger, E.P.; Purnell, J.Q. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 2002, 346, 1623–1630. [Google Scholar] [CrossRef]
- Horvath, T.L.; Diano, S.; Sotonyi, P.; Heiman, M.; Tschop, M. Minireview: Ghrelin and the regulation of energy balance--a hypothalamic perspective. Endocrinology 2001, 142, 4163–4169. [Google Scholar] [CrossRef]
- Gahete, M.D.; Rincon-Fernandez, D.; Villa-Osaba, A.; Hormaechea-Agulla, D.; Ibanez-Costa, A.; Martinez-Fuentes, A.J.; Gracia-Navarro, F.; Castano, J.P.; Luque, R.M. Ghrelin gene products, receptors, and GOAT enzyme: Biological and pathophysiological insight. J. Endocrinol. 2014, 220, R1–R24. [Google Scholar] [CrossRef]
- Khatib, M.N.; Shankar, A.H.; Kirubakaran, R.; Gaidhane, A.; Gaidhane, S.; Simkhada, P.; Syed, Z.Q. Ghrelin for the management of cachexia associated with cancer. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef][Green Version]
- Chen, J.A.; Splenser, A.; Guillory, B.; Luo, J.; Mendiratta, M.; Belinova, B.; Halder, T.; Zhang, G.; Li, Y.P.; Garcia, J.M. Ghrelin prevents tumour- and cisplatin-induced muscle wasting: Characterization of multiple mechanisms involved. J. Cachexia Sarcopenia Muscle 2015, 6, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Waseem, T.; Duxbury, M.; Ito, H.; Ashley, S.W.; Robinson, M.K. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 2008, 143, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.D.; Schaffer, E.M.; Pyle, R.S.; Collins, G.D.; Sakthivel, S.K.; Palaniappan, R.; Lillard, J.W., Jr.; Taub, D.D. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Investig. 2004, 114, 57–66. [Google Scholar] [CrossRef]
- Date, Y.; Kojima, M.; Hosoda, H.; Sawaguchi, A.; Mondal, M.S.; Suganuma, T.; Matsukura, S.; Kangawa, K.; Nakazato, M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141, 4255–4261. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Adams, E.F.; Huang, B.; Buchfelder, M.; Howard, A.; Smith, R.G.; Feighner, S.D.; van der Ploeg, L.H.; Bowers, C.Y.; Fahlbusch, R. Presence of growth hormone secretagogue receptor messenger ribonucleic acid in human pituitary tumors and rat GH3 cells. J. Clin. Endocrinol. Metab. 1998, 83, 638–642. [Google Scholar] [CrossRef]
- Howard, A.D.; Feighner, S.D.; Cully, D.F.; Arena, J.P.; Liberator, P.A.; Rosenblum, C.I.; Hamelin, M.; Hreniuk, D.L.; Palyha, O.C.; Anderson, J.; et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996, 273, 974–977. [Google Scholar] [CrossRef]
- Gnanapavan, S.; Kola, B.; Bustin, S.A.; Morris, D.G.; McGee, P.; Fairclough, P.; Bhattacharya, S.; Carpenter, R.; Grossman, A.B.; Korbonits, M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002, 87, 2988. [Google Scholar] [CrossRef]
- Papotti, M.; Ghe, C.; Cassoni, P.; Catapano, F.; Deghenghi, R.; Ghigo, E.; Muccioli, G. Growth hormone secretagogue binding sites in peripheral human tissues. J. Clin. Endocrinol. Metab. 2000, 85, 3803–3807. [Google Scholar] [CrossRef]
- Nokhbehsaim, M.; Memmert, S.; Damanaki, A.; Nanayakkara, S.; Zhou, X.; Jager, A.; Deschner, J. Effect of interleukin-1beta on ghrelin receptor in periodontal cells. Clin. Oral Investig. 2019, 23, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Nokhbehsaim, M.; Nogueira, A.V.B.; Memmert, S.; Damanaki, A.; Eick, S.; Cirelli, J.A.; Deschner, J. Regulation of ghrelin receptor by microbial and inflammatory signals in human osteoblasts. Braz. Oral Res. 2019, 33, e025. [Google Scholar] [CrossRef] [PubMed]
- Nokhbehsaim, M.; Damanaki, A.; Nogueira, A.V.B.; Eick, S.; Memmert, S.; Zhou, X.; Nanayakkara, S.; Gotz, W.; Cirelli, J.A.; Jager, A.; et al. Regulation of Ghrelin Receptor by Periodontal Bacteria In Vitro and In Vivo. Mediat. Inflamm. 2017, 2017, 4916971. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.F.R.; Arnold, N.; Richter, V.; Deschner, J.; Kantyka, T.; Eick, S. Salivary, gingival crevicular fluid and serum levels of ghrelin and chemerin in patients with periodontitis and overweight. J. Periodontal. Res. 2017, 52, 1050–1057. [Google Scholar] [CrossRef]
- Mohamed, H.G.; Idris, S.B.; Mustafa, M.; Ahmed, M.F.; Astrom, A.N.; Mustafa, K.; Ibrahim, S.O. Impact of Chronic Periodontitis on Levels of Glucoregulatory Biomarkers in Gingival Crevicular Fluid of Adults with and without Type 2 Diabetes. PLoS ONE 2015, 10, e0127660. [Google Scholar] [CrossRef]
- Yilmaz, G.; Kirzioglu, F.Y.; Doguc, D.K.; Kocak, H.; Orhan, H. Ghrelin levels in chronic periodontitis patients. Odontology 2014, 102, 59–67. [Google Scholar] [CrossRef]
- Warzecha, Z.; Kownacki, P.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Dembinski, A. Ghrelin accelerates the healing of oral ulcers in non-sialoadenectomized and sialoadenectomized rats. J. Physiol. Pharmacol. 2013, 64, 657–668. [Google Scholar]
- Kang, W.; Jia, Z.; Tang, D.; Zhang, Z.; Gao, H.; He, K.; Feng, Q. Fusobacterium nucleatum Facilitates Apoptosis, ROS Generation, and Inflammatory Cytokine Production by Activating AKT/MAPK and NF-kappaB Signaling Pathways in Human Gingival Fibroblasts. Oxid. Med. Cell Longev. 2019, 2019, 1681972. [Google Scholar] [CrossRef]
- Rath-Deschner, B.; Memmert, S.; Damanaki, A.; Nokhbehsaim, M.; Eick, S.; Cirelli, J.A.; Gotz, W.; Deschner, J.; Jager, A.; Nogueira, A.V.B. CXCL1, CCL2, and CCL5 modulation by microbial and biomechanical signals in periodontal cells and tissues-in vitro and in vivo studies. Clin. Oral Investig. 2020, 24, 3661–3670. [Google Scholar] [CrossRef]
- Khazaei, M.; Tahergorabi, Z. Serum inflammatory markers in obese mice: Effect of ghrelin. Adv. Biomed. Res. 2015, 4, 145. [Google Scholar] [CrossRef]
- Deng, B.; Fang, F.; Yang, T.; Yu, Z.; Zhang, B.; Xie, X. Ghrelin inhibits AngII -induced expression of TNF-alpha, IL-8, MCP-1 in human umbilical vein endothelial cells. Int. J. Clin. Exp. Med. 2015, 8, 579–588. [Google Scholar] [PubMed]
- Qu, R.; Chen, X.; Hu, J.; Fu, Y.; Peng, J.; Li, Y.; Chen, J.; Li, P.; Liu, L.; Cao, J.; et al. Ghrelin protects against contact dermatitis and psoriasiform skin inflammation by antagonizing TNF-alpha/NF-kappaB signaling pathways. Sci. Rep. 2019, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Z.; He, X.; Yu, H.; Feng, J. Ghrelin Attenuates Neuroinflammation and Demyelination in Experimental Autoimmune Encephalomyelitis Involving NLRP3 Inflammasome Signaling Pathway and Pyroptosis. Front. Pharmacol. 2019, 10, 1320. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Feng, L. Ghrelin Regulates Cyclooxygenase-2 Expression and Promotes Gastric Cancer Cell Progression. Comput. Math. Methods Med. 2021, 2021, 5576808. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, J.; Li, H.; Yang, Z.; Zeng, Y.; Liu, J.; Hao, Y.; Li, R. Ghrelin accelerates wound healing through GHS-R1a-mediated MAPK-NF-kappaB/GR signaling pathways in combined radiation and burn injury in rats. Sci. Rep. 2016, 6, 27499. [Google Scholar] [CrossRef]
- Liu, C.; Hao, Y.; Huang, J.; Li, H.; Yang, Z.; Zeng, Y.; Liu, J.; Li, R. Ghrelin accelerates wound healing in combined radiation and wound injury in mice. Exp. Dermatol. 2017, 26, 186–193. [Google Scholar] [CrossRef]
- He, J.; Huang, W.; Pan, Z.; Cui, H.; Qi, G.; Zhou, X.; Chen, H. Quantitative analysis of microbiota in saliva, supragingival, and subgingival plaque of Chinese adults with chronic periodontitis. Clin. Oral Investig. 2012, 16, 1579–1588. [Google Scholar] [CrossRef]
- Signat, B.; Roques, C.; Poulet, P.; Duffaut, D. Fusobacterium nucleatum in periodontal health and disease. Curr. Issues Mol. Biol. 2011, 13, 25–36. [Google Scholar]
- Dabija-Wolter, G.; Cimpan, M.R.; Costea, D.E.; Johannessen, A.C.; Sornes, S.; Neppelberg, E.; Al-Haroni, M.; Skaug, N.; Bakken, V. Fusobacterium nucleatum enters normal human oral fibroblasts in vitro. J. Periodontol. 2009, 80, 1174–1183. [Google Scholar] [CrossRef]
- Groschl, M.; Topf, H.G.; Bohlender, J.; Zenk, J.; Klussmann, S.; Dotsch, J.; Rascher, W.; Rauh, M. Identification of ghrelin in human saliva: Production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin. Chem. 2005, 51, 997–1006. [Google Scholar] [CrossRef]
- Nogueira, A.V.; de Molon, R.S.; Nokhbehsaim, M.; Deschner, J.; Cirelli, J.A. Contribution of biomechanical forces to inflammation-induced bone resorption. J. Clin. Periodontol. 2017, 44, 31–41. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Rath-Deschner, B.; Nogueira, A.V.B.; Beisel-Memmert, S.; Nokhbehsaim, M.; Eick, S.; Cirelli, J.A.; Deschner, J.; Jager, A.; Damanaki, A. Interaction of periodontitis and orthodontic tooth movement-an in vitro and in vivo study. Clin. Oral Investig. 2022, 26, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Weusmann, J.; Deschner, J.; Imber, J.C.; Damanaki, A.; Leguizamon, N.D.P.; Nogueira, A.V.B. Cellular effects of glycine and trehalose air-polishing powders on human gingival fibroblasts in vitro. Clin. Oral Investig. 2021, 26, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Memmert, S.; Nokhbehsaim, M.; Damanaki, A.; Nogueira, A.V.B.; Papadopoulou, A.K.; Piperi, C.; Basdra, E.K.; Rath-Deschner, B.; Gotz, W.; Cirelli, J.A.; et al. Role of cathepsin S In periodontal wound healing-an in vitro study on human PDL cells. BMC Oral Health 2018, 18, 60. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, A.V.B.; Nokhbehsaim, M.; Damanaki, A.; Eick, S.; Beisel-Memmert, S.; Kirschneck, C.; Schröder, A.; Cirelli, T.; Leguizamón, N.D.P.; Cirelli, J.A.; et al. Effect of Bacterial Infection on Ghrelin Receptor Regulation in Periodontal Cells and Tissues. Int. J. Mol. Sci. 2022, 23, 3039. https://doi.org/10.3390/ijms23063039
Nogueira AVB, Nokhbehsaim M, Damanaki A, Eick S, Beisel-Memmert S, Kirschneck C, Schröder A, Cirelli T, Leguizamón NDP, Cirelli JA, et al. Effect of Bacterial Infection on Ghrelin Receptor Regulation in Periodontal Cells and Tissues. International Journal of Molecular Sciences. 2022; 23(6):3039. https://doi.org/10.3390/ijms23063039
Chicago/Turabian StyleNogueira, Andressa V. B., Marjan Nokhbehsaim, Anna Damanaki, Sigrun Eick, Svenja Beisel-Memmert, Christian Kirschneck, Agnes Schröder, Thamiris Cirelli, Natalia D. P. Leguizamón, Joni A. Cirelli, and et al. 2022. "Effect of Bacterial Infection on Ghrelin Receptor Regulation in Periodontal Cells and Tissues" International Journal of Molecular Sciences 23, no. 6: 3039. https://doi.org/10.3390/ijms23063039
APA StyleNogueira, A. V. B., Nokhbehsaim, M., Damanaki, A., Eick, S., Beisel-Memmert, S., Kirschneck, C., Schröder, A., Cirelli, T., Leguizamón, N. D. P., Cirelli, J. A., & Deschner, J. (2022). Effect of Bacterial Infection on Ghrelin Receptor Regulation in Periodontal Cells and Tissues. International Journal of Molecular Sciences, 23(6), 3039. https://doi.org/10.3390/ijms23063039






