BMP-2 Long-Term Stimulation of Human Pre-Osteoblasts Induces Osteogenic Differentiation and Promotes Transdifferentiation and Bone Remodeling Processes
Abstract
:1. Introduction
2. Results
2.1. Effect of BMP-2 on Viability and ALP Activity in Human Pre-Osteoblasts
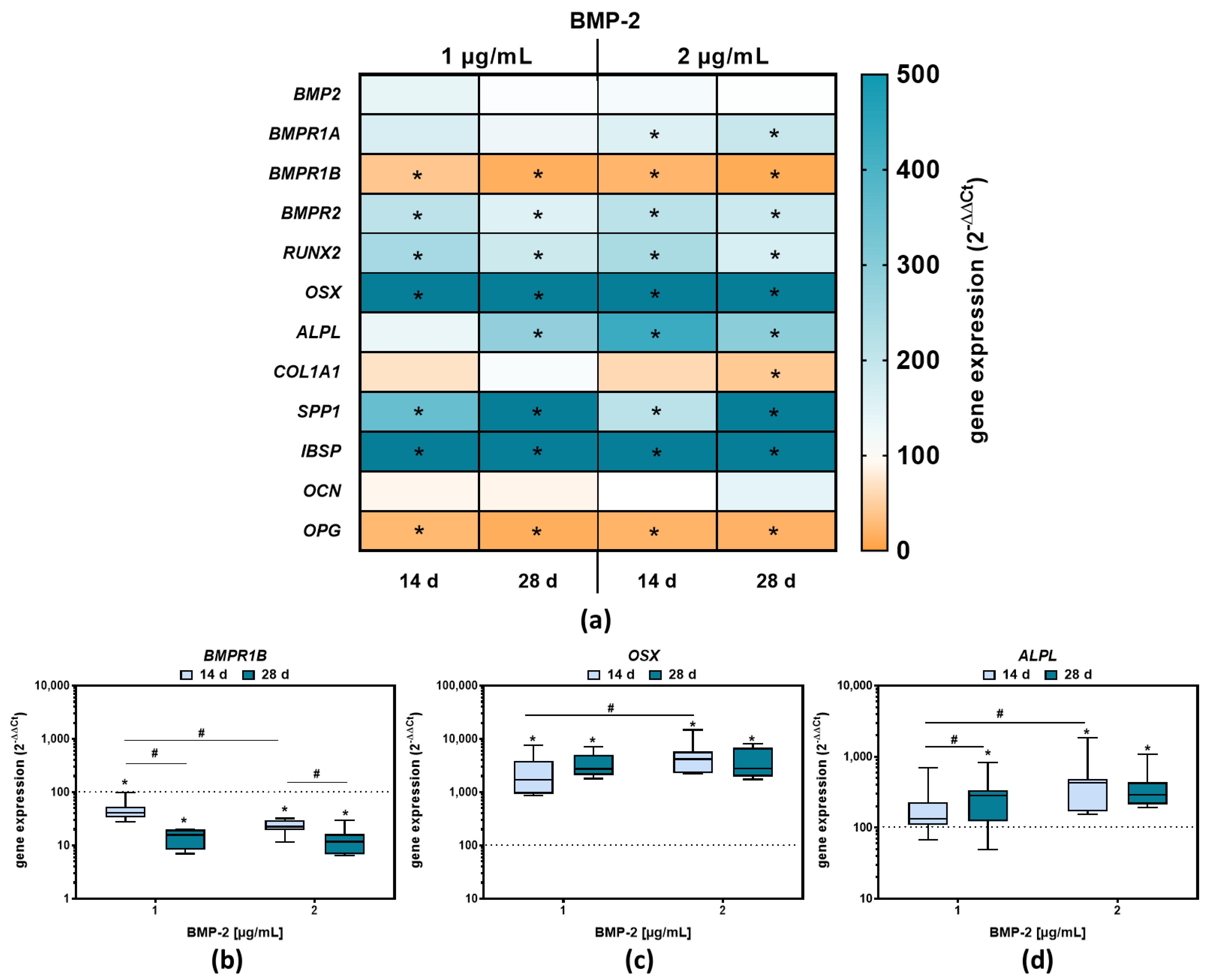
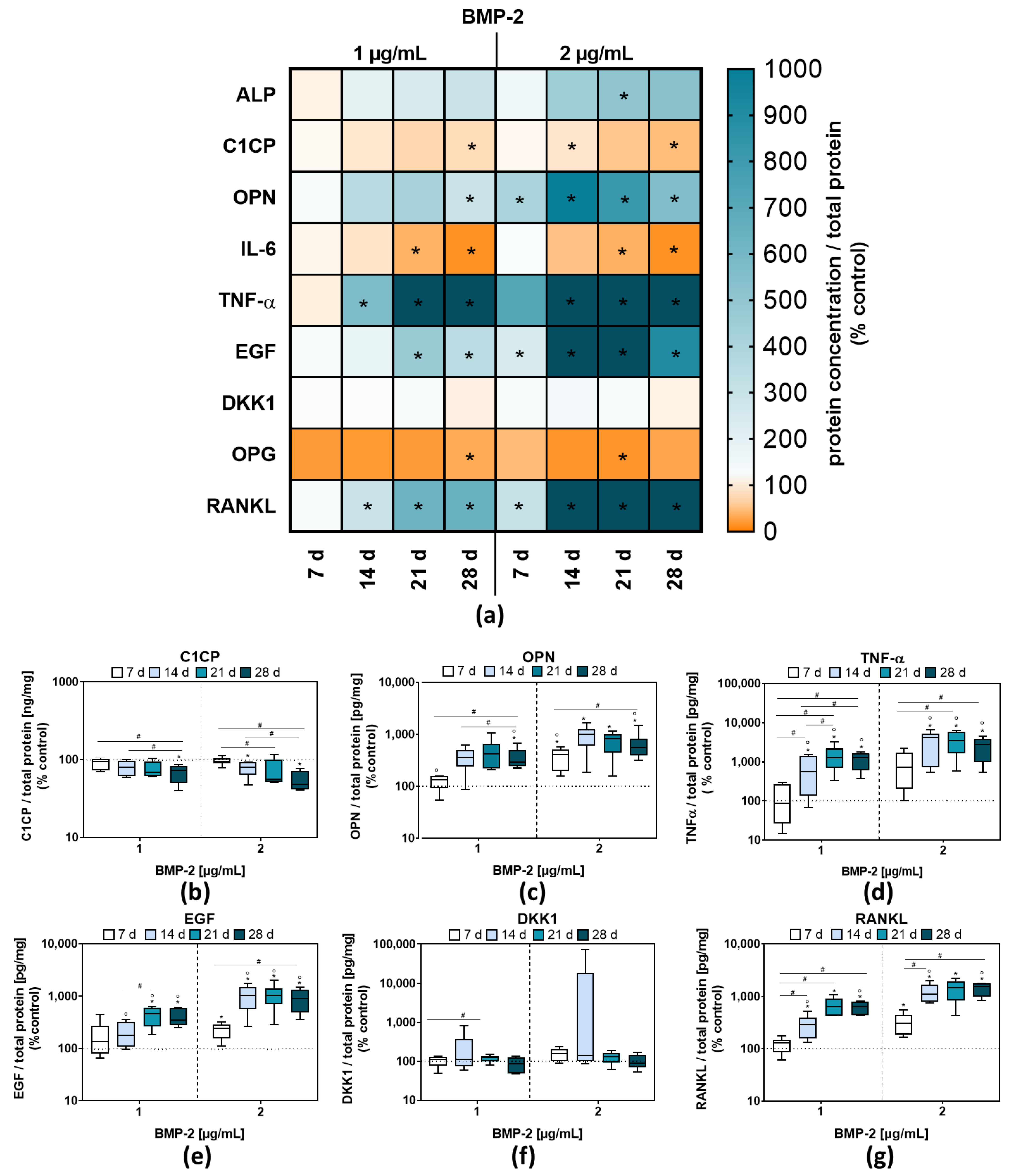
2.2. Osteoblastic Differentiation and Induction of Bone Remodeling Markers following BMP-2 Exposure
2.3. Morphological Changes of Human Pre-Osteoblasts Due to Long-Term BMP-2 Stimulation
3. Discussion
3.1. Influence of BMP-2 on Osteogenic Differentiation Capacity
3.2. Enhanced Inflammation and Osteoclastic Activation Potential Caused by BMP-2 Exposure in Human Pre-Osteoblasts
3.3. Intracellular Inclusions in Human Pre-Osteoblasts after Long-Term BMP-2 Exposure
4. Materials and Methods
4.1. Isolation and Culture of Human Primary Pre-Osteoblasts
4.2. Exposure of Pre-Osteoblasts to Exogenous rh BMP-2
4.3. Analysis of Metabolic Activity
4.4. Quantification of Enzymatic ALP Activity
4.5. Analysis of Gene Expression
4.6. Quantification of Protein Secretion in Cell Culture Supernatants
4.7. Quantification of Reactive Oxygen Species
4.8. Cytoskeleton Staining
4.9. Lipid Droplet Staining
4.10. Imaging by Transmission Electron Microscopy and Scanning Electron Microscopy
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tatara, A.M.; Koons, G.L.; Watson, E.; Piepergerdes, T.C.; Shah, S.R.; Smith, B.T.; Shum, J.; Melville, J.C.; Hanna, I.A.; Demian, N.; et al. Biomaterials-aided mandibular reconstruction using in vivo bioreactors. Proc. Natl. Acad. Sci. USA 2019, 116, 6954–6963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zho, M.; Shao, Z.; Zhu, Y.; Liu, B.; Wu, T. Comparison of Complicated and Simple Guiding Templates in Mandibular Reconstruction Using Vascularized Iliac Crest Flap. BioMed Res. Int. 2019, 2019, 7496538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, B.P.; Venkatesh, V.; Kumar, K.A.J.; Yadav, B.Y.; Mohan, S.R. Mandibular Reconstruction: Overview. J. Maxillofac. Oral Surg. 2016, 15, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Cabral, J.; da Silva, C.; Vashishth, D. Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix. Polymers 2021, 13, 1095. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, D.A.; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; Lang, R.A.; et al. Canonical wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Cremers, S.; Garnero, P.; Seibel, M.J. Biochemical Markers of Bone Metabolism. In Principles of Bone Biology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1857–1881. ISBN 978-0-12-373884-4. [Google Scholar]
- Komori, T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010, 339, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, T.M.; Jensen, E.D.; Westendorf, J.J. Runx2: A master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res. Part C Embryo Today Rev. 2005, 75, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Kim, H.-J.; Li, Q.; Chi, X.-Z.; Ueta, C.; Komori, T.; Wozney, J.M.; Kim, E.-G.; Choi, J.-Y.; Ryoo, H.-M.; et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. 2000, 20, 8783–8792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.; Gao, Y.-H.; Inada, M.; et al. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation owing to Maturational Arrest of Osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, A.; Srinaath, N.; Rohini, M.; Selvamurugan, N. Regulation of Runx2 by MicroRNAs in osteoblast differentiation. Life Sci. 2019, 232, 116676. [Google Scholar] [CrossRef] [PubMed]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev. 2012, 23, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2010, 147, 35–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nohe, A.; Hassel, S.; Ehrlich, M.; Neubauer, F.; Sebald, W.; Henis, Y.I.; Knaus, P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 2002, 277, 5330–5338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibuya, H.; Yamaguchi, K.; Shirakabe, K.; Tonegawa, A.; Gotoh, Y.; Ueno, N.; Irie, K.; Nishida, E.; Matsumoto, K. TAB1: An activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science 1996, 272, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Chuong, C.-M. Activation of protein kinase A is a pivotal step involved in both BMP-2- and cyclic AMP-induced chondrogenesis. J. Cell. Physiol. 1997, 170, 153–165. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, S.; Zhou, G.; Yang, J.; Ji, D.; Sabatakos, G.; Zhu, T. Downregulation of cAMP-dependent protein kinase inhibitor gamma is required for BMP-2-induced osteoblastic differentiation. Int. J. Biochem. Cell Biol. 2006, 38, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Hyzy, S.L.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. BMP2 induces osteoblast apoptosis in a maturation state and noggin-dependent manner. J. Cell. Biochem. 2012, 113, 3236–3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, A.W.; Lachaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- El Bialy, I.; Jiskoot, W.; Nejadnik, M.R. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation In Vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, K.; Kobayashi, Y.; Koide, M.; Uehara, S.; Okamoto, M.; Ishihara, A.; Kayama, T.; Saito, M.; Marumo, K. The Regulation of Bone Metabolism and Disorders by Wnt Signaling. Int. J. Mol. Sci. 2019, 20, 5525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonanantanasarn, K.; Lee, H.-L.; Baek, K.; Woo, K.M.; Ryoo, H.-M.; Baek, J.-H.; Kim, G.-S. EGF Inhibits Wnt/β-Catenin-Induced Osteoblast Differentiation by Promoting β-Catenin Degradation. J. Cell. Biochem. 2015, 116, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Waletzko-Hellwig, J.; Pohl, C.; Riese, J.; Schlosser, M.; Dau, M.; Engel, N.; Springer, A.; Bader, R. Effect of High Hydrostatic Pressure on Human Trabecular Bone Regarding Cell Death and Matrix Integrity. Front. Bioeng. Biotechnol. 2021, 9, 730266. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Raza, S.; Zhao, C.; Cheng, G.; Zan, L. Overexpression of PLIN1 Promotes Lipid Metabolism in Bovine Adipocytes. Animals 2020, 10, 1944. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Z.; Yoshida, C.A.; Furuichi, T.; Amizuka, N.; Ito, M.; Fukuyama, R.; Miyazaki, T.; Kitaura, H.; Nakamura, K.; Fujita, T.; et al. Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev. Dyn. 2007, 236, 1876–1890. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Jiang, Q.; Miyazaki, T.; Komori, T. Runx2 regulates cranial suture closure by inducing hedgehog, Fgf, Wnt and Pthlh signaling pathway gene expressions in suture mesenchymal cells. Hum. Mol. Genet. 2019, 28, 896–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liedtke, D.; Hofmann, C.; Jakob, F.; Klopocki, E.; Graser, S. Tissue-Nonspecific Alkaline Phosphatase—A Gatekeeper of Physiological Conditions in Health and a Modulator of Biological Environments in Disease. Biomolecules 2020, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2006, 17, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Landis, W.; Song, M.; Leith, A.; Mc Ewen, L.; Mc Ewen, B.F. Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J. Struct. Biol. 1993, 110, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Bourne, L.E.; Wheeler-Jones, C.P.; Orriss, I.R. Regulation of mineralisation in bone and vascular tissue: A comparative review. J. Endocrinol. 2021, 248, R51–R65. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.C.; Huesa, C.; Narisawa, S.; Hoylaerts, M.F.; Moreau, A.; Farquharson, C.; Millán, J.L. Ablation of Osteopontin Improves the Skeletal Phenotype of Phospho1−/− Mice. J. Bone Miner. Res. 2014, 29, 2369–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laflamme, C.; Curt, S.; Rouabhia, M. Epidermal growth factor and bone morphogenetic proteins upregulate osteoblast proliferation and osteoblastic markers and inhibit bone nodule formation. Arch. Oral Biol. 2010, 55, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shimizu, E.; Zhang, X.; Partridge, N.; Qin, L. EGFR signaling suppresses osteoblast differentiation and inhibits expression of master osteoblastic transcription factors Runx2 and osterix. J. Cell. Biochem. 2011, 112, 1749–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, V.; Bryant, H.U.; MacDougald, O. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.; Park, H.; Lee, Y.L.; Oh, Y.; Park, J.; Kim, S.Y.; Weon, S.; Choi, S.H.; Yang, J.-H.; Jo, S.; et al. TGFβ1 Suppressed Matrix Mineralization of Osteoblasts Differentiation by Regulating SMURF1–C/EBPβ–DKK1 Axis. Int. J. Mol. Sci. 2020, 21, 9771. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-N.; Jeong, H.-S.; Park, K.-J.; Kim, H.-S.; Kim, E.-H.; Jin, H.-M.; Jung, H.-J.; Ju, J.K.; Choi, S.-E.; Kang, J.-H.; et al. Altered distribution and enhanced osteoclastogenesis of mucosal-associated invariant T cells in gouty arthritis. Rheumatology 2020, 59, 2124–2134. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Li, F.; Li, X.; Wang, Z.-G.; Zhang, B. TNF-α and RANKL promote osteoclastogenesis by upregulating RANK via the NF-κB pathway. Mol. Med. Rep. 2018, 17, 6605–6611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniele, S.; Natali, L.; Giacomelli, C.; Campiglia, P.; Novellino, E.; Martini, C.; Trincavelli, M.L. Osteogenesis Is Improved by Low Tumor Necrosis Factor Alpha Concentration through the Modulation of Gs-Coupled Receptor Signals. Mol. Cell. Biol. 2017, 37, e00442-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerstenfeld, L.; Cho, T.-J.; Kon, T.; Aizawa, T.; Cruceta, J.; Graves, B.; Einhorn, T. Impaired Intramembranous Bone Formation during Bone Repair in the Absence of Tumor Necrosis Factor-Alpha Signaling. Cells Tissues Organs 2001, 169, 285–294. [Google Scholar] [CrossRef]
- David, J.-P.; Schett, G. TNF and Bone. Curr. Dir. Autoimmun. 2010, 11, 135–144. [Google Scholar] [PubMed]
- Standal, T.; Borset, M.; Sundan, A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp. Oncol. 2004, 26, 179–184. [Google Scholar]
- Duong, L.T.; Lakkakorpi, P.; Nakamura, I.; Rodan, G.A. Integrins and signaling in osteoclast function. Matrix Biol. 2000, 19, 97–105. [Google Scholar] [CrossRef]
- Singh, A.; Gill, G.; Kaur, H.; Amhmed, M.; Jakhu, H. Role of osteopontin in bone remodeling and orthodontic tooth movement: A review. Prog. Orthod. 2018, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Rho, J.; Takami, M.; Choi, Y. Osteoimmunology: Interactions of the immune and skeletal systems. Mol. Cells 2004, 17, 1–9. [Google Scholar]
- Walsh, M.C.; Kim, N.; Kadono, Y.; Rho, J.; Lee, S.Y.; Lorenzo, J.; Choi, Y. Osteoimmunology: Interplay between the immune system and bone metabolism. Annu. Rev. Immunol. 2006, 24, 33–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, J.; Begun, D.; Westendorf, J.; McGee-Lawrence, M. Defining osteoblast and adipocyte lineages in the bone marrow. Bone 2019, 118, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Kim, H.-Y.; Kang, D.-C.; Park, J.-H.; Park, J.H. Comparison of Bone Regeneration in Different Forms of Bovine Bone Scaffolds with Recombinant Human Bone Morphogenetic Protein-2. Int. J. Mol. Sci. 2021, 22, 11121. [Google Scholar] [CrossRef]
- Tencerova, M.; Kassem, M. The Bone Marrow-Derived Stromal Cells: Commitment and Regulation of Adipogenesis. Front. Endocrinol. 2016, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Yang, L.; Luo, Z.-J. Transdifferentiation between bone and fat on bone metabolism. Int. J. Clin. Exp. Pathol. 2014, 7, 1834–1841. [Google Scholar]
- McGee-Lawrence, M.; Carpio, L.R.; Schulze, R.J.; Pierce, J.; McNiven, M.A.; Farr, J.N.; Khosla, S.; Oursler, M.J.; Westendorf, J.J. Hdac3 Deficiency Increases Marrow Adiposity and Induces Lipid Storage and Glucocorticoid Metabolism in Osteochondroprogenitor Cells. J. Bone Miner. Res. 2016, 31, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochner, K.; Fritsche, A.; Jonitz, A.; Hansmann, D.; Mueller, P.; Mueller-Hilke, B.; Bader, R. The potential role of human osteoblasts for periprosthetic osteolysis following exposure to wear particles. Int. J. Mol. Med. 2011, 28, 1055–1063. [Google Scholar] [CrossRef] [PubMed]



| Primer | Sequences (5′-3′) |
|---|---|
| Alkaline Phosphatase, Biomineralization Associated (ALPL) | fwd: CATTGTGACCACCACGAGAG |
| rev: CCATGATCACGTCAATGTCC | |
| Bone Morphogenetic Protein 2 (BMP-2) | fwd: GCGTGAAAAGAGAGACTGCG |
| rev: ACCATGGTCGACCTTTAGGAG | |
| Bone Morphogenetic Protein Receptor Type 1A (BMPR1A) | fwd: AGCCACATCTTGGAGGAGTCG |
| rev: ATGTTTCCTGTGTACTGTCACCTT | |
| Bone Morphogenetic Protein Receptor Type 1B (BMPR1B) | fwd: TGCCTTGTTGATAAAGGTTCAGAC |
| rev: TTCCTGCACTTCGCAAAAGC | |
| Bone Morphogenetic Protein Receptor Type 2 (BMPR2) | fwd: GGGTAAGCTCTTGCCGTCTT |
| rev: CTGATAGTGCCAACCTCGCT | |
| Caspase 8 (CASP8) | fwd: TGTTTTCACAGGTTCTCCTCCTTT |
| rev: GAGAATATAATCCGCTCCACCCTT | |
| Collagen Type I Alpha 1 Chain (COL1A1) | fwd: ACGAAGACATCCCACCAATC |
| rev: AGATCACGTCATCGCACAAC | |
| Fas Associated Via Death Domain (FADD) | fwd: ACCGAGCTCAAGTTCCTATGC |
| rev: AAATGCTGCACACAGGTCTTC | |
| Fas Cell Surface Death Receptor (FAS) | fwd: TGACCCTTGCACCAAATGTGA |
| rev: AGACAAAGCCACCCCAAGTT | |
| Hypoxanthine Phosphoribosyl-transferase 1 (HPRT) | fwd: CCCTGGCGTCGTGATTAGTG |
| rev: TCGAGCAAGACGTTCAGTCC | |
| Integrin Binding Sialoprotein (IBSP) | fwd: ATTTTGGGAATGGCCTGTGC |
| rev: GTCACTACTGCCCTGAACTGG | |
| Osteocalcin (OCN) | fwd: TCAGCCAACTCGTCACAGTC |
| rev: GGTGCAGCCTTTGTGTCC | |
| Osteoprotegerin (OPG) | fwd: TGTGGAATAGATGTTACCCTGTGTG |
| rev: ACACTAAGCCAGTTAGGCGT | |
| Osterix (OSX) | fwd: TAGGACTGTAGGACCGGAGC |
| rev: CCATAGTGAACTTCCTCCTCAAG | |
| Perilipin 1 (PLIN1) | fwd: AAGGGAAGAAGTTGAAGCTTGAGG |
| rev: CACGCCCTTCTCATAGGCAT | |
| Peroxisome Proliferator Activated Receptor Gamma (PPARG) | fwd: AGTCAGCCTTTAACGAAATGACC |
| rev: CACGGAGCTGATCCCAAAGT | |
| RUNX Family Transcription Factor 2 (RUNX2) | fwd: CGCCTCACAAACAACCACAG |
| rev: ACTGCTTGCAGCCTTAAATGAC | |
| Secreted Phosphoprotein 1 (SPP1) | fwd: AACGCCGACCAAGGAAAACT |
| rev: GCACAGGTGATGCCTAGGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingwersen, L.-C.; Frank, M.; Naujokat, H.; Loger, K.; Bader, R.; Jonitz-Heincke, A. BMP-2 Long-Term Stimulation of Human Pre-Osteoblasts Induces Osteogenic Differentiation and Promotes Transdifferentiation and Bone Remodeling Processes. Int. J. Mol. Sci. 2022, 23, 3077. https://doi.org/10.3390/ijms23063077
Ingwersen L-C, Frank M, Naujokat H, Loger K, Bader R, Jonitz-Heincke A. BMP-2 Long-Term Stimulation of Human Pre-Osteoblasts Induces Osteogenic Differentiation and Promotes Transdifferentiation and Bone Remodeling Processes. International Journal of Molecular Sciences. 2022; 23(6):3077. https://doi.org/10.3390/ijms23063077
Chicago/Turabian StyleIngwersen, Lena-Christin, Marcus Frank, Hendrik Naujokat, Klaas Loger, Rainer Bader, and Anika Jonitz-Heincke. 2022. "BMP-2 Long-Term Stimulation of Human Pre-Osteoblasts Induces Osteogenic Differentiation and Promotes Transdifferentiation and Bone Remodeling Processes" International Journal of Molecular Sciences 23, no. 6: 3077. https://doi.org/10.3390/ijms23063077






